Abstract
The biofilms and rugose colony morphology of Salmonella enterica serovar Typhimurium strains are usually associated with at least two different exopolymeric substances (EPS), curli and cellulose. In this study, another EPS, a capsular polysaccharide (CP) synthesized constitutively in S. enterica serovar Typhimurium strain DT104 at 25 and 37°C, has been recognized as a biofilm matrix component as well. Fluorophore-assisted carbohydrate electrophoresis (FACE) analysis indicated that the CP is comprised principally of glucose and mannose, with galactose as a minor constituent. The composition differs from that of known colanic acid-containing CP that is isolated from cells of Escherichia coli and other enteric bacteria grown at 37°C. The reactivity of carbohydrate-specific lectins conjugated to fluorescein isothiocyanate or gold particles with cellular carbohydrates demonstrated the cell surface localization of CP. Further, lectin binding also correlated with the FACE analysis of CP. Immunoelectron microscopy, using specific antibodies against CP, confirmed that CP surrounds the cells. Confocal microscopy of antibody-labeled cells showed greater biofilm formation at 25°C than at 37°C. Since the CP was shown to be produced at both 37°C and 25°C, it does not appear to be significantly involved in attachment during the early formation of the biofilm matrix. Although the attachment of S. enterica serovar Typhimurium DT104 does not appear to be mediated by its CP, the capsule does contribute to the biofilm matrix and may have a role in other features of this organism, such as virulence, as has been shown previously for the capsules of other gram-negative and gram-positive bacteria.
Salmonella enterica serovar Typhimurium DT104 is a food-borne pathogen that emerged during the 1980s. It has a wide animal reservoir and is now recognized as an important pathogen of humans and animals (22, 32). Infection of humans results in enterocolitis with diarrhea and abdominal pain between 8 and 72 h after ingestion. Serovar Typhimurium DT104 carries chomosomally integrated resistance genes to ampicillin, chloramphenicol, streptomycin, sulfonamides and tetracyclines (ACSSuT) (6), making it a great risk to public health.
Anriany et al. (3) has reported a strain of serovar Typhimurium DT104, Rv, that forms wrinkled (rugose) colonies on nutrient-rich agar plates with low osmolarity at 25°C (Rv/25) but not at 37°C (Rv/37). Cells exhibiting this phenotype are more resistant to low pH and hydrogen peroxide than an isolate incapable of rugosity (Stv). Other food-borne pathogens such as Vibrio cholerae O1 El Tor, V. cholerae O139 Bengal, Salmonella enterica serovar Enteriditis, and non-DT104 serovar Typhimurium (1, 2, 27, 33, 36) have also been reported to form rugose colonies associated with some type of resistance to adverse environments.
In Salmonella, the phenomenon of rugosity (also called rdar or red, dry, and rough phenotype) has been investigated and shown to require curli (28, 37), proteinaceous fibrils (7), also referred to as thin aggregative fimbriae or Tafi (35), and cellulose, which largely comprise the organism's exopolymeric substances (EPS) (37). The curli are synthesized at stationary phase below 30°C (28), while cellulose is assembled only at temperatures below 30°C because it requires the temperature-sensitive regulatory protein AdrA for complete biosynthesis (37). The cellulose component is reported to stabilize or otherwise interact with the fibrils. Additionally, a second curli-interacting polysaccharide has been reported (35). The curli are thought to be instrumental in biofilm formation by causing the organisms to adhere to a surface and to one another. On agar at 25°C, rugose colonies are a manifestation of such aggregation.
In fact, species that form biofilms generally have long, narrow macromolecules—termed adhesins—that are able to penetrate repelling forces (e.g., van der Waal's [25]) near the surface prior to adhering to the surface. They are normally composed of protein, such as fimbriae; however, some capsular polysaccharides (CP) have been shown to be not only the dominant component of biofilm matrices but also the primary adhesin (25). In nature, the survival value of CP in the biofilm matrix has been recognized for more than a decade (8, 10), with the complexity of biofilm (29) and cell-cell interactions becoming increasingly apparent (16).
It has long been documented (9) that capsules are associated with virulence (23). Their functions include barrier protection (21), recognition, stress resistance (34), and defense against the host immune system (e.g., complement and phagocytes) (14) in addition to their role in the formation of biofilms. Capsules are highly hydrated structures (99% water) and are usually composed of one type of repeating polysaccharide subunit (18).
Unlike the Vi antigen of S. enterica serovar Typhi (4), the capsules of other salmonellae have not been nearly as well studied, being removed by boiling for the purpose of serologic typing based on O and H antigens. The present study tested the hypothesis that serovar Typhimurium DT104 synthesizes a capsule and that CP is a third component of extracellular matrices (biofilms) (24) in addition to cellulose (30) and curli (35, 37).
