Abstract
This report presents a fluorescent carboxyfluorescein diacetate (CFDA)-modified microdilution method used for the susceptibility testing of Candida albicans to amphotericin B, fluconazole, ketoconazole, itraconazole, voriconazole, and flucytosine. Four different broth microdilution susceptibility testing methods were simultaneously evaluated at 24 and 48 h. The MICs determined using the CFDA-modified method (MICcfda) were compared to those obtained by the standard broth microdilution method (MICvisual) and a procedure employing the indicator Alamar blue (MICalamar). The reference MIC was determined visually as recommended by the NCCLS M27-A protocol, and then quantified spectrophotometrically following agitation (MICspec). The CFDA-modified microdilution method was demonstrated to effectively determine the MICs for all the antifungal drugs tested at both 24 and 48 h. The results from both the MICspec and MICcfda methods yielded >80% agreement within ±1 dilution and >90% agreement within ±2 dilutions at 24 h in comparison to the reference MICvisual method, respectively. The trailing growth phenomenon that occurs with azole antifungal drugs and many strains of C. albicans did not inhibit the effectiveness of the MICspec and MICcfda methods. The MICspec and MICcfda methods shared 92.8% agreement within ±1 dilution at 24 h and 87.6% agreement within ±1 dilution at 48 h.
Reliable antifungal susceptibility testing of pathogenic and opportunistic fungi is of growing importance due to the emergence of antifungal-drug resistance (26), the development of new antifungal drugs (3, 12), and the increased prevalence of serious fungal infections in critically ill and immunocompromised patients (17). In 1997 the NCCLS published reference guidelines for susceptible testing of Candida spp. and Cryptococcus neoformans against fluconazole (FLC), ketoconazole (KTC), itraconazole (ITC), flucytosine (5FC), and amphotericin B (AMB) (11). The broth antifungal susceptibility testing guidelines have provided greater interlaboratory reproducibility and served as a reference for the development of more-efficient modifications and alternative approaches. The determination of MIC endpoints remains a major source of interlaboratory variability (5, 19, 24). The trailing-growth phenomenon is often responsible for subjective and variable endpoint interpretations (5, 8, 14, 15, 24). Trailing growth is caused by the partial inhibition of fungal growth over the range of antifungal concentrations, especially with fungistatic drugs. Several studies have used the colorimetric (4, 9, 16, 25) and spectrophotometric (1, 5, 13, 18) determination of broth microdilution endpoints to address this problem. Previous work has also demonstrated the utility of using the fluorescent dye carboxyfluorescein diacetate (CFDA) in the standardized NCCLS M27-A broth microdilution method to successfully assess susceptibility of Candida to FLC (11). In this study we evaluated the applicability of the CFDA-modified microdilution method for susceptibility testing of clinical Candida albicans isolates to 5FC, AMB, ITC, KTC, FLC, and voriconazole (VRC). We simultaneously compared the MIC endpoints determined using the CFDA-modified microdilution method (MICcfda) with the microdilution MICs obtained using Alamar blue endpoints (MICalamar), spectrophotometric endpoints (MICspec), and the standard visual endpoints (MICvisual).
MATERIALS AND METHODS
Antifungal drugs.
Analytical-grade powders of the following six antifungal drugs to be tested were provided by the indicated manufacturers: AMB (Bristol Myers-Squibb Co., Princeton, N.J.), FLC and VRC (Pfizer, Inc., Groton, Conn.), KTC and ITC (Research Diagnostics, Inc., Flanders, N.J.), and 5FC (Hoffmann-La Roche, Inc., Nutley, N.J.). Stock solutions of FLC, VRC, and 5FC were prepared in distilled water, and those of AMB, ITC, and KTC were prepared in 100% dimethyl sulfoxide. Stock solutions of antifungal agents were made to 10,000 μg/ml and frozen at −70°C. Each of the stock solutions was thawed once, and fresh dilutions were used.
Yeast isolates.
Yeast isolates were obtained from the National Centre for Mycology, Edmonton, Alberta, Canada. One strain of C. albicans was kindly supplied by John H. Rex from the Center for the Study of Emerging Pathogens and Reemerging Pathogens, Laboratory of Mycology Research, University of Texas Medical School, Houston. The identity of the isolates was confirmed by standard methods (27). Isolates were stored in skim milk at −70°C and then subcultured twice on Sabouraud dextrose agar (Difco, Sparks, Md.) before use. Additional reference strains of C. albicans (ATCC 90028 and ATCC 24433) were included.
Antifungal susceptibility testing.
The reference NCCLS broth microdilution method was performed as described in the M27-A document (11). Antifungal concentrations were diluted in RPMI 1640 medium with l-glutamine and morpholinepropanesulfonic acid (MOPS) buffer at 165 mM and pH 7 (Angus Buffers & Biochemicals, Niagara Falls, N.Y.), and 100-μl aliquots were placed into the wells of 96-well microtiter Linbro plates (Flow Laboratories Inc., McLean, Va.) with clear, U-shaped well bottoms. The final concentration ranges used were 0.125 to 64 μg/ml for 5FC and FLC and 0.03 to 64 μg/ml for ITC, KTC, VRC, and AMB. Six C. albicans strains were tested per 96-well plate, which allowed the outermost wells to be filled with sterile water to minimize evaporation.
