Abstract
Ten strains of lactobacilli were assessed for their capacity to degrade inulin-type fructans, which are well-known prebiotics. Both oligofructose and inulin were tested. The dairy isolate Lactobacillus acidophilus IBB 801 degraded only oligofructose. The human isolate Lactobacillus paracasei subsp. paracasei 8700:2 degraded oligofructose and long-chain inulin and grew rapidly on both energy sources. In both cases, fractions of different degrees of polymerization were fermented. Moreover, large and short fractions of oligofructose were degraded simultaneously. When L. paracasei subsp. paracasei 8700:2 grew on oligofructose-enriched inulin, oligofructose was preferentially metabolized. In all cases, lactic acid was the main metabolic end product. Significant amounts of acetic acid, formic acid, and ethanol were produced when long-chain inulin or oligofructose-enriched inulin was used as the sole energy source.
Consumers are becoming increasingly aware of the importance of the human gut microbiota in well-being and health (26, 30). The modulation of the intestinal microbiota by diet, toward a composition with increased proportions of beneficial bacteria such as bifidobacteria and lactobacilli, has recently gained a lot of interest (29). Prebiotics are nondigestible food ingredients that beneficially affect the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria in the colon (15, 16). Certain criteria need to be fulfilled to classify a food ingredient as prebiotic (15, 16): (i) resistance to gastric acidity, to hydrolysis by mammalian digestive enzymes, and to gastrointestinal absorption; (ii) fermentation by the colonic microbiota; and (iii) selective stimulation of the colon bacteria that contribute to health and well-being. According to these criteria and based on existing scientific data, only three types of carbohydrates can be classified as prebiotics, namely the inulin-type fructans (inulin and oligofructose), transgalactooligosaccharides, and lactulose (15).
Inulin and its partially hydrolyzed derivative oligofructose are made up of linear β-(2→1) glycosidic bonds of d-fructose, often with a terminal glucose moiety that is linked by an α-(1→2) glycosidic bond, as in sucrose. Oligofructose can contain both chains of fructose (Fm type) and fructose chains with a terminal glucose unit (GFn type) (39). The β-(2→1) linkages prevent the digestion of these fructans in the upper part of the human gastrointestinal tract. Once they arrive in the colon, β-fructosidase-producing bacteria can hydrolyze these prebiotics (39). Inulin-type fructans are known for their so-called bifidogenic effect, meaning their ability to selectively increase the number of bifidobacteria in the human colon, as bifidobacteria are able to use inulin-type fructans as the sole energy source (40, 46). This property has been demonstrated in various clinical trials with human volunteers (25, 27, 38, 42, 43). The in vitro fermentation kinetics of bifidobacteria grown on inulin-type fructans has been studied to some extent (12, 20, 36) but only for Bifidobacterium animalis subsp. lactis DN-173 010 on a quantitative basis (44). It has been shown that short oligofructose fractions are preferentially metabolized (44).
Data on the growth of members of the colonic microbiota, other than bifidobacteria, on inulin-type fructans have been reported (11, 18, 23, 25). It has been demonstrated that certain Lactobacillus strains, including strains of Lactobacillus acidophilus and Lactobacillus paracasei, are able to grow on these prebiotics (6, 23, 31, 32, 33, 47). Further, an operon involved in oligofructose utilization has been described for L. acidophilus NCFM (3). Some in vivo studies with animal models or clinical trials have demonstrated an increase of the number of lactobacilli when inulin-type fructans are applied (25, 27), but in other reports the number of lactobacilli remains stable after administration of such prebiotics (14, 42). These results indicate that the ability to ferment inulin-type fructans is strain specific for lactobacilli, in contrast with bifidobacteria, where this property is more widespread (4, 20).
Despite the increasing evidence of the fermentation of inulin-type fructans by certain lactobacilli, no information on the kinetics of the degradation of and growth on such substrates by lactobacilli is available. The aim of this work was to investigate the ability of lactobacilli to ferment inulin-type fructans and to study their kinetics of growth and fructan degradation.
MATERIALS AND METHODS
Bacterial strains, media, and substrates.
Ten Lactobacillus strains were used in this study (Table 1). All strains were stored at −80°C in de Man-Rogosa-Sharpe (MRS) broth (Oxoid, Basingstoke, United Kingdom), supplemented with 25% (vol/vol) glycerol as a cryoprotectant. Customized MRS medium (8), without glucose and supplemented with 0.5 g liter−1 l-cysteine hydrochloride (VWR International, Darmstadt, Germany), hereafter referred to as mMRS medium, was used as the fermentation medium throughout this study. The pH of the medium was adjusted to 6.5 before sterilization (at 121°C and 2.1 × 105 Pa for 20 min). Glucose, fructose, galactose, lactulose, inulin-type fructans (oligofructose [Raftilose P95], long-chain inulin [Raftiline HP], and oligofructose-enriched inulin [Raftilose Synergy 1]), and transgalactooligosaccharides (Oligomate 55) were used as the sole energy sources (in concentrations of 10 and 20 g liter−1). In all cases, the energy source was sterilized through membrane filtration using Sartolab-P20 filters (pore size, 0.20 μm; Sartorius AG, Goettingen, Germany) and added aseptically to the sterile mMRS medium. Solid medium was prepared by adding 1.5% (wt/vol) agar (Oxoid) to the broth.
TABLE 1.