Here we report the constitutive synthesis of a CP that surrounds the cell and is also a component of serovar Typhimurium DT104 biofilms, functioning in a way other than as a primary adhesin. This is the first report of a capsule in serovar Typhimurium DT104.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
Frozen stocks of S. enterica serovar Typhimurium DT104 strain 11601 and E. coli DH5α were maintained at −80°C in Trypticase soy broth (TSB) containing 10% glycerol. Stocks of Hyphomonas adhaerens were maintained and grown in marine broth at 37°C (25). Serovar Typhimurium DT104 and E. coli were cultured on Trypticase soy agar and Luria-Bertani (LB) agar and broth (Becton Dickinson, Franklin Lakes, NJ) at 25°C and 37°C for the formation of rugose (Rv/25)- and smooth (Rv/37)-colony variant phenotypes, respectively (3). A stable, smooth, spontaneous mutant of serovar Typhimurium DT104 (Stv) (3), which forms smooth colonies at both temperatures, was used as a negative control in all experiments. Capsules were observed in negatively stained India ink preparations following the standard Duguid protocol (19).
Screening Rv and Stv for production of exopolymeric substances.
The ability of Rv/25 and Rv/37 to produce an exopolysaccharide containing (1-3)-β and (1-4)-β-glucopyranosyl units was tested. Five microliters of overnight cultures grown at 37°C in LB broth were plated in triplicate onto LB agar without salt and supplemented with 200 μg/ml of Calcofluor (fluorescent brightener 28; Sigma-Aldrich, St. Louis, MO). Plates were incubated at 25 and 37°C for 4 and 2 days, respectively. Colony fluorescence was inspected using a hand-held UV light source and photographed in a Bio-Rad GelDoc 100 chamber equipped with a digital camera (Rainbow TV zoom lens PH6X8-II; MACRO 8 to 48 mm [Bio-Rad, Hercules, CA]).
LB agar without salt, supplemented with Congo red (40 μg/ml) and Coomassie brilliant blue (20 μg/ml), was inoculated and incubated as described above to test for production of Congo red-binding fibrillar extracellular organelles by Rv/25 and Rv/37 (28).
Adhesion assay.
Sterile 12-mm round microscope coverslips (Fisher Scientific, Hampton, NH) were aseptically placed inside tissue culture plate wells with diameters of 22 mm (Corning, Inc., Corning, NY). Each well was filled with 4 ml of LB broth and inoculated with 10 μl of an overnight culture of strain Rv/37 of serovar Typhimurium DT104. Tissue culture plates were incubated for 24 h with gentle shaking. Coverslips were aseptically transferred to a petri dish using forceps and washed three times with 2 ml of distilled water (dH2O) for 1 min to remove nonadhered cells. For improved visualization, cells attached to the glass surface were stained with a 1% acridine orange solution for 5 min and washed with dH2O. Coverslips were inverted onto a glass slide and viewed under an epifluorescent microscope (Axiophot microscope; Carl Zeiss Microimaging, Thornwood, NY). Cells attached to 0.016-mm2 areas were counted. Seven separate areas were counted on three different coverslips per experiment for a sample size of 21.
A second set of adhesion experiments compared the amounts of biofilm formed by Rv/25 and Rv/37 after extended incubation. Polystyrene centrifuge tubes containing 5 ml of LB broth were inoculated three times with 5 μl of overnight cultures of each organism grown in the same broth at 37°C. The tubes were incubated at 25 and 37°C for 9 days. The spent medium was discarded, the tubes were rinsed twice with phosphate-buffered saline (PBS), and the biofilms were stained with a 0.4% solution of crystal violet in dH2O for 5 min. The dye solution was discarded, the tubes were rinsed with PBS, and the crystal violet bound to the biofilms was solubilized with an ethanol-acetone solution (80:20). Absorbance readings were measured at 570 nm.
Purification of CP.
Since serovar Typhimurium DT104 assembles curli and cellulose at temperatures below 30°C, and since the capsule was produced at both 25 and 37°C, the CP purification was initiated from cultures growing at 37°C to minimize contamination with other EPS. Two liters of TSB each were inoculated with an overnight culture of serovar Typhimurium DT104 and incubated with aeration for 5 days. The spent medium was exchanged for sterile TSB after 48 h. Cells and flocs of biofilm adhering to the walls of flasks were harvested by centrifugation (7,000 × g) for 20 min. The supernatant was discarded after failing to yield a precipitate after extraction with 2-propanol, indicating the absence of significant quantities of soluble polysaccharides. The pellet was blended in a Waring blender (Dynamics Corporation of America, Hartford, CT) with a solution of 10 mM EDTA and 3% NaCl for 1 min (1:10 ratio of sample to EDTA-NaCl solution) to shear off the integral CP from cells. The resulting suspension was centrifuged (7,000 × g) for 20 min. The supernatant was precipitated with 4 volumes of ice-cold 2-propanol and then centrifuged (16,000 × g) for 20 min (17).