Five colonies of C. albicans with diameters of ≥1 mm were suspended in sterile 0.85% saline and adjusted to a final concentration (after inoculation) of 0.5 × 103 to 2.5 × 103 cells per ml in RPMI 1640-MOPS medium. The inoculum was directly added to the antifungal-containing trays and incubated at 35°C without agitation. Endpoints were recorded visually, spectrophotometrically, and fluorometrically at 24 and 48 h. All measurements were performed in triplicate for each yeast strain assayed.
(i) MICvisual.
The 96-well microdilution plates were first read visually without agitation as recommended by the reference NCCLS M27-A protocol (11). The reference broth microdilution method was scored by comparing the growth in each well with that in the growth control (drug-free) well. The MICs of the azole antifungals and 5FC were defined as the minimum drug concentration at which visual growth was determined to be 80% relative to that of the growth control (Fig. 1d) (11). The MIC of AMB was defined as the lowest drug concentration at which there was 100% inhibition of growth compared with the growth for the drug-free control (11).
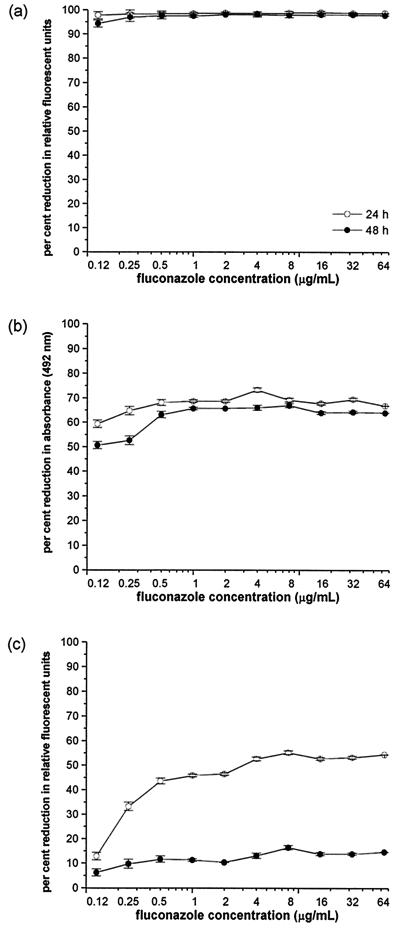
FIG. 1.
FLC MIC endpoints at 24 and 48 h for the low-high-phenotype C. albicans strain MY894as determined by four microdilution MIC methods: the MICcfda method (a), the MICspec method (b), the MICalamar method (c), and the reference NCCLS microdilution (MICvisual) method (d). The results for the MICcfda, MICspec, and MICalamar methods are shown graphically. The fluorescence or optical density of the growth in the drug-free control was defined as 100%, and the FLC-exposed wells werescaled to this value. The results of the MICvisual reference M27-A method are shown as a digital image of the unagitated growth in the 96-well plate at 24 and 48 h. The error bars represent standard error.
(ii) MICspec.
The supernates were first removed from the microdilution plate wells using a multichannel pipettor, and the remaining organisms were resuspended to 200 μl per well in 35°C-warmed 0.1 M MOPS buffer at pH 3.5 with 50 mM citric acid. The buffer resuspension step was included to allow the same plate that was evaluated using the MICvisual and MICspec methods to also be subsequently evaluated using the CFDA-modified microdilution method. A multichannel pipettor was used to resuspend well contents by pipetting, alternately filling and emptying the wells 20 times. The MICspec method was performed using a microtiter plate spectrophotometer (Diagnostics Pasteur, Chaska, Minn.) set at 492 nm. The absorbance of the drug-free control was defined as 100%, and the absorbance of the antifungal-exposed wells were reported proportionally to this value (Fig. 1b). The MIC endpoints for the azoles, 5FC, and AMB were defined as the lowest concentration of antifungal drug at which the absorbance was reduced to ≤50% in comparison to that for the drug-free growth control (1, 13, 18).
(iii) MICcfda.
The CFDA-modified microdilution method (8) employs a vitality-specific fluorescent dye at 24 and 48 h with the 96-well microdilution plates prepared according to the NCCLS M27-A protocol (11). After the removal of supernates and resuspension in 0.1 M MOPS buffer at pH 3.5 with 50 mM citric acid, 5 μl of a stock of 5(6)-CFDA (5 mg/ml; Molecular Probes, Eugene, Oreg.) in dimethyl sulfoxide was added to each well for a final concentration of 122 μg/ml. A multichannel pipettor was used to resuspend well contents by repetitive pipetting 20 times. The trays were then incubated without shaking in the dark at 35°C for 1 h. The well contents were resuspended again, as before, and the trays were assayed for relative fluorescence intensity with a FL500 microplate fluorescence reader (Bio-Tek Instruments Inc., Winooski, Vt.). 5(6)-CFDA was evaluated using excitation and emission wavelengths of 485 and 530 nm, respectively. The fluorescence of the drug-free control was defined as 100%, and the fluorescence of the antifungal-exposed wells was reported proportionally to this value. The MIC endpoints were defined as the lowest concentration of antifungal drug at which the fluorescence was reduced to 80% of that of the drug-free growth control (Fig. 1a and 2). This endpoint criterion was inclusive for all antifungal drugs tested.