Growth of lactobacilli on different energy sources, including prebiotics
| Lactobacillus strain | Origin (and/or source)a | Growth by energy sourceb
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Glc | Fru | Gal | Lat | TOS | Ogf | Ogf-Inu | Inu | ||
| L. acidophilus ACC | Actimel cholesterol control (Danone, France) | + | + | + | + | + | − | − | − |
| L. acidophilus IBB 801 | Dairy isolate (48) | + | ± | + | + | + | + | ± | − |
| L. amylovorus DCE 471 | Corn steep liquor (10) | + | + | + | + | + | − | − | − |
| L. casei Shirota | Yakult (Yakult Honsha Co., Japan) | + | + | + | + | + | − | − | − |
| L. fermentum IMDO-3 | Healthy infant's faeces isolate | + | + | + | + | + | − | − | − |
| L. johnsonii La1 | LC1 (Nestlé, Switzerland) | + | + | + | + | + | − | ± | − |
| L. gasseri K7 | Healthy infant's faeces isolate (5) | + | + | + | + | + | ± | ± | − |
| L. paracasei subsp. paracasei 8700:2 | Human colonic mucosa isolate (1) | + | + | + | + | + | + | + | + |
| L. plantarum ACA-DC 287 | Greek xynotyri cheese isolate (10) | + | + | + | + | + | ± | − | − |
| L. rhamnosus GG | Gefilus (Valio, Finland) | + | + | + | − | ± | − | − | − |
The probiotic strains L. acidophilus ACC, L. casei Shirota, L. johnsonii La1, and L. rhamnosus GG were isolated from their commercial products.
Glc, glucose; Fru, fructose; Gal, galactose; Lat, lactulose; TOS, transgalactooligosaccharides (Oligomate 55); Ogf, oligofructose (Raftilose P95); Ogf-Inu, oligofructose-enriched inulin (Raftilose synergy 1); Inu, long-chain inulin (Raftiline HP). +, clear color change of the agar medium; ±, slight color change of the agar medium; −, no color change of the agar medium.
Glucose, fructose, galactose, and lactulose were purchased from VWR International; Raftiline HP, Raftilose P95, and Raftilose Synergy 1 were kindly provided by Orafti N.V. (Tienen, Belgium); and Oligomate 55 was kindly provided by Yakult Belgium (Brussels, Belgium). Raftiline HP (long-chain inulin), a commercial powder derived from chicory roots, contains inulin (≥99.5%, wt/wt) and minor amounts of glucose, fructose, and sucrose (<0.5%, wt/wt). The average degree of polymerization (DP) of the inulin chains exceeds 23, due to removal of the smaller molecules during processing. Raftilose P95 (oligofructose) is a commercial powder produced through enzymatic hydrolysis of chicory inulin. The powder contains oligofructose (≥93.2%, wt/wt) with a little glucose, fructose, and sucrose (<6.8%, wt/wt). The DP of the oligofructose fractions varies between 2 and 8, with an average of 4. Raftilose Synergy 1 (oligofructose-enriched inulin) is a commercial powder containing oligofructose and long-chain inulin (90 to 94%, wt/wt), glucose and fructose (4 to 6%, wt/wt), and sucrose (2 to 4%, wt/wt). Oligomate 55 (transgalactooligosaccharides [TOS]) is a commercial powder, produced through transgalactosylation of lactose, containing transgalactooligosaccharides (55%, wt/wt). Its DP varies between 2 and 5, with an average of 3.
Growth of lactobacilli on prebiotics. (i) Agar plate assays.
A first screening of the growth of the 10 Lactobacillus strains studied on different energy sources, including prebiotics, was performed with an agar plate assay as described previously (23), with some modifications. Briefly, mMRS agar medium containing the appropriate energy source (1%, wt/vol) and 300 mg liter−1 bromocresol purple (Sigma-Aldrich Chemie Gmbh, Steinheim, Germany) as a color indicator was used. The Lactobacillus strains were propagated twice in MRS broth (Oxoid), and cultures obtained after 12 h of growth at 37°C were centrifuged (at 5,500 × g for 10 min). The pellet was washed once with phosphate-buffered saline (0.8% NaCl, 0.02% KH2PO4, 0.115% Na2HPO4 [pH 7.4]) and resuspended in phosphate-buffered saline, followed by spotting of 10 μl of this suspension on mMRS agar plates. The plates were incubated at 37°C for 48 h. All incubations took place anaerobically in a modular atmosphere-controlled system (MG Anaerobic Work Station; Don Withley Scientific, Ltd., Shipley, United Kingdom) that was continuously under a stream of a mixture of 80% N2, 10% CO2, and 10% H2 (Air Liquide, Paris, France). Plates were checked for color changes around the developing colonies. These experiments were performed in triplicate.
(ii) Fermentation experiments.
To confirm the growth of Lactobacillus strains that were positive for growth on inulin-type fructans, small-scale fermentations in glass bottles (100 ml) containing mMRS medium with fructose, oligofructose, oligofructose-enriched inulin, or long-chain inulin as the sole energy source (1%, wt/vol), were carried out in duplicate. Therefore, lactobacilli were propagated twice in mMRS medium with glucose as the sole energy source for 12 h. These precultures were inoculated (2%, vol/vol) in mMRS medium containing the energy source to be studied. All incubations took place anaerobically (in a modular atmosphere-controlled system) at 37°C. During fermentation, samples were withdrawn at regular time intervals to measure the optical density at 600 nm (OD600) and pH.
Kinetic analysis of the growth of L. paracasei subsp. paracasei 8700:2 on fructose and inulin-type fructans as the sole energy sources (2%, wt/vol) was performed in 10 liters of mMRS medium in duplicate. Therefore, fermentations were carried out in a 15-liter laboratory fermentor (Biostat C; B. Braun Biotech International, Melsungen, Germany). The fermentor was sterilized in situ at 121°C for 20 min; the energy source was sterilized separately by membrane filtration and added aseptically to the fermentor. The fermentation temperature was kept constant at 37 ± 0.1°C, and the stirring rate was fixed at 100 rpm to keep the medium homogeneous. The pH was kept constant at 6.5 ± 0.05, which is the average pH of the colon, by automatic addition of 10 N NaOH. The medium was sparged with a mixture of 90% N2 and 10% CO2 (Air Liquide) at a rate of 0.5 l min−1. Temperature, pH, agitation, and airflow were controlled on line (Micro-MFCS for Windows NT software, B. Braun Biotech International). During fermentation, samples were withdrawn aseptically for further analysis (see below). To prepare the inoculum, three subcultures of 48, 18, and 12 h of incubation (anaerobically at 37°C) were carried out in 10 ml, 20 ml, and 200 ml of mMRS medium, respectively, containing the energy source to be studied. The transfer inoculum was always 2% (vol/vol).