The exopolysaccharide was suspended in a minimal volume of dH2O, dialyzed exhaustively against dH2O for 3 days using a Spectrapor 1 membrane with a 6,000- to 8,000-molecular-weight cutoff (Spectrum Medical Industries, Los Angeles, CA), and lyophilized. The resulting solute was suspended in a minimal volume of 0.1 M MgCl2 and incubated for 4 h at 37°C with a 0.1-mg/ml solution of DNase and RNase (Sigma-Aldrich) (25, 26). This was followed by an overnight treatment in 0.1 mg/ml of proteinase K (Sigma-Aldrich) at 37°C.
The enzyme-treated EPS was subjected to a hot phenol-chloroform extraction to further purify the sample from protein and lipopolysaccharide (LPS) contamination. Briefly, the partially purified carbohydrate was heated to 70°C in a water bath for 10 min and then added to an equal volume of 90% phenol preheated to the same temperature. The mixture was vigorously stirred for 15 min and cooled in ice, and the emulsion was centrifuged (30,000 × g) for 30 min.
The aqueous layer was extracted with methanol-chloroform (5). Briefly, 20 ml of the aqueous layer from the phenol extraction was mixed with 60 ml of a chloroform-methanol solution (1:2), and the mixture was vigorously vortexed for 1 min. An additional 20 ml of chloroform was added, the mixture was vortexed (30 s), and 20 ml of dH2O was added with an additional 30 s of vortexing. The chloroform and the methanol layers were allowed to separate, and the methanol layer was exhaustively dialyzed against dH2O and lyophilized. The final purified EPS material was white and fluffy. It was analyzed for carbohydrate versus protein and LPS content as described below.
Carbohydrate assay.
The hot phenol-sulfuric acid assay was performed to quantify the sugar content of the purified EPS (11). Briefly, 0.5 ml of a glucose standard (10 to 100 μg/ml) and a 1-mg/ml solution of the purified EPS were mixed with an equal volume of phenol. Next, 2.5 ml of sulfuric acid was added to each carbohydrate solution, and the mixture was vortexed. Tubes were incubated in the dark at room temperature for 18 h. Absorbances were measured at 490 nm.
Bicinchoninic acid assay.
The amount of protein in the final EPS extract was measured using a bicinchoninic acid protein assay (Pierce, Rockford, IL) according to the manufacturer's instructions. Briefly, 0.1-ml aliquots of the EPS solution (1 mg/ml) and a bovine serum albumin standard solution (10 to 100 μg/ml) were mixed with 2 ml of the bicinchoninic acid reagent in glass tubes. Samples were incubated at 37°C for 30 min, and their absorbances were measured at 562 nm.
Limulus assay.
The Limulus assay was performed according to the manufacturer's instructions to determine the amount of LPS contaminant in the EPS extract (Associates of Capecod, Inc., Falmouth, MA). Briefly, the 5 ml of the Limulus amoebocyte reagent was added to 0.5 μg of E. coli endotoxin standard or the isolated CP. The mixture was allowed to stand at room temperature for 1 h and then was vortexed for 1 min every 10 min. Serial dilutions of the tube contents were incubated at 25°C for 1 h, and then the tubes were gently inverted. The detection of endotoxin was recorded as positive if the mixture solidified.
Immunoagglutination.
The immunoagglutination procedure was also performed to evaluate the EPS extract for possible LPS contamination. Forty micrograms of the purified EPS was resuspended in 200 μl water and then further diluted from 10−1 to 10−6. The positive controls were boiled serovar Typhimurium DT104 strain Rv/37 cells. Twenty microliters of sample from each dilution was reacted with 1 drop of antiserum (Bacto Salmonella group B antiserum; specific for the LPS O-side chain) on a slide. Negative controls (test for autoagglutination) used saline instead of antisera. The slides were gently rocked, and agglutination was scored after 1 min.
Fluorophore-assisted carbohydrate electrophoresis (FACE) monosaccharide composition analysis of EPS.
For sugar composition analysis (31), the extracted EPS was hydrolyzed according to the protocol provided by Glyco, Inc. Briefly, neutral sugar hydrolysis was tested by incubating 50 μl of a 2:5 (wt/vol) solution of purified CP preparation in dH2O with 50 μl trifluoroacetic acid (4 N) at 100°C for 5 h. Amine sugar hydrolysis was performed with hydrochloric acid (8 N) at 100°C for 1 h. After hydrolysis, samples were dried in a SpeedVac, and the sample that underwent amine hydrolysis was reacetylated (according to the instructions of Glyco, Inc.). The hydrolyzed CP was incubated with MONO labeling dye overnight at 37°C, dried in a SpeedVac evaporator, and resuspended in labeling solvent. The sample was mixed in loading buffer, and 6-μl volumes were loaded onto a polyacrylamide gel for electrophoresis and photographed in a GelDoc 100 chamber equipped with a digital camera (Rainbow TV zoom lens X6X8-II; 8 to 48 mm).
Production of anti-EPS polyclonal antibodies.