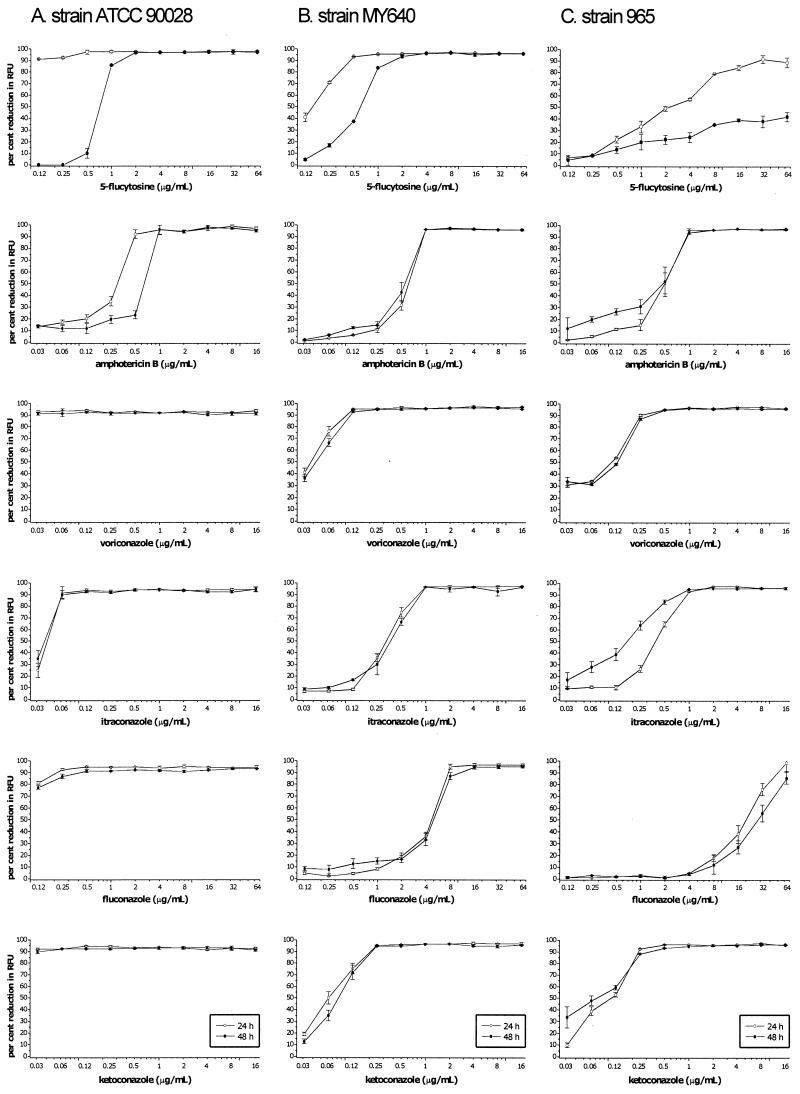
FIG. 2.
MICcfdas of 5FC, AMB, KTC, VRC, ITC, and FLC for three representative strains of C. albicans—ATCC 90028 (A), MY640 (B), and 965 (C)—at 24 and 48 h. The fluorescence of the growth in the drug-free control is defined as 100%. The error bars represent standard errors.
(iv) MICalamar.
The Alamar blue method was also identical to the reference method in terms of inoculum, drug dilution, and incubation, with the exception of the addition of the oxidation-reduction indicator Alamar blue. Prior to the inoculation of each plate, 5 μl of 10× Alamar blue (Trek Diagnostic Systems, Inc., Westlake, Ohio) was added to each well. The colorimetric MIC endpoints were read successively at 24 and 48 h. The growth in each well was visually indicated by a color change from blue to red. Fungal isolates which exhibit trailing growth will also produce hues of purple color in transition from blue to red. To accommodate the trailing color transition and to provide an objective and quantifiable endpoint, Alamar blue plates were assayed for fluorescence with a FL500 microplate fluorescence reader (Bio-Tek Instruments Inc.). Alamar blue was evaluated using the optimal excitation and emission wavelengths of 530 and 590 nm, respectively. The fluorescence of the drug-free control was defined as 100%, and the fluorescence of the antifungal-exposed wells were reported proportionally to this value. The MIC endpoints for the azoles, 5FC, and AMB were defined as the lowest concentrations at which the absorbance was reduced to ≤50% of that of the drug-free growth control (Fig. 1c).