Analysis of microbial growth and metabolites.
Cell growth was measured by OD600 and biomass determinations. The OD600 of the samples, appropriately diluted if necessary, was measured twice. Biomass, expressed as cell dry mass, was obtained through filtration of a fixed volume of sample through 0.45-μm-pore-size filters (cellulose nitrate filter; Sartorius AG), dried at 105°C for 48 h, and weighed.
To determine metabolites, samples were centrifuged (16,060 × g for 20 min), and the amounts of fructose, lactic acid, acetic acid, formic acid, and ethanol in the cell-free culture supernatants were determined by high-performance liquid chromatography analysis. A Waters chromatograph (Milford, MA), equipped with a differential refractometer, a controller, a column oven, and an autosampler was used. An ICSep ICE-ORH-801 column (Interchim, Montluçon, France) was used with 10 mM H2SO4 as mobile phase at a flow rate of 0.4 ml min−1. The column temperature was kept at 35°C. The experimental error on the measurements of fructose, lactic acid, acetic acid, formic acid, and ethanol was 7.5%, 5.8%, 3.1%, 6.2%, and 11.8%, respectively.
The maximal specific growth rate, μmax, was determined by plotting ln([X]/[X0]) as a function of time, where X0 refers to the biomass concentration (g of cell dry mass liter−1) at the beginning of the fermentations.
Analysis of fructans.
Quantitative analysis of fructans was carried out by gas chromatography (GC) as described previously (19, 22, 45). The first gas chromatograph (GC HRGC 5300-HT Mega; Carlo Erba, Rodina, Italy) was used to analyze oligofructose and prehydrolyzates (see below) of oligofructose-enriched inulin. It contained an oven, a column (SGE Aluminum Clad-5 capillary column; Achrom NV/SA, Zulte, Belgium), an autosampler, a cold on-column injector, a flame ionization detector, and an integrator (HP 3393A coupled to the data processing system Chromoperfect). The oven temperature was programmed from 105 to 440°C at 10°C min−1. The second gas chromatograph (GC HRGC 5160 Mega; Carlo Erba), used to analyze the hydrolysates of long-chain inulin and oligofructose-enriched inulin, was equipped with an AT1-capillary column (Alltech, Cleveland, OH). For both gas chromatographs, helium was used as a carrier gas; a mixture of air and hydrogen was used as a detector gas, and nitrogen was used as a make-up gas.
For the analysis of the samples containing oligofructose or oligofructose-enriched inulin, a derivatization procedure involving oxymation and silylation of the sugars was carried out (22). The oxime-trimethylsilyl sugar derivatives were extracted using iso-octane, and the resulting iso-octane phase was injected into the GC to determine sugars up to DP 8. The same procedure was carried out for a reference sample of Raftilose P95 (Orafti N.V.) and external standards (glucose, fructose, and sucrose).
For the analysis of the samples containing long-chain inulin or oligofructose-enriched inulin, a procedure involving the preparation of prehydrolyzates and enzymatic hydrolysis with inulinase (Fructozyme SP 230; Novozymes, Bagsvaerd, Denmark) was performed (19). The derivatization of the samples before and after hydrolysis was carried out as described above. Raftiline HP (Orafti N.V.) was used as a reference sample. The amounts of sugars up to DP 8 before hydrolysis and of glucose and fructose after hydrolysis were determined by GC analysis. The amount of fructans was calculated from the concentrations of glucose and fructose as described previously (19). The average DP (DPav) of long-chain inulin was calculated using the following equation: DPav = (F/G) + 1, where F and G are the amounts of fructose and glucose, respectively, released from the fructans after hydrolysis.
RESULTS
Growth of lactobacilli on prebiotics.
Using the agar plate assay, Lactobacillus strains that fermented the prebiotics tested as the sole energy source gave a yellow zone against a purple background, due to the production of significant amounts of organic acids, while the nonfermenting strains did not cause any color change of the agar medium. All strains fermented the monosaccharides glucose, fructose, and galactose (Table 1). Moreover, all lactobacilli fermented lactulose and TOS, with the exception of Lactobacillus rhamnosus GG, which did not ferment lactulose, while TOS were slightly fermented. Both L. acidophilus IBB 801 and L. paracasei subsp. paracasei 8700:2 fermented oligofructose. Interestingly, L. paracasei subsp. paracasei 8700:2 also fermented oligofructose-enriched inulin and long-chain inulin as the sole energy sources. Only slight color changes were observed with Lactobacillus gasseri K7 and Lactobacillus plantarum ACA-DC 287 with oligofructose and with L. acidophilus IBB 801, Lactobacillus johnsonii La1, and L. gasseri K7 with oligofructose-enriched inulin.
During small-scale fermentations (100 ml), both L. acidophilus IBB 801 and L. paracasei subsp. paracasei 8700:2 grew fast on oligofructose and oligofructose-enriched inulin and acidified the mMRS medium, lowering the pH below 4.5 after 12 h of growth. Interestingly, L. paracasei subsp. paracasei 8700:2 grew very well on long-chain inulin, too, and the acidification profile was comparable to that obtained during fermentation of fructose (data not shown). Limited growth of L. johnsonii La1, L. gasseri K7, and L. plantarum ACA-DC 287 was observed on inulin-type fructans. The pH drop observed in fermentations with these bacteria was attributed to growth on residual glucose and fructose concentrations. No pH drop was observed in mMRS medium without an energy source for all lactobacilli tested.
Kinetic analysis of the growth of L. paracasei subsp. paracasei 8700:2 on fructose and inulin-type fructans.