Purified CP was sent to Sigma Genosys (The Woodlands, TX) for production of rabbit polyclonal antibodies. Upon receipt, the antibodies were tested by enzyme-linked immunosorbent assay and Western analyses and were adsorbed nine times with E. coli DH5α acetone-treated powder.
Sodium dodecyl sulfate-PAGE of EPS.
Samples of cell lysates and exopolysaccharide were subjected to polyacrylamide gel electrophoresis (PAGE) with sodium dodecyl sulfate on a 12% Tris-glycine gel (iGels; Gradipore Ltd., Australia) in Tris-glycine buffer (Bio-Rad; 25 mM Tris, 192 nM glycine, and 20% [vol/vol] methanol, pH 8.3). Briefly, EPS extracts of Rv/37 and H. adhaerens (negative control for nonspecific CP adhesion) (25) were dissolved in PIPES [piperazine-1,4-bis(2-ethanosulfonic acid)] buffer to a final concentration of 4 mg/ml. Whole-cell lysates prepared from late logarithmic cultures of Rv/25, Rv/37 Stv, and E. coli were adjusted to an optical density at 600 nm of 1.0. Cells were harvested, washed in PIPES, and resuspended in the same buffer. Aliquots of 100 μl were stored at −70°C.
Polysaccharide and cell lysate samples were diluted in Laemmli sample buffer (Bio-Rad) (1:2) containing β-mercaptoethanol (βME; 1:20), heated for 5 min at 95°C, and allowed to cool to room temperature. Samples were treated with a proteinase K solution (20 mg/ml) at 37°C for 18 h before loading. Wells were loaded with 35 μl and 20 μl of polysaccharide and cell lysate samples, respectively. Kaleidoscope prestained standard (Bio-Rad) was included (5 μl).
Electroblotting, protein staining, and Western polysaccharide blotting.
Samples were electrotransferred onto an Immobilon-P membrane (Millipore Corp., Bedford, MA) in a Tris-glycine-methanol buffer (0.025 M Tris, 0.19 M glycine, 20% methanol) at a constant voltage of 100 V for 1 h. After transfer, membranes were submerged in methanol for 1 min, allowed to air dry, and stained for proteins using SYPRO Ruby protein blot stain (Molecular Probes, Inc., Eugene, OR) according to the protocol provided by the manufacturer.
Briefly, membranes were floated on a solution of 7% acetic acid and 20% methanol for 15 min at room temperature with gentle rocking. Membranes were rinsed four times in dH2O for 5 min per rinse and incubated in 25 ml of SYPRO Ruby blot stain for 15 min at room temperature. After staining, membranes were double rinsed in dH2O, air dried, visualized, and photographed as described previously.
Membranes were destained in methanol and blocked in 5% PBS-Tween, containing 2% skim milk (1:500), for 18 h at 4°C. Membranes were triple washed in PBS-Tween (0.1%) and incubated for 1 h at room temperature with 50 ml of anti-EPS polyclonal antibodies in PBS-Tween containing 2% skim milk (1:500). Blots were washed three times with PBS-Tween and incubated for 1 h with horseradish peroxidase-labeled goat anti-rabbit immunoglobulin G (IgG) secondary antibodies (1:5,000) (Poti-4CN detection kit; Bio-Rad). The blots were washed twice in PBS-Tween and developed.
Immunoelectron microscopy.
Cultures of Rv/25, Rv/37, and Stv/25 in late logarithmic phase, grown in LB broth, were washed three times in PIPES to remove the medium and resuspended in 1 ml of the same buffer. Drops of each culture were applied to a small piece of Parafilm. Cells were attached to nickel Formvar-coated copper grids (200 mesh) by floating the grids on drops of sample for 1 min. The grids were blotted dry, blocked with 1% skim milk for 15 min, and serially washed in three successive drops of PIPES. The grids were then inverted on a drop of 1:100 anti-EPS polyclonal antibodies in PIPES containing 0.1% of skim milk for 30 min at room temperature. The grids were blotted dry, successively rinsed in 3 drops of PIPES, and inverted on a drop of 1:100 goat anti-rabbit secondary antibodies conjugated to 12-nm colloidal gold (Electron Microscopy Sci., Washington, PA) for 20 min. The grids were rinsed by inverting them on 3 drops of dH2O and observed with a Zeiss EM10 CA electron microscope (Carl Zeiss SMT, Inc., Thornwood, NY).
Characterization of DT104 CP by lectin specificity.