RESULTS
A comparative evaluation of the MICs determined using four different broth microdilution methods for six antifungal drugs was conducted with 16 C. albicans strains (14 clinical isolates and two reference strains). The four methods included endpoint determinations by the standard visual estimation (MICvisual), a spectrophotometric measurement (MICspec), and fluorescent measurements after incubation with either CFDA (MICcfda) or Alamar blue (MICalamar). The C. albicans isolates chosen covered a broad range of susceptibilities determined using the reference method: for 5FC, 0.12 to 64 μg/ml; for AMB, 0.25 to 1 μg/ml; for KTC, 0.03 to 16 μg/ml; for VRC, 0.03 to 16 μg/ml; for ITC, 0.06 to >16 μg/ml; and for FLC, 0.12 to >64 μg/ml). The MICs at which 50% of the isolates tested were inhibited (MIC50s) and MIC90s for C. albicans were as follows, respectively: 0.25 and >64 μg/ml for 5FC, 0.5 and 1 μg/ml for AMB, 0.12 and 16 μg/ml for KTC, 0.12 and >16 μg/ml for VRC, 0.25 and >16 μg/ml for ITC, and 1 and 64 μg/ml for FLC. The MICs for two control strains, ATCC 90028 and ATCC 24433, were within the accepted limits.
The CFDA-modified microdilution method allowed the simple quantification of antifungal inhibition and the production of dose-response curves (Fig. 1a and 2). The MICcfda were easily visualized for all the antifungals tested using these dose-response curves. Table 1 summarizes the overall distribution of difference and the percent agreement in the MICs of all six antifungals by comparing the four microdilution methods. The MICspec and MICcfda methods both yielded >80% agreement within ±1 dilution compared to the reference MICvisual method at 24 h and >80% agreement within ±2 dilutions at 48 h. The MICspec and MICcfda endpoints were both lower than the MICvisual endpoints at 24 and 48 h. The highest percentages of agreement of endpoints between the MICvisual method compared to the MICspec and MICcfda methods were both at 24 h within ±2 dilutions, with 97.9 and 94.7% agreement, respectively. Further comparison between the MIC methods using the individual antifungal drugs (Table 2) showed that the MICcfda endpoints for 5FC and AMB had >90% agreement within ±1 dilution compared to the MICvisual endpoints at both 24 and 48 h. The results were similar for the MICspec method, comparing the MICspec endpoints for 5FC and AMB to the MICvisual endpoints, with the exception that the percentage agreement within ±1 dilution was lower; and noticeably so with AMB at 24 h. The greatest discrepancies between the MICspec and MICcfda endpoints compared to the MICvisual endpoints were due to the azole antifungals at 48 h (Table 2). These discrepancies at 48 h were not further evaluated to see if they persisted.
TABLE 1.
Distribution of differences between the broth microdilution MIC endpoints for all antifungal agents in this studya
| Methods compared | Incubation time (h) | % Discrepancies between MICsb
|
% Agreement within no. of dilutions
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| < −2 | −2 | −1 | 0 | 1 | 2 | >2 | 1 | 2 | ||
| MICcfda and MICspec | 24 | 1 | 3.1 | 19.8 | 66.7 | 6.3 | 3.1 | 0 | 92.8 | 99 |
| 48 | 1 | 7.3 | 16.7 | 63.6 | 7.3 | 3.1 | 1 | 87.6 | 98 | |
| MICcfda and MICvisual | 24 | 5.2 | 8.3 | 20.8 | 62.5 | 2.1 | 1 | 0 | 85.4 | 94.7 |
| 48 | 16.7 | 12.5 | 17.7 | 51 | 2.1 | 0 | 0 | 70.8 | 83.3 | |
| MICalamar and MICcfda | 24 | 0 | 0 | 1 | 22.9 | 24 | 17.7 | 34.4 | 47.9 | 65.6 |
| 48 | 0 | 0 | 0 | 14.6 | 12.5 | 7.3 | 65.6 | 27.1 | 34.4 | |
| MICspec and MICvisual | 24 | 1 | 11.5 | 13.5 | 67.7 | 5.2 | 0 | 1 | 86.4 | 97.9 |
| 48 | 11.5 | 9.4 | 17.7 | 57.3 | 3.1 | 1 | 0 | 78.1 | 88.5 | |
| MICalamar and MICspec | 24 | 0 | 0 | 0 | 22.9 | 29.2 | 16.7 | 31.3 | 52.1 | 68.8 |
| 48 | 0 | 0 | 0 | 13.6 | 9.4 | 6.3 | 70.8 | 23 | 29.3 | |
| MICalamar and MICvisual | 24 | 0 | 0 | 5.2 | 24 | 22.9 | 24 | 24 | 52.1 | 76.1 |
| 48 | 0 | 0 | 4.2 | 20 | 13.5 | 7.3 | 55.2 | 37.7 | 45 | |
Broth microdilution MIC endpoints for 16 strains of C. albicans for all six antifungal agents at 24 and 48 h as determined by the comparison of alternative methods (MICspec, MICalamar, and MICcfda) with the MICvisual.
Dilution differences (log2) from < −2 to > 2 are indicated.
TABLE 2.