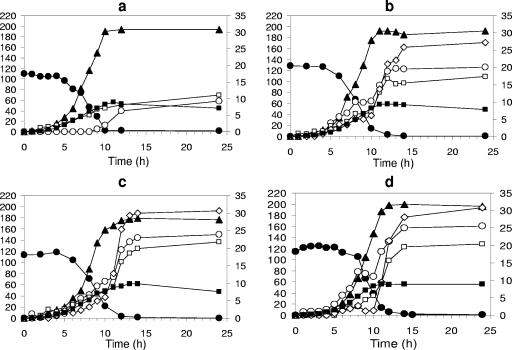
To better understand the growth of L. paracasei subsp. paracasei 8700:2 on fructose and inulin-type fructans as the sole energy sources, large-scale fermentations (10 liters) under controlled conditions were performed (Fig. 1). The strain grew fast (μmax = 0.61 h−1; r2 = 0.99) on fructose, which was almost completely depleted after 12 h of fermentation (Fig. 1a). Lactic acid was the main metabolic end product, with a concentration of 196 ± 5 mM after 12 h of fermentation. Small amounts of acetic acid and ethanol were produced as well, while no formic acid was detected.
FIG. 1.
Fermentation of L. paracasei subsp. paracasei 8700:2 in mMRS medium with a 2% (wt/vol) concentration of fructose (a), oligofructose (Raftilose P95) (b), oligofructose-enriched inulin (Raftilose Synergy 1) (c), and long-chain inulin (Raftiline HP) (d). Left axis: •, total carbohydrate concentration (in mmol liter−1); ▴, lactic acid concentration (in mmol liter−1). Right axis: ○, acetic acid concentration (in mmol liter−1); ⋄, formic acid concentration (in mmol liter−1); □, ethanol concentration (in mmol liter−1); ▪, OD600. In the case of inulin-type fructans, total carbohydrate concentration is expressed as mmol liter−1 of fructose and glucose released after total hydrolysis. The graphs are representative of the results of two experiments.
The fermentation profile of L. paracasei subsp. paracasei 8700:2 on oligofructose was similar to that on fructose (Fig. 1b). The energy source was depleted after 12 h of growth, when the cells entered the stationary phase. GC analysis of the samples revealed that L. paracasei subsp. paracasei 8700:2 first metabolized the free amounts of glucose and fructose (Table 2). After 4 h of growth, when the monosaccharides were depleted, the oligofructose was metabolized. The rapid degradation of the large oligofructose fractions led to the accumulation of fructose, F2 (inulobiose), and GF2 (kestose), indicating the activity of an extracellular β-fructosidase. These components were then also quickly metabolized (Table 2). Lactic acid was the main metabolic end product, and the final concentration of lactic acid in the fermentation medium was the same as that reached with fructose as the sole energy source (192 ± 3 mM). However, higher amounts of acetic acid, formic acid, and ethanol were produced, compared with fermentation with fructose. The production of these compounds was higher both at the beginning and at the end of the fermentation, indicating shifts between homolactic and heterolactic fermentation.
TABLE 2.
Fructan degradation during the growth of L. paracasei subsp. paracasei 8700:2 on oligofructose as Raftilose P95 (2%, wt/vol)
| Componenta | Concentration (g/liter) at time (h):
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 24 | |
| F | 0.9 | 0.6 | 0.0 | 0.7 | 2.4 | 1.4 | 0.2 | 0.1 | 0.1 |
| G | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 | 0.1 | 0.1 |
| S | 0.1 | 0.1 | 0.1 | 0.1 | 0.4 | 0.6 | 0.3 | 0.1 | 0.0 |
| F2 | 0.9 | 1.0 | 1.0 | 2.6 | 4.2 | 0.1 | 0.0 | 0.0 | 0.0 |
| GF2 | 0.2 | 0.2 | 0.2 | 0.5 | 1.3 | 0.3 | 0.0 | 0.0 | 0.0 |
| F3 | 5.8 | 5.8 | 6.0 | 4.9 | 1.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| GF3 | 0.8 | 0.8 | 0.9 | 0.8 | 0.4 | 0.0 | 0.0 | 0.0 | 0.0 |
| F4 | 5.4 | 5.4 | 5.5 | 3.4 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 |
| GF4 | 1.5 | 1.5 | 1.5 | 1.1 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| F5 | 2.3 | 2.3 | 2.4 | 1.7 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 |
| GF5 | 1.1 | 1.1 | 1.2 | 0.9 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| F6 | 1.5 | 1.5 | 1.4 | 1.2 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| GF6 | 0.6 | 0.5 | 0.6 | 0.4 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| F7 | 0.5 | 0.5 | 0.5 | 0.4 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| F8 | 0.1 | 0.2 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Oligofructoseb | 20.5 | 20.6 | 21.1 | 17.7 | 8.8 | 0.4 | 0.0 | 0.0 | 0.0 |
F, fructose; G, glucose; S, sucrose.
Oligofructose: the sum of the amounts of F2 to F8 and GF2 to GF6.
The fermentation profiles of L. paracasei subsp. paracasei 8700:2 on oligofructose-enriched inulin and long-chain inulin were very similar (Fig. 1c and d). Growth was fast on both prebiotics (μmax = 0.56 and 0.55 h−1 on oligofructose-enriched inulin and long-chain inulin, respectively; r2 = 0.99), and lactic acid was the main metabolic end product (179 ± 8 and 200 ± 4 mM on oligofructose-enriched inulin and long-chain inulin, respectively). Higher amounts of acetic acid, formic acid, and ethanol were produced, compared with the fermentation of oligofructose. The accumulation of these mixed-acid fermentation products was higher both at the beginning and at the end of the fermentations. For instance, when L. paracasei subsp. paracasei 8700:2 grew on long-chain inulin, the production of acetic acid and formic acid stopped after 8 h of fermentation, followed by the production of lactic acid and small amounts of ethanol during the next 2 h, which in turn was followed by a restart of the production of acetic acid and formic acid (Fig. 1d). This pattern was reproducible for all fermentations. When oligofructose-enriched inulin was used as a sole energy source for L. paracasei subsp. paracasei 8700:2, consumption of the free fructose present in the fermentation medium occurred during the first 4 h of fermentation (Table 3). Then, the oligofructose and inulin were also degraded, leading to an accumulation of fructose, glucose, and sucrose, which, in turn, were depleted very fast. The oligofructose part was metabolized preferentially during the exponential growth phase. Long-chain inulin was rapidly degraded as well, however, in a later stage of the fermentation. A similar pattern was followed when long-chain inulin was used as the sole energy source. The fast degradation of long-chain inulin led to an increased concentration of fructose and sucrose after 8 h of growth (Table 4). Thereafter, these mono- and disaccharides were rapidly depleted.