Aliquots of 100 μl of serovar Typhimurium DT104 CP were air dried on glass slides. Thirty microliters of a 1:100 fluorescein isothiocyanate (FITC)-labeled lectin solution in dH2O was added to each sample, and the slides were incubated at 37°C for 30 min in a chamber prehumidified with distilled water and protected from light. The CP-lectin mixtures were gently washed three times with 25 ml of PBS for 10 min per rinse. Lectin binding was scored semiquantitatively based on biofilm delineation and intensity of fluorescence under epifluorescence microscopy (Zeiss Axiophot; 63× objective; numerical aperture, 1.32; Zeiss filter set 9). Lectins labeled with FITC (EY Laboratories, Inc., San Mateo, CA) were selected based on a previously reported composition of the capsules of enteric bacteria. The reported predominant carbohydrate specificities of the lectins used in this study are as follows: Canavilis ensiformis (concanavalin A [ConA]), α-Man, α-Glucose (Glc), α-GlcNAc (N-acetylglucosamine), and branched Man with α-Fucose (Fuc) as the determinant; Vicia faba (VFA), α-Man, α-Glc, α-GlcNAc, and branched Man with α-Fuc as the determinant; Bauhinia purpurea (BPA), α-GalNac and β-GalNAc; Anguilla anguilla (AAA), α-Fuc; and Helix pomatia (HPA), terminal N-acetyl-GalNAc.
Lectin-gold labeling of cells.
Gold-labeled ConA (ConA-Au; 20-nm-diameter gold particles; EY Labs, San Mateo, CA)and Helix pomatia (HP-Au; 20-nm-diameter gold particles; EY Labs) lectins were used to label the CP of serovar Typhimurium DT104 from an Rv/37 culture in the late logarithmic phase of growth. Cells were then attached to Formvar-coated copper grids. The sample was blocked as previously described. Grids were incubated in a 1:10 dilution of each lectin in PIPES containing 0.1% of skim milk for 50 min at room temperature and rinsed three times with dH2O. Cells were observed as described above.
Scanning confocal laser microscopy.
Scanning confocal laser microscopy analysis of biofilms attached to coverslips was performed using a Zeiss inverted microscope and a dual laser scanning confocal imaging system equipped with a 100-mW argon ion laser and a 5-mW krypton-argon laser (Zeiss LSM 510; Carl Zeiss, Inc.). Biofilms of Rv/25, Rv/37, and Stv were allowed to accrue for 45 h on 12-mm round glass coverslips as previously described (see “Adhesion assay” above). Planktonic cells were gently removed by submerging the glass slides three times in PBS.
Biofilms were observed using probes. Adherent bacteria were stained with SYTO 9, a fluorescent nucleic acid stain that is part of the Live-Dead staining kit (Molecular Probes Inc.) that excites at 488 nm and emits at 522 nm. One hundred microliters of SYTO 9 (1 part stain to 3 parts water) was gently applied to the biofilm and incubated at room temperature in the dark for 15 min. The coverslip was rinsed twice for 1 min with PBS. Biofilms were then stained with anti-DT104 CP polyclonal antibodies (1:200 in PBS) for 30 min at 23°C, washed three times, and then incubated with Alexa Fluor 546 goat anti-rabbit IgG (excitation, 556 nm; emission, 573 nm) (Molecular Probes Inc.) in PBS (1:3,000) for 20 min at 23°C. Twice-washed biofilms were mounted on glass slides and analyzed with a confocal scanning laser microscope. Analysis of digital thin sections of images was used to determine the thickness of biofilms, bacterial cell area, and localization of CP-producing organisms within the biofilm.
RESULTS
Salmonella enterica serovar Typhimurium DT104 forms a wrinkled (rugose) colony type late in stationary phase at lower temperatures on eugonic media with low osmolarity and a smooth colony type at 37°C (3). Thin aggregative fimbriae (curli) (28) and cellulose (37) are manifested in the rugose colony type. Consistent with the notion that rugosity is associated with adhesiveness to inanimate surfaces and between cells, serovar Typhimurium DT104 adhered better to polystyrene surfaces and formed thicker, more stable pellicles under rugose-permissive conditions than otherwise (data not shown).
CP production.
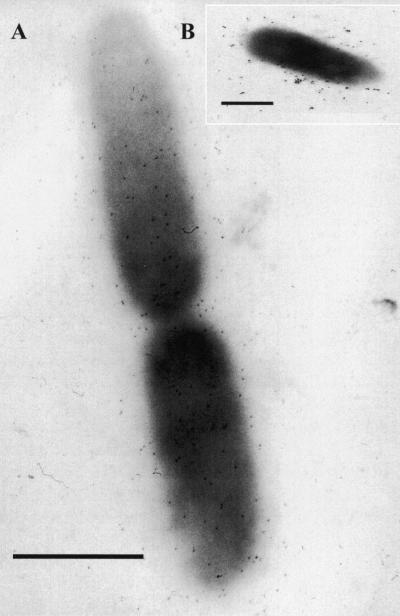
After negative staining with India ink, serovar Typhimurium DT104 was observed microscopically to have capsules that completely surrounded the cells (Fig. 1 A) at temperatures between 25°C (which allows rugose formation) and 37°C (which inhibits rugosity) (Table 1). These observations included filamentous forms, which comprised approximately 5% of the population in very late stationary phase (Fig. 1B, inset). Serovar Typhimurium DT104 makes this capsule during all growth phases, as observed by capsular stain periodically during the first 24 h of growth (data not shown). Both wild-type RV and Stv, the spontaneous mutant deficient in the ability to form rugose colonies, make this capsule.