Distribution of differences between the broth microdilution MIC endpoints for each antifungal agent used in this study
| Methods compared | Drug | Incubation time (h) | % Discrepancies between MICsb
|
% Agreement within no. of dilutions
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| < −2 | −2 | −1 | 0 | 1 | 2 | > 2 | 1 | 2 | |||
| MICcfda and MICspec | 5FC | 24 | 6.3 | 6.3 | 68.8 | 18.8 | 93.8 | 93.8 | |||
| 48 | 87.5 | 6.3 | 6.3 | 87.5 | 93.8 | ||||||
| AMB | 24 | 12.5 | 62.5 | 12.5 | 12.5 | 87.5 | 100 | ||||
| 48 | 56.3 | 43.8 | 100 | 100 | |||||||
| KTC | 24 | 6.3 | 87.5 | 6.3 | 100 | 100 | |||||
| 48 | 6.3 | 75 | 18.8 | 100 | 100 | ||||||
| VRC | 24 | 18.8 | 81.3 | 100 | 100 | ||||||
| 48 | 6.3 | 25 | 68.8 | 93.8 | 93.8 | ||||||
| ITC | 24 | 6.3 | 12.5 | 68.8 | 6.3 | 6.3 | 87.5 | 100 | |||
| 48 | 18.8 | 12.5 | 68.8 | 81.3 | 81.3 | ||||||
| FLC | 24 | 6.3 | 56.3 | 37.5 | 93.8 | 100 | |||||
| 48 | 25 | 43.8 | 31.3 | 75 | 100 | ||||||
| MICcfda and MICvisual | 5FC | 24 | 6.3 | 6.3 | 75 | 12.5 | 93.8 | 93.8 | |||
| 48 | 12.5 | 87.5 | 100 | 100 | |||||||
| AMB | 24 | 6.3 | 43.8 | 50 | 93.8 | 100 | |||||
| 48 | 18.8 | 68.8 | 12.5 | 100 | 100 | ||||||
| KTC | 24 | 6.3 | 12.5 | 81.3 | 93.8 | 100 | |||||
| 48 | 25 | 12.5 | 18.8 | 43.8 | 62.5 | 75 | |||||
| VRC | 24 | 6.3 | 25 | 68.8 | 93.8 | 100 | |||||
| 48 | 18.8 | 12.5 | 25 | 43.8 | 68.8 | 81.3 | |||||
| ITC | 24 | 6.3 | 12.5 | 6.3 | 68.8 | 6.3 | 75 | 93.8 | |||
| 48 | 31.3 | 12.5 | 18.8 | 37.5 | 56.3 | 68.8 | |||||
| FLC | 24 | 12.5 | 18.8 | 37.5 | 31.3 | 68.8 | 87.5 | ||||
| 48 | 25 | 43.8 | 6.3 | 25 | 31.3 | 75 | |||||
| MICspec and MICvisual | 5FC | 24 | 6.3 | 6.3 | 18.8 | 62.5 | 6.3 | 87.5 | 93.8 | ||
| 48 | 6.3 | 6.3 | 12.5 | 75 | 87.5 | 93.8 | |||||
| AMB | 24 | 6.3 | 18.8 | 31.3 | 43.8 | 75 | 93.8 | ||||
| 48 | 6.3 | 43.8 | 50 | 93.8 | 100 | ||||||
| KTC | 24 | 6.3 | 87.5 | 6.3 | 93.8 | 100 | |||||
| 48 | 18.8 | 12.5 | 12.5 | 43.8 | 6.3 | 6.3 | 62.5 | 81.3 | |||
| VRC | 24 | 6.3 | 6.3 | 87.5 | 93.8 | 100 | |||||
| 48 | 12.5 | 12.5 | 6.3 | 68.8 | 75 | 87.5 | |||||
| ITC | 24 | 12.5 | 6.3 | 81.3 | 87.5 | 100 | |||||
| 48 | 12.5 | 12.5 | 12.5 | 62.5 | 75 | 87.5 | |||||
| FLC | 24 | 18.8 | 25 | 37.5 | 18.8 | 81.3 | 100 | ||||
| 48 | 18.8 | 12.5 | 18.8 | 37.5 | 12.5 | 68.8 | 81.3 | ||||
| MICalamar and MICvisual | 5FC | 24 | 6.3 | 43.8 | 31.3 | 12.5 | 6.3 | 81.3 | 93.8 | ||
| 48 | 6.3 | 50 | 37.5 | 6.3 | 93.8 | 93.8 | |||||
| AMB | 24 | 18.8 | 31.3 | 37.5 | 12.5 | 87.5 | 100 | ||||
| 48 | 6.3 | 18.8 | 37.5 | 37.5 | 62.5 | 100 | |||||
| KTC | 24 | 31.3 | 12.5 | 25 | 31.3 | 43.8 | 68.8 | ||||
| 48 | 12.5 | 6.3 | 81.3 | 18.8 | 18.8 | ||||||
| VRC | 24 | 31.3 | 6.3 | 18.8 | 43.8 | 37.5 | 56.3 | ||||
| 48 | 12.5 | 87.5 | 12.5 | 12.5 | |||||||
| ITC | 24 | 6.3 | 25 | 31.3 | 37.5 | 31.3 | 62.5 | ||||
| 48 | 12.5 | 87.5 | 12.5 | 12.5 | |||||||
| FLC | 24 | 6.3 | 6.3 | 31.3 | 31.3 | 25 | 43.8 | 75 | |||
| 48 | 18.8 | 6.3 | 6.3 | 68.8 | 25 | 31.3 | |||||
Broth microdilution MIC endpoints for 16 strains of C. albicans for each of six antifungal agents at 24 and 48 h as determined by the comparison of alternative methods (MICspec, MICalamar, and MICcfda) with the MICvisual.