TABLE 3.
Fructan degradation during the growth of L. paracasei subsp. paracasei 8700:2 on a mixture of oligofructose and long-chain inulin (oligofructose-enriched inulin) as Raftilose Synergy 1 (2%, wt/vol)
| Componenta | Concentration (g/liter) at time (h):
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 24 | |
| F | 1.0 | 0.9 | 0.2 | 1.2 | 1.4 | 0.9 | 0.2 | 0.1 | 0.1 |
| G | 0.2 | 0.2 | 0.2 | 0.0 | 0.5 | 0.3 | 0.2 | 0.1 | 0.0 |
| S | 0.9 | 0.8 | 1.0 | 1.1 | 1.7 | 1.3 | 0.2 | 0.0 | 0.0 |
| F2 | 0.7 | 0.8 | 0.9 | 2.0 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 |
| GF2 | 0.7 | 0.7 | 0.9 | 1.7 | 0.9 | 0.1 | 0.0 | 0.0 | 0.0 |
| F3 | 2.6 | 2.5 | 2.8 | 1.2 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| GF3 | 1.4 | 1.3 | 1.5 | 0.8 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| F4 | 1.7 | 1.6 | 1.7 | 0.5 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| GF4 | 1.5 | 1.4 | 1.5 | 0.7 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| F5 | 0.5 | 0.5 | 0.5 | 0.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| GF5 | 1.1 | 1.0 | 1.1 | 0.5 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 |
| F6 | 0.3 | 0.3 | 0.3 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| GF6 | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Oligofructoseb | 10.4 | 10.2 | 11.2 | 7.8 | 1.6 | 0.1 | 0.0 | 0.0 | 0.0 |
| Long-chain inulin | 6.1 | 6.9 | 6.8 | 7.2 | 6.5 | 1.2 | 0.1 | 0.1 | 0.1 |
F, fructose; G, glucose; S, sucrose.
Oligofructose: the sum of the amounts of F2 to F6 and GF2 to GF6 components.
TABLE 4.
Fructan degradation during the growth of L. paracasei subsp. paracasei 8700:2 on long-chain inulin as Raftiline HP (2%, wt/vol)
| Component | Concentration (g/liter) at time (h):
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 24 | |
| Fructose | 0.1 | 0.1 | 0.1 | 0.2 | 0.5 | 0.4 | 0.1 | 0.0 | 0.0 |
| Glucose | 0.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.1 | 0.0 | 0.0 |
| Sucrose | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.5 | 0.1 | 0.0 | 0.0 |
| Long-chain inulin | 19.6 | 20.2 | 19.8 | 16.9 | 9.9 | 1.7 | 0.1 | 0.1 | 0.1 |
| DPav | 24.1 | 24.8 | 24.3 | 26.7 | 27.5 | 19.0 | NRa | NR | NR |
NR, not relevant.
DISCUSSION
In this paper, a screening among lactobacilli for their ability to ferment inulin-type fructans showed that two strains, namely, L. acidophilus IBB 801 and L. paracasei subsp. paracasei 8700:2, were able to ferment oligofructose as well as oligofructose-enriched inulin, but only the latter strain also fermented long-chain inulin.
Interestingly, L. acidophilus IBB 801 belongs to the L. acidophilus complex that includes species that are part of the human intestinal microbiota (9, 17). Strains belonging to this complex have been previously reported to grow on short fractions of oligofructose (23), and the genes encoding enzymes of the β-fructosidase family have been found in the genome of L. acidophilus NCFM recently (3). Such genes are not found in the genome of L. johnsonii NCC 533 (37), a strain identical to L. johnsonii La1, hence confirming the inability of the latter strain to grow on inulin-type fructans. In this study, only one strain among the strains of the L. acidophilus complex tested, namely, L. acidophilus IBB 801, showed significant growth on oligofructose, confirming that this property is not common among lactobacilli. The ability of L. acidophilus IBB 801 to metabolize oligofructose (this study), its production of a bacteriocin named acidophilin 801 (48), and the antagonistic activity of the strain against the intestinal pathogen Salmonella enterica serovar Typhimurium SL1344 (10) support the idea to further investigate potential health benefits of this strain for use as a probiotic.
The human isolate L. paracasei subsp. paracasei 8700:2 showed some interesting features. Small-scale fermentations revealed the ability of this strain to grow on oligofructose and, interestingly, on long-chain inulin. Up to now, the degradation of inulin has been shown for lactobacilli isolated from silage only (31), while bifidobacteria do not grow on long-chain inulin at all (4, 12, 21, 35). Strains belonging to the species L. paracasei have been found to degrade inulin-type fructans before (23, 31, 33, 34, 47). For the silage isolate, L. paracasei subsp. paracasei P 4134, this degradation has been ascribed to the production of a β-d-fructohydrolase, which has been purified and characterized (32). Further, an ATP-dependent transport system has been suggested for the uptake of short fractions of oligofructose by L. paracasei 1195 (24). These findings indicate that an extracellular enzyme is responsible for the degradation of large fractions of oligofructose and inulin, leading to the extracellular accumulation of short fractions that can be taken up before further intracellular hydrolysis. With respect to bifidobacteria, no extracellular β-fructosidases have been found up to now, and the β-fructosidases characterized to date on enzymatic and genetic levels are intracellular (13). However, a permease transport system has been suggested for the uptake of short fractions of oligofructose only (41).