FIG. 1.
(A) India ink capsular stain of serovar Typhimurium DT104 grown on Luria-Bertani agar plates. Both DT104 at 25°C (Rv/25) and DT104 at 37°C (Rv/37) (not shown) elaborated capsules. Arrows indicate cells with thick capsules. (B) Inset. Late stationary growth phase, filamentous form.
TABLE 1.
Population of serovar Typhimurium DT104 expressing an integral capsule after growth on LB broth at 25 and 37°Ca
| Incubation temp (°C)b | % Capsulated cells (SD)c |
|---|---|
| 25 (n = 4) | 29.8 (4.5) |
| 37 (n = 6) | 37.6 (8.2) |
| Both temps (n = 10) | 33.7 (7.8) |
Ten regions from four separate experiments (two at each temperature) were visually counted three times, and the average value was used to estimate the percentage of encapsulated bacteria in the total population.
Cells were allowed to accrue on coverslip surfaces incubated at 25 and 37°C under gentle rotation for 19 h. n, number of regions examined.
Numbers represent the percentages of cells of the total population that expressed a capsule, estimated using a Petroff-Hausser chamber and confirmed by anti-DT104 capsular polyclonal antibodies under confocal microcopy (see Fig. 5).
Isolation of the CP.
To correlate the observations of capsules with biochemical and immunological evidence, the CP of serovar Typhimurium DT104 was extracted from cultures grown at 37°C (to maximize the ratio of CP to curli fibers and cellulose), purified, and used as antigen. SYPRO-stained PAGE gels did not detect proteins, nor did the Limulus amoebocyte assay (for lipid endotoxin) or immunoprecipitation analysis using anti-Salmonella LPS antibodies (versus O antigen) detect significant LPS in the CP antigen preparation. In Western blots (Fig. 2), the antibodies detected a diffuse band above 250 kDa in serovar Typhimurium DT104 preparations from cultures grown at both 25°C and 37°C (lanes 2 and 3) and also in the lane containing purified CP (lane 5). They did not react with E. coli (lane 1) nor with H. adhaerens preparations (not shown). While enhancing the specificity of the antiserum, these antibodies, adsorbed against E. coli, retained specificity for DT104 CP.
FIG. 2.
Electroblots. Lane 1, E. coli DH5α; lane 2, DT104 (Rv/25) at 25°C, 24 h; lane 3, DT104 (Rv/37) at 37°C, 24 h; lane 4, standard protein (STP), kaleidoscope prestained standards; lane 5, DT104 (Rv/37) purified CP, versus anti-DT104 (Rv/37) CP antibodies.
Analysis and cell localization of CP.
In immunoelectron micrographs, the antibodies targeted a structure that envelops the cell, which was interpreted to be the capsule (Fig. 3). Consistent with light microscopic evidence, cells grown at both 25 and 37°C and Stv produced this capsule. Gold-conjugated ConA lectin, specific for glucose and mannose residues (Table 2), also targeted this structure (Fig. 3, inset).
FIG. 3.
Immunoelectron micrograph of serovar Typhimurium DT104. (A) Stv/25 cells versus rabbit anti-DT104 CP probed with goat anti-rabbit IgG, conjugated with 12-nm gold. Rv/37 and Rv/25 (not shown) displayed the same pattern. (B) Inset. Rv/37 versus ConA lectin conjugated with 20-nm colloidal gold. Gold particles surrounded cells at a 34:1 ratio over background.
TABLE 2.
Reactions of FITC-labeled lectins with capsule produced by DT104 wild-type biofilms
| Specificity | Lectina | Reactivityb |
|---|---|---|
| Man, branched Man, and Glc-specific groups | ConA | ++c |
| VFA | + | |
| Fuc and branched Fuc-specific groups | AAA | − |
| GalNAc | BPA | − |
| HPA | −d |
Carbohydrate specificities were as follows: Canavalis ensiformis (ConA): α-Man, α-Glc, α-GlcNAc, and branched Man; Vicia faba (VFA): α-Man, α-Glc, α-GlcNAc, and branched Man with α-fucose as a determinant; Anguilla anguilla (AAA): α-fucose; Bauhinia purpurea (BPA), α-GalNAc and β-GalNAc; Helix pomatia (HPA); terminal N-acetyl-GalNAc. Lectins were obtained from EY Laboratories, Inc.
++, biofilms of all sizes fluoresced with high intensity and architecture was very well defined; +, biofilms of all sizes fluoresced with moderate intensity (Biofilm structure was usually visible for large biofilms but not for smaller ones); −, little fluorescence observed.
ConA, conjugated to 20-nm colloidal gold, also bound to the capsular material of DT104 (Fig. 5, inset).
HPA, conjugated to colloidal gold, did not bind to the capsule of the DT104 rugose variant (scanning electron microscopy; data not shown).