Dilution differences (log2) from < −2 to > 2 are indicated.
Between all of the MIC methods, the highest percentage agreement was between the MICcfda and MICspec methods, yielding 92.8% agreement within ±1 dilution and 99% agreement within ±2 dilutions at 24 h and 87.6% agreement within ±1 dilution and 98% agreement within ±2 dilutions at 48 h (Table 1). The greatest discrepancy between the MICcfda and MICspec methods (Tables 1 and 2) was due to the MICcfda endpoints for FLC at 48 h being lower than the endpoints obtained by the MICspec method. Additional comparison between the MICspec and MICcfda methods for the drugs with established interpretive breakpoints (FLC, ITC, and 5FC) (11) showed that all of the endpoint differences were by definition minor errors.
There was very low overall agreement between the endpoints obtained using the MICalamar method compared to those endpoints obtained using the MICvisual, MICspec, and MICcfda methods (Table 1). This discrepancy was due to the consistently higher values obtained using the MICalamar method. The MICalamar endpoints for 5FC and AMB had >90% agreement within ±2 dilutions compared to the MICvisual endpoints at both 24 and 48 h (Table 2). The MICalamar endpoints for all of the azole antifungals had very low agreement in comparison to the MICvisual endpoints. The best agreement that could be obtained using the MICalamar method in comparison to the MICvisual, MICspec, and MICcfda methods was obtained at 24 h (Table 1).
Two of the C. albicans strains, MY894 and 707-15 (Fig. 1), tested by the reference NCCLS microdilution method using visual endpoint determinations produced extreme trailing endpoints with KTC, VRC, ITC, and FLC. These strains which manifested partial inhibition of growth over the entire range of azoles resulted in discordant MICs at 24 (sensitive) and 48 h (resistant). These strains, when evaluated using the both MICcfda and MICspec methods, were not discordant at 24 versus 48 h but were susceptible to all of the azoles at 24 and 48 h. The MICs for the low-high-phenotype strain MY894 are shown in Fig. 1. The MICs obtained by the MICcfda method (80% inhibition) and the MICspec method (50% inhibition) were ≤0.12 μg/ml at both 24 and 48 h. However, the MIC obtained using the MICalamar method (50% inhibition) was 4.0 μg/ml at 24 h and >64 μg/ml at 48 h. The MIC obtained using the reference MICvisual method (80% inhibition) was 0.5 μg/ml at 24 h and trailed to >64 μg/ml at 48 h.
DISCUSSION
We have previously demonstrated the utility of CFDA for susceptibility testing of FLC with C. albicans (8). The use of CFDA for assessing the vitality of C. albicans exposed to AMB under different conditions has also been previously demonstrated (7). The CFDA-modified microdilution format does not alter the M27-A microdilution protocol (11). The fluorescent dye CFDA is added to the microdilution tray at the 24- or 48-h endpoint and does not interfere in any way with the interaction between the fungal cells and antifungal agent. In this manner, the different endpoints can be sequentially determined visually (MICvisual), spectrophotometrically (MICspec), and fluorescently (MICcfda), all with the same 96-well microdilution plate. The subjective nature of visual endpoint determination and the partial inhibition of fungal growth that occurs with azole antifungals were responsible for the higher endpoints derived using the MICvisual method, especially at 48 h (Table 2). Interestingly, between the MICspec and MICcfda methods, the MICspec endpoints had lower agreement to the MICvisual endpoints for AMB and 5FC, especially AMB at 24 h. Due to the relative ease in determining visual endpoints for 5FC and AMB, this result suggests an advantage of the MICcfda method over the MICspec method.
The spectrophotometric measurement of turbidity to evaluate the inhibition of fungal growth (MICspec) has been previously shown to provide an objective measurement and correlate well with the reference method (1, 5, 13, 18). The MICcfda microdilution method is a completely different methodological way of evaluating viability. CFDA is a fluorescent, nonpolar substrate that diffuses across the cell membrane and is hydrolyzed by nonspecific intracellular esterases to the fluorescent anion carboxyfluorescein (20). The MICcfda and MICspec endpoints are also differentially measured at 80 and 50% inhibition compared to the growth control, respectively. Thus, the MICcfda method provides an objective and independent confirmation of the MICspec at both 24 and 48 h. The greatest difference in endpoints between the MICcfda and MICspec methods was observed with FLC and ITC at 48 h (Table 2). The CFDA-modified method utilizes the CFDA vitality dye, which has been shown to be a good measure of C. albicans viability (7). Thus, one interesting possibility is that the lower MICs of FLC and ITC determined using the CFDA method were due to the detection of decreased viability that could not be determined spectrophotometrically, since the MICspec method is a measure of cell number.