Remarkably, the growth of L. paracasei subsp. paracasei 8700:2 on oligofructose, long-chain inulin, or oligofructose-enriched inulin was very fast. The μmax values for the growth on inulin-type fructans were comparable to the values obtained when fructose was used as the sole energy source. The fructan analyses demonstrated a rapid degradation and metabolism of oligofructose and long-chain inulin. These data may be ascribed to the production and extracellular release of a β-fructosidase, as mentioned above. In the Western diet, the daily per capita intake of oligofructose and inulin has been estimated to range from 1 to 10 g (45). It has been demonstrated that the average recovery of such prebiotics in the colon varies between 86 and 89% of the material fed (7). Thus, the extracellular fructan degradation by L. paracasei subsp. paracasei 8700:2 could be an important advantage for this strain to survive in the competitive environment of the colon. The degradation of oligofructose by bifidobacteria is much slower, and a preferential metabolism of the short fractions of oligofructose has been reported (36, 44). This has been ascribed to the induced, cell envelope-located hydrolysis of large fractions and the uptake of shorter fractions in a preferential order, i.e., F2 > F3 > F4 (44). In contrast, hydrolysis of the short and large fractions of oligofructose by L. paracasei subsp. paracasei 8700:2 occurred simultaneously, and, in addition, this strain rapidly metabolized the inulin fractions with a high degree of polymerization. The application of this strain in a probiotic preparation could, hence, be useful in increasing substrate availability for bifidobacteria in the gut, as the latter display no degradation of (highly purified) long-chain inulin (4, 12, 21, 35). Nevertheless, further coculture studies are needed to investigate the interactions between L. paracasei subsp. paracasei 8700:2 and bifidobacteria in the presence of inulin-type fructans.
Lactic acid was the major metabolic end product of L. paracasei subsp. paracasei 8700:2, but significant amounts of other metabolic end products such as acetic acid, formic acid, and ethanol were also produced, in particular when oligofructose and/or long-chain inulin were used as the sole energy sources. In the case of bifidobacteria, it has been shown before that slowly degraded inulin-type fructans switch their metabolism toward more acetic acid and formic acid at the cost of lactic acid (44). Under sugar-limiting conditions and hence slow growth, acetate is preferentially produced through pyruvate metabolism by lactic acid bacteria, because of extra ATP generation, while redox equilibrium (NAD+ regeneration) is achieved through the production of ethanol (2, 28). Indeed, in the beginning of the fermentations with inulin-type fructans by L. paracasei subsp. paracasei 8700:2, small amounts of monosaccharides were available, and the production of acetic acid was high. During the exponential growth phase, however, degradation of the fructans led to the accumulation of mono- and disaccharides, and slow or no production of acetic acid was observed. At the end of the fermentation, when almost all sugar was depleted, relatively high amounts of acetic acid, as well as formic acid and ethanol, were produced. The shift in the metabolite production pattern, which is favored under the anoxic and neutral pH conditions that were applied in this study (2), was less pronounced than in the case of bifidobacteria. This is probably due to the faster degradation of the inulin-type fructans by L. paracasei subsp. paracasei 8700:2 in comparison with bifidobacteria. For instance, fermentation of the oligofructose-enriched inulin by B. animalis subsp. lactis DN-173 010 lasts more that 40 h and results in a ratio of acetic acid to lactic acid that is much higher than the one that is theoretically expected (44).
In conclusion, the results presented here show that the ability to ferment inulin-type fructans is not a unique feature of bifidobacteria, nor is it common among lactobacilli. L. acidophilus IBB 801 metabolized oligofructose, while L. paracasei subsp. paracasei 8700:2 rapidly degraded both oligofructose and long-chain inulin. To our knowledge, this is the first report on the kinetics of growth and fructan degradation of a Lactobacillus strain on inulin-type fructans. Knowledge on the fermentation kinetics of (probiotic) Lactobacillus strains growing on prebiotics is useful in understanding their physiological role and commercial importance, as well as the physiology of the colon ecosystem in general. Therefore, the physiological relevance of the degradation of inulin-type fructans by L. paracasei subsp. paracasei 8700:2 needs to be further studied using in vitro gut simulation models and in vivo trials. All studies together will allow the investigation of the interactions between L. paracasei subsp. paracasei 8700:2 with other colon bacteria and the assessment of possible applications of this strain in probiotic and synbiotic preparations.
Acknowledgments
This study has been carried out with financial support from the Commission of the European Communities, specific RTD program “Quality of Life and Management of Living Resources, QLK1-2001-01179-PROPATH—Molecular analysis and mechanistic elucidation of the functionality of probiotics and prebiotics in the inhibition of pathogenic microorganisms to combat gastrointestinal disorders and to improve human health.” This study does not necessarily reflect the Commission's views and in no way anticipates the Commission's future policy in this area.
This work was also supported by the Research Council of the Vrije Universiteit Brussel, the Fund for Scientific Research-Flanders, the Flemish Institute for the Encouragement of Scientific and Technological Research in the Industry, in particular, the GBOU project “Development of a fast, noninvasive technological tool to investigate the functionality and effectivity of pro- and prebiotics in normal healthy humans: the use of a labeled biomarker.”
REFERENCES
- 1.Ahrné, S., S. Nobaek, B. Jeppsson, I. Adlerberth, A. E. Wold, and G. Molin. 1998. The normal Lactobacillus flora of healthy human rectal and oral mucosa. J. Appl. Microbiol. 85:88-94. [DOI] [PubMed] [Google Scholar]
- 2.Axelsson, L. 2004. Lactic acid bacteria: classification and physiology, p. 1-66. In S. Salminen, A. von Wright, and A. Ouwehand (ed.), Lactic acid bacteria: microbiological and functional aspects. Marcel Dekker, Inc., New York, N.Y.