In fact, FACE gels revealed the major sugars in the CP to be comprised principally of glucose and mannose, with galactose being a minor constituent (Fig. 4). Since CP bands in the neutral (lane 1) and amine sugar (lane 2)-containing lanes were the same size, it is concluded that the CP extract does not contain N-acetylated sugars. The composition of the CP of DT104 is supported by lectin probe analysis (Table 2). ConA and VFA, specific for mannose and glucose, bound to DT104 CP, while lectins specific for N-acetylgalactosamine did not. Anguilla anguilla, specific for fucose linkages, a common constituent of colanic acid, also did not bind, providing further evidence that the DT104 capsule is not colanic acid. The enrichment for CP during purification and the chemical composition of CP (13) suggest that it is capsular and distinct from other envelope polysaccharides (20).
FIG. 4.
FACE monosaccharide compositional analysis of serovar Typhimurium DT104 (Rv/37) purified CP. Lanes: 1, CP; 2, CP analyzed for acetylated sugars; 3, MONO dye-positive reacetylation control (note lower GlcNAc band); 4, MONO dye control ladder standard (100 pmol of each monosaccharide). The densities of the bands corresponding to the Man and Glu standards suggest the presence of a higher concentration than that of the Gal band. N-acetylated sugars were not detected. The faint high band in lane 1 is described (Glyko FACE monosaccharide composition kit GK90100) as a common anomalous dye contaminant.
Biofilm formation.
DT104 colonies that exhibit rugosity bind to surfaces better than those that do not, form better-developed pellicles, and, as would be predicted, form thicker biofilms more rapidly (Fig. 5). At 37°C, and in the case of Stv at 25 and 37°C, biofilm formation occurred more slowly and attained a depth of only one or two cell layers. Most notably, cells did not form a confluent layer but were restricted to circular “colonial” zones. This is more apparent in earlier stages of colonization (Fig. 5C, phase-contrast inset c). Rugose-type cells, however, formed biofilms that were 14 μm thick within 45 h. Significantly, CP (Fig. 5A, stained red by 2o antibody, contrasting with green cells) was present in the matrixes of both biofilms.
FIG. 5.
Sagittal (A, B, and C) and overhead (D, E, and F) images of serovar Typhimurium DT104 Rv/25, Rv/37, and Stv biofilms on glass coverslips. Cultures were grown in LB broth for 45 h. Coverslips were removed from the culture flasks and rinsed with sterile distilled water to remove unattached cells. Biofilms were dually stained with the nucleic acid stain SYTO 9 and serovar Typhimurium DT104 anti-CP polyclonal antibodies followed by Alexa Fluor-labeled secondary antibodies. Stained biofilms were examined by confocal scanning laser microscopy (numerical aperture, 1.4). Sagittal and overhead images were acquired from a collection of 36 consecutive Z-series scans of each biofilm, taken at 0.4-μm and 0.9-μm intervals, respectively. Cells are stained green; CP is stained red. Insets a and c are phase-contrast micrographs of Rv/25 and Stv/25 cells, respectively, attached to glass coverslips in 18-h cultures.
DISCUSSION
Our findings indicate that a constitutively produced capsule, though not part of the rugose matrix, is an integral component of the exopolymeric substances and biofilm produced by serovar Typhimurium DT104 at 37°C. The observations suggest that the capsule alone is not sufficient for optimal adhesion. Curli, which are produced at 25°C but not at 37°C, are more likely to be the primary adhesin. Additional adhesion studies involving strains that lack capsule would further clarify the role of the capsule in adhesion, if any, and in biofilm formation.
CP structural composition.
Using FACE, a sensitive carbohydrate analysis method that has been shown to be comparable to high-performance liquid chromatography analysis (31), the composition of the CP carbohydrate was characterized as having repeating units of glucose, mannose, and, to a lesser extent, galactose. Though it awaits confirmatory nuclear magnetic resonance analysis, the chemical composition of serovar Typhimurium DT104 CP appears to differ from CP synthesized by other gram-negative pathogens (13, 24), including enterohemorrhagic E. coli (15). serovar Typhimurium DT104 CP also does not share the same composition with any of the many E. coli K capsules (14) or other Salmonella capsules (12), which are composed of colanic acid. Furthermore, serovar Typhimurium DT104 CP appears to be uniquely different from the Vi capsules of S. enterica serovar Typhi, S. enterica serovar Paratyphi, and even some strains of serovar Dublin, which are linear homopolymers of α-1,4,2-deoxy-2-N-acetylgalactosamine uronic acid (4). Finally, the spatiality, composition, and presence of serovar Typhimurium DT104 capsule at 37°C suggest that it is different from that recently reported for another Salmonella serovar (35).
CP spatiality.
Bright-field and immunoelectron microscopy clearly reveal that serovar Typhimurium DT104 produces a capsule under all conditions studied. We conclude that the isolated polysaccharide is the CP based on the following lines of evidence. (i) It was isolated from cells grown at 37°C, a temperature at which a minimum amount of another polysaccharide (cellulose) is produced. (ii) The isolation procedure largely excluded other polymers such as LPS. (iii) The antibody against the isolated polysaccharide binds to the structure that envelops the cells. Finally, (iv) the structure binds lectins that specifically target mannose and glucose (two of the components of the isolated polysaccharide) and does so with capsule-like spatiality.