Colorimetric endpoint determination of microdilution susceptibility testing has been previously demonstrated to improve endpoint reading under some circumstances (4, 9, 16, 25). However, the colorimetric microdilution method has also been shown to be difficult to evaluate at 48 h due to the partial inhibition of growth with azole antifungals (16, 25), and some species-specific discrepancies have been observed (9, 16). We also have shown extensive trailing of the azole endpoints with the MICalamar method at 48 h (Table 1). There was good agreement between the MICalamar and MICvisual methods for AMB and 5FC endpoints (Table 2). However, there was also less than 50% agreement within ±1 dilution at 24 h for azole endpoints determined by the MICalamar method compared to those determined by each of the three other MIC methods.
The trailing growth phenomenon is exacerbated over time with low-high-phenotype strains, producing discordant MICs (MICvisual) of <8 μg/ml (susceptible) at 24 h and of ≥64 μg/ml (resistant) at 48 h (2, 8, 21, 22). In this study, we included 2 strains (MY894 and 707-15) with such discordant MICs (MICvisual) of FLC, KTC, ITC, and VRC. We previously showed that the CFDA-modified microdilution method could identify 12 low-high-phenotype strains of C. albicans to be susceptible to FLC at both 24 and 48 h (8). The two low-high-phenotype strains examined in this study using the MICcfda method were also shown to be susceptible at both 24 and 48 h to FLC, KTC, ITC, and VRC. The MICspec method confirmed these results. These observations are supported by current evidence that suggests that the lower MIC at 24 h obtained by the reference method (MICvisual) correlates best with the in vivo outcome (10, 21, 22). The MICalamar method determined both of the low-high-phenotype strains to have endpoints at 48 h which were resistant to the azole antifungals.
The endpoints for susceptibility testing of Candida spp. to 5FC and azole antifungals were established by the M27-A protocol with a criterion of 80% reduction in growth after 48 h of incubation (11). It has been suggested that the trailing azole MIC endpoint phenomenon may be reduced by lowering the MIC endpoint to a 50% reduction in growth and by shortening the incubation time to 24 h (16, 22, 23). The requirement of a 48-h incubation for optimal testing conditions may represent a barrier to this change (6, 18, 22). It is important that the selection of a 24- or 48-h incubation time be based on the best agreement with clinical outcome, without overdue consideration for difficulties encountered with in vitro trailing endpoints to the azole antifungals. The results described here demonstrate that the trailing-growth phenomenon does not effect the MICcfda or MICspec endpoint determinations at 48 h, unlike both the MICalamar and MICvisual methods. Interestingly, for all six of the antifungals tested, only 33% of all endpoints differed from 24 to 48 h when the MICcfda method was used. In comparison, 46% of all the endpoints tested differed from 24 to 48 h when the MICspec method was used.
In summary, there was excellent agreement between the reference broth microdilution method (MICvisual) and the CFDA-modified (MICcfda) and spectrophotometric methods (MICspec). The MICcfda and MICspec methods provided objective, clearly demarcated and reproducible endpoints for antifungal susceptibility testing at 24 and 48 h, while maintaining the integrity of the NCCLS protocol. Both the MICcfda and MICspec methods eliminated the ambiguity of the trailing growth phenomenon for all of the azole antifungal drugs, including the two low-high-phenotype strains. These two methods used in combination could provide a powerful tool for antifungal susceptibility testing and increase interlaboratory reproducibility.
REFERENCES
- 1.Anaissie, E. J., V. L. Paetznick, L. G. Ensign, A. Espinel-Ingroff, J. N. Galgiani, C. A. Hitchcock, M. LaRocco, T. Patterson, M. Pfaller, J. H. Rex, and M. G. Rinaldi. 1996. Microdilution antifungal susceptibility testing of Candida albicans and Cryptococcus neoformans with and without agitation: an eight-center collaborative study. Antimicrob. Agents Chemother. 40:2387-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthington-Skaggs, B. A., D. W. Warnock, and C. J. Morrison. 2000. Quantitation of Candida albicans ergosterol content improves the correlation between in vitro antifungal susceptibility test results and in vivo outcome after fluconazole treatment in a murine model of invasive candidiasis. Antimicrob. Agents Chemother. 44:2081-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ernst, E. J. 2001. Investigational antifungal agents. Pharmacotherapy 21:165S-174S. [DOI] [PubMed]
- 4.Espinel-Ingroff, A., J. L. Rodriguez-Tudela, and J. V. Martinez-Suarez. 1995. Comparison of two alternative microdilution procedures with the National Committee for Clinical Laboratory Standards reference macrodilution method M27-P for in vitro testing of fluconazole-resistant and -susceptible isolates of Candida albicans. J. Clin. Microbiol. 33:3154-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinel-Ingroff, A., T. White, and M. A. Pfaller. 1999. Antifungal agents and susceptibility tests, p. 1640-1652. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.