- 3.Barrangou, R., E. Altermann, R. Hutkins, R. Cano, and T. R. Klaenhammer. 2003. Functional and comparative genomic analyses of an operon involved in fructooligosaccharide utilization by Lactobacillus acidophilus. Proc. Natl. Acad. Sci. USA 100:8957-8962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bielecka, M., E. Biedrzycka, and A. Majkowska. 2002. Selection of probiotics and prebiotics for synbiotics and confirmation of their in vivo effectiveness. Food Res. Int. 35:125-131. [Google Scholar]
- 5.Bogovic-Matijašic, B., and I. Rogelj. 2000. Lactobacillus K7—a new candidate for a probiotic strain. Food Technol. Biotechnol. 38:113-119. [Google Scholar]
- 6.Cebeci, A., and C. Gurakan. 2003. Properties of potential probiotic Lactobacillus plantarum strains. Food Microbiol. 20:511-518. [Google Scholar]
- 7.Cummings, J. H., G. T. Macfarlane, and H. N. Englyst. 2001. Prebiotic digestion and fermentation. Am. J. Clin. Nutr. 73:S415-S420. [DOI] [PubMed] [Google Scholar]
- 8.de Man, J., M. Rogosa, and M. Sharpe. 1960. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 23:130-135. [Google Scholar]
- 9.De Vuyst, L., L. Avonts, and L. Makras. 2004. Probiotics, prebiotics and gut health, p. 416-482. In C. Remacle and B. Reusens (ed.), Functional foods, ageing and degenerative disease. Woodhead Publishing Ltd., Cambridge, United Kingdom.
- 10.De Vuyst, L., L. Makras, L. Avonts, H. Holo, Q. Yi, A. Servin, D. Fayol-Messaoudi, C. Berger, G. Zoumpopoulou, E. Tsakalidou, D. Sgouras, B. Martinez-Gonzales, E. Panayotopoulou, A. Mentis, D. Smarandache, L. Savu, P. Thonart, and I. Nes. 2004. Antimicrobial potential of probiotic or potentially probiotic lactic acid bacteria, the first results of the international European research project PROPATH of the PROEUHEALTH cluster. Microb. Ecol. Health Dis. 16:125-130. [Google Scholar]
- 11.Duncan, S., K. Scott, A. Ramsay, H. Harmsen, G., Welling, C. Stewart, and H. Flint. 2003. Effects of alternative dietary substrates on competition between human colonic bacteria in an anaerobic fermentor system. Appl. Environ. Microbiol. 69:1136-1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durieux, A., C. Fougnies, H. Jacobs, and J. P. Simon. 2001. Metabolism of chicory fructooligosaccharides by bifidobacteria. Biotechnol. Lett. 23:1523-1527. [Google Scholar]
- 13.Ehrmann, M. A., M. Korakli, and R. F. Vogel. 2003. Identification of the gene for β-fructofuranosidase of Bifidobacterium lactis DSM10140T and characterization of the enzyme expressed in Escherichia coli. Curr. Microbiol. 46:391-397. [DOI] [PubMed] [Google Scholar]
- 14.Gibson, G. R., E. R. Beatty, X. Wang, and J. H. Cummings. 1995. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology 108:975-982. [DOI] [PubMed] [Google Scholar]
- 15.Gibson, G. R., H. Probert, J. Van Loo, R. Rastall, and M. B. Roberfroid. 2004. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr. Res. Rev. 17:259-275. [DOI] [PubMed] [Google Scholar]
- 16.Gibson, G. R., and M. B. Roberfroid. 1995. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J. Nutr. 125:1401-1412. [DOI] [PubMed] [Google Scholar]
- 17.Gomes, A. M. P., and F. X. Malcata. 1999. Bifidobacterium spp. and Lactobacillus acidophilus: biological, biochemical, technological and therapeutical properties relevant for use as probiotics. Trends Food Sci. Technol. 10:139-157. [Google Scholar]
- 18.Hidaka, H., T. Eida, T. Takizawa, T. Tokunaga, and, Y. Tashiro. 1986. Effects of fructooligosaccharides on intestinal flora and human health. Bifidobacteria Microflora 5:37-50. [Google Scholar]
- 19.Hoebregs, H. 1997. Fructans in foods and food products, ion-exchange chromatographic method: collaborative study. J. AOAC Int. 80:1029-1036. [Google Scholar]
- 20.Hopkins, M. J., J. H. Cummings, and G. T. Macfarlane. 1998. Inter-species differences in maximum specific growth rates and cell yields of bifidobacteria cultured on oligosaccharides and other simple carbohydrate sources. J. Appl. Microbiol. 85:381-386. [Google Scholar]
- 21.Janer, C., L. M. Rohr, C. Peláez, M. Laloi, V. Cleusix, T. Requena, and L. Meile. 2004. Hydrolysis of oligofructoses by the recombinant β-fructofuranosidase from Bifidobacterium lactis. Syst. Appl. Microbiol. 27:279-285. [DOI] [PubMed] [Google Scholar]
- 22.Joye, D., and H. Hoebregs. 2000. Determination of oligofructose, a soluble dietary fiber, by high-temperature capillary gas chromatography. J. AOAC Int. 83:1020-1025. [PubMed] [Google Scholar]
- 23.Kaplan, H., and R. W. Hutkins. 2000. Fermentation of fructooligosaccharides by lactic acid bacteria and bifidobacteria. Appl. Environ. Microbiol. 66:2682-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan, H., and R. W. Hutkins. 2003. Metabolism of fructooligosaccharides by Lactobacillus paracasei 1195. Appl. Environ. Microbiol. 69:2217-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleessen, B., L. Hartmann, and M. Blaut. 2001. Oligofructose and long-chain inulin: influence on the gut microbial ecology of rats associated with a human faecal flora. Br. J. Nutr. 86:291-300. [DOI] [PubMed] [Google Scholar]
- 26.Lähteenmäki, L. 2004. Consumers and health: Getting the probiotic message across. Microb. Ecol. Health Dis. 16:145-149. [Google Scholar]
- 27.Langlands, S. J., M. J. Hopkins, N. Coleman, and J. H. Cummings. 2004. Prebiotic carbohydrates modify the mucosa associated microflora of the human large bowel. Gut 53:1610-1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu, S. Q. 2003. Practical implications of lactate and pyruvate metabolism by lactic acid bacteria in food and beverage fermentations. Int. J. Food Microbiol. 83:115-131. [DOI] [PubMed] [Google Scholar]
- 29.Mai, V., and J. G. Morris. 2003. Colonic bacterial flora: changing understandings in the molecular age. J. Nutr. 134:459-464. [DOI] [PubMed] [Google Scholar]
- 30.Mattila-Sandholm, T., M. Blaut, C. Daly, L. De Vuyst, J. Doré, G. Gibson, H. Goosens, D. Knorr, J. Lucas, L. Lähteenmäki, A. Mercenier, M. Saarela, F. Shanahan, and W. M. de Vos. 2002. Food, GI-tract functionality and human health cluster: PROEUHEALTH. Microb. Ecol. Health Dis. 14:65-74. [Google Scholar]
- 31.Müller, M., and D. Lier. 1994. Fermentation of fructans by epiphytic lactic acid bacteria. J. Appl. Bacteriol. 76:406-411. [DOI] [PubMed] [Google Scholar]
- 32.Müller, M., and W. Seyfarth. 1997. Purification and substrate specificity of an extracellular fructanhydrolase from Lactobacillus paracasei ssp. paracasei P 4134. New Phytol. 136:89-96. [Google Scholar]
- 33.Müller, M., and J. Steller. 1995. Comparative studies of the degradation of grass fructan and inulin by strains of Lactobacillus paracasei subsp. paracasei and Lactobacillus plantarum. J. Appl. Bacteriol. 78:229-236. [Google Scholar]
- 34.Ohlson, K., S. Bjorneholm, R. Fonden, and U. Svensson. 2002. Lactobacillus F19: a probiotic strain suitable for consumer products. Microb. Ecol. Health Dis. 3(Suppl):27-32. [Google Scholar]
- 35.Perrin, S., C. Fougnies, J. P. Grill, H. Jacobs, and F. Schneider. 2002. Fermentation of chicory fructo-oligosaccharides in mixtures of different degrees of polymerization by three strains of bifidobacteria. Can. J. Microbiol. 48:759-763. [DOI] [PubMed] [Google Scholar]
- 36.Perrin, S., M. Warchol, J. P. Grill, and F. Schneider. 2001. Fermentations of fructo-oligosaccharides and their components by Bifidobacterium infantis ATCC 15697 on batch culture in semi-synthetic medium. J. Appl. Microbiol. 90:859-865. [DOI] [PubMed] [Google Scholar]
- 37.Pridmore, R. D., B. Berger, F. Desiere, D. Vilanova, C. Barretto, A. C. Pittet, M. C. Zwahlen, M. Rouvet, E. Altermann, R. Barrangou, B. Mollet, A. Mercenier, T. Klaenhammer, F. Arigoni, and M. A. Schell. 2004. The genome sequence of the probiotic intestinal bacterium Lactobacillus johnsonii NCC 533. Proc. Natl. Acad. Sci. USA 101:2512-2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao, V. 2001. The probiotic properties of oligofructose at low intake levels. Nutr. Res. 21:843-848. [Google Scholar]
- 39.Roberfroid, M. B., and N. M. Delzenne. 1998. Dietary fructans. Annu. Rev. Nutr. 18:117-143. [DOI] [PubMed] [Google Scholar]
- 40.Roberfroid, M. B., J. A. E. Van Loo, and G. R. Gibson. 1998. The bifidogenic nature of chicory inulin and its hydrolysis products. J. Nutr. 128:11-19. [DOI] [PubMed] [Google Scholar]
- 41.Schell, M. A., M. Karmirantzou, B. Snel, D. Vilanova, B. Berger, G. Pessi, M. C. Zwahlen, F. Desiere, P. Bork, M. Delley, R. D. Pridmore, and F. Arigoni. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. USA 99:14422-14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tuohy, K. M., R. K. Finlay, A. G. Wynne, and G. R. Gibson. 2001. A human volunteer study on the prebiotic effects of HP-inulin: faecal bacteria enumerated using fluorescent in situ hybridisation (FISH). Anaerobe 7:113-118. [Google Scholar]
- 43.Tuohy, K. M., S. Kolida, A. M. Lustenberger, and G. R. Gibson. 2001. The probiotic effects of biscuits containing partially hydrolysated guar gum and fructo-oligosaccharides—a human volunteer study. Br. J. Nutr. 86:341-348. [DOI] [PubMed] [Google Scholar]
- 44.Van der Meulen, R., L. Avonts, and L. De Vuyst. 2004. Short fractions of oligofructose are preferentially metabolized by Bifidobacterium animalis DN-173 010. Appl. Environ. Microbiol. 70:1923-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Loo, J., P. Coussement, L. De Leenheer, H. Hoebregs, and G. Smits. 1995. On the presence of inulin and oligofructose as natural ingredients in the western diet. Crit. Rev. Food Sci. 35:525-552. [DOI] [PubMed] [Google Scholar]
- 46.Van Loo, J., J. Cummings, N. Delzenne, H. Englyst, A. Franck, M. Hopkins, N. Kok, G. Macfarlane, D. Newton, M. Quigley, M. Roberfroid, T. van Vliet, and E. van den Heuvel. 1999. Functional food properties of nondigestible oligosaccharides: a consensus report from the ENDO project (DGXII AIRII-CT94-1095). Br. J. Nutr. 81:121-132. [DOI] [PubMed] [Google Scholar]
- 47.Winters, A. L., R. J. Merry, M. Müller, D. R. Davies, G. Pahlow, and T. Müller. 1998. Degradation of fructans by epiphytic and inoculant lactic acid bacteria during ensilage of grass. J. Appl. Microbiol. 84:304-312. [Google Scholar]
- 48.Zamfir, M., R. Callewaert, P. C. Cornea, L. Savu, I. Vatafu, and L. De Vuyst. 1999. Purification and characterization of a bacteriocin produced by Lactobacillus acidophilus IBB 801. J. Appl. Microbiol. 87:923-931. [DOI] [PubMed] [Google Scholar]