- 6.Fromtling, R. A., J. N. Galgiani, M. A. Pfaller, A. Espinel-Ingroff, K. F. Bartizal, M. S. Bartlett, B. A. Body, C. Frey, G. Hall, G. D. Roberts, F. B. Nolte, F. C. Odds, M. G. Rinaldi, A. M. Sugar, and K. Villareal. 1993. Multicenter evaluation of a broth macrodilution antifungal susceptibility test for yeasts. Antimicrob. Agents Chemother. 37:39-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao, R. S., R. P. Rennie, and J. A. Talbot. 1999. Assessment of the effect of amphotericin B on the vitality of Candida albicans. Antimicrob. Agents Chemother. 43:1034-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liao, R. S., R. P. Rennie, and J. A. Talbot. 2001. Novel fluorescent broth microdilution method for fluconazole susceptibility testing of Candida albicans. J. Clin. Microbiol. 39:2708-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Jodra, O., J. M. Torres-Rodriguez, R. Mendez-Vasquez, E. Ribas-Forcadell, Y. Morera-Lopez, T. Baro-Tomas, and C. Alia-Aponte. 2000. In vitro susceptibility of Cryptococcus neoformans isolates to five antifungal drugs using a colorimetric system and the reference microbroth method. J. Antimicrob. Chemother. 45:645-649. [DOI] [PubMed] [Google Scholar]
- 10.Marr, K. A., T. R. Rustad, J. H. Rex, and T. C. White. 1999. The trailing endpoint phenotype in antifungal susceptibility testing is pH dependent. Antimicrob. Agents Chemother. 43:1383-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National committee for Clinical Laboratory Standards. 1997. Reference method for broth dilution antifungal susceptibility testing of yeasts. Document M27-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 12.Neely, M. N., and M. A. Ghannoum. 2000. The exciting future of antifungal therapy. Eur. J. Clin. Microbiol. Infect. Dis. 19:897-914. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen, M. H., and C. Y. Yu. 1999. Influence of incubation time, inoculum size, and glucose concentrations on spectrophotometric endpoint determinations for amphotericin B, fluconazole, and itraconazole. J. Clin. Microbiol. 37:141-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Odds, F. C. 1980. Laboratory evaluation of antifungal agents: a comparative study of five imidazole derivatives of clinical importance. J. Antimicrob. Chemother. 6:749-761. [DOI] [PubMed] [Google Scholar]
- 15.Odds, F. C. 1985. Laboratory tests for the activity of imidazole and triazole antifungal agents in vitro. Semin. Dermatol. 4:260-279. [Google Scholar]
- 16.Pfaller, M. A., and A. L. Barry. 1994. Evaluation of a novel colorimetric broth microdilution method for antifungal susceptibility testing of yeast isolates. J. Clin. Microbiol. 32:1992-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfaller, M. A., R. N. Jones, G. V. Doern, H. S. Sader, S. A. Messer, A. Houston, S. Coffman, R. J. Hollis, and The Sentry Participant Group. 2000. Bloodstream infections due to Candida species: SENTRY antimicrobial surveillance program in North America and Latin America, 1997-1998. Antimicrob. Agents Chemother. 44:747-751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfaller, M. A., S. A. Messer, and S. Coffmann. 1995. Comparison of visual and spectrophotometric methods of MIC endpoint determinations by using broth microdilution methods to test five antifungal agents, including the new triazole D0870. J. Clin. Microbiol. 33:1094-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfaller, M. A., and M. G. Rinaldi. 1993. Antifungal susceptibility testing: current state of technology, limitations, and standardization. Infect. Dis. Clin. N. Am. 7:435-444. [PubMed] [Google Scholar]
- 20.Pringle, J., R. Preston, A. Adams, T. Stearns, D. Drubin, B. Haarer, and E. Jones. 1989. Fluorescence microscopy methods for yeast. Methods Cell Biol. 31:357-435. [DOI] [PubMed] [Google Scholar]
- 21.Revankar, S. G., W. R. Kirkpatrick, R. K. McAtee, A. W. Fothergill, S. W. Redding, M. G. Rinaldi, and T. F. Patterson. 1998. Interpretation of trailing endpoints in antifungal susceptibility testing by the National Committee for Clinical Laboratory Standards method. J. Clin. Microbiol. 36:153-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rex, J. H., P. W. Nelson, V. L. Paetznick, M. Lozano-Chiu, and A. Espinel-Ingroff. 1998. Optimizing the correlation between results of testing in vitro and therapeutic outcome in vivo for fluconazole by testing clinical isolates in a murine model of invasive candidiasis. Antimicrob. Agents Chemother. 42:129-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, and A. L. Barry for the Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 24.Rex, J. H., M. A. Pfaller, M. G. Rinaldi, A. Polak, and J. N. Galgiani. 1993. Antifungal susceptibility testing. Clin. Microbiol. Rev. 6:367-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.To, W., A. W. Fothergill, and M. G. Rinaldi. 1995. Comparative evaluation of macrodilution and Alamar colorimetric microdilution broth methods for antifungal susceptibility testing of yeast isolates. J. Clin. Microbiol. 33:2660-2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanden Bossche, H., F. Dromer, I. Improvisi, M. Lozano-Chiu, J. H. Rex, and D. Sanglard. 1998. Antifungal drug resistance in pathogenic fungi. Med. Mycol. 36(Suppl. 1):119-128. [PubMed] [Google Scholar]
- 27.Warren, N. G., and K. C. Hazen. 1999. Candida, Cryptococcus, and other yeasts of medical importance, p. 1184-1199. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. American Society for Microbiology, Washington, D.C.