Functions of CP in serovar Typhimurium DT104.
Rugosity is correlated with curli and cellulose (24, 37). The curli may represent adhesions, which in combination with cellulose cause cells to adhere to one another to yield the wrinkled colony type (3) and which also assist in attachment of the cells to surfaces. Since their expression is temperature dependent, curli would function in serovar Typhimurium DT104's water habitats, where temperatures are below 30°C. Unlike the curli, serovar Typhimurium DT104 CP is not believed to be a primary adhesin; however, it may be a constituent of the biofilm matrix, along with curli and cellulose, and thus a major component in the biofilm matrix. Biofilm CP has been shown to confer enhanced survival in the environment, endowing protection from predation and harmful chemicals and enabling the sequestering of nutrients (10). Unlike the E. coli M antigen, colanic acid, which is expressed in response to the lower temperature of its water habitat (20), the serovar Typhimurium DT104 capsule appears to be expressed at both lower and higher temperatures, suggesting that this structure may be important for survival in the matrix of biofilms in environments both inside and outside of the host (25). As shown with other enteric pathogens, the capsule of serovar Typhimurium DT104 may function as a virulence mechanism by protecting the organism against host defense mechanisms, e.g., phagocytosis.
Acknowledgments
This research was supported by a grant from the Joint Institute for Food Safety and Applied Nutrition (JIFSAN), University of Maryland, College Park, and by the Technology Development Corporation (Tedco), Maryland.
Footnotes
This paper is dedicated to the memory of Lewis E. Carr.
The electron microscopy work described herein is contribution 101 from the Laboratory of Biological Ultrastructure, College of Life Sciences, University of Maryland, College Park.
REFERENCES
- 1.Ali, A., J. A. Johnson, A. A. Franco, D. J. Metzger, T. D. Connell, J. G. Morris Jr., and S. Sozhamannan. 2000. Mutation in the extracellular protein secretion pathway genes (esp) interfere with rugose polysaccharide production in and motility of Vibrio cholerae. Infect. Immun. 68:1967-1974. (Erratum, 68: 3792.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen-Vercoe, E., M. Dibb-Fuller, C. J. Thorns, and M. J. Woodward. 1997. SEF17 fimbriae are essential for the convoluted colonial morphology of Salmonella enteritidis. FEMS Microbiol. Lett. 153:33-42. [DOI] [PubMed] [Google Scholar]
- 3.Anriany, Y. A., R. M. Weiner, J. A. Johnson, C. E. De Rezende, and S. W. Joseph. 2001. Salmonella enterica serovar Typhimurium DT104 displays a rugose phenotype. Appl. Environ. Microbiol. 67:4048-4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker, E. E., R. E. Whiteside, R. Basch, and M. A. Derow. 1959. The Vi antigens of the Enterobacteriaceae. I. Purification and chemical properties. J. Immunol. 83:680-696. [PubMed] [Google Scholar]
- 5.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Med. Sci. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 1997. Multidrug resistant Salmonella serotype Typhimurium—United States, 1996. Morb. Mortal. Wkly. Rep. 46:308-310. [PubMed] [Google Scholar]
- 7.Chapman, M. R., L. S. Robinson, J. S. Pinkner, R. Roth, J. Heuser, M. Hammar, S. Normark, and S. J. Hultgren. 2002. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295:851-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen, B. E. 1989. The role of extracellular polysaccharides in biofilms. J. Biotechnol. 10:181-202. [Google Scholar]
- 9.Cross, A. S. 1990. The biologic significance of bacterial encapsulation. Curr. Top. Microbiol. Immunol. 150:87-95. [DOI] [PubMed] [Google Scholar]
- 10.Decho, A. W. 1990. Microbial exopolymer secretions in ocean environments: their role(s) in food webs and marine processes. Oceanogr. Mar. Biol. Annu. Rev. 28:73-153. [Google Scholar]
- 11.Dubois, M., K. A. Gilles, J. K. Hamilton, P. A. Rebers, and F. Smith. 1956. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28:350-356. [Google Scholar]
- 12.Grant, W. D., I. W. Sutherland, and J. F. Wilkinson. 1969. Exopolysaccharide colonic acid and its occurrence in the Enterobacteriaceae. J. Bacteriol. 100:1187-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hancock, L. E., and M. S. Gilmore. 2002. The capsular polysaccharide of Enterococcus faecalis and its relationship to other polysaccharides in the cell wall. Proc. Natl. Acad. Sci. USA 99:1574-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jann, B., and K. Jann. 1990. Structure and biosynthesis of the capsular antigens of Escherichia coli. Curr. Top. Microbiol. Immunol. 150:19-41. [DOI] [PubMed] [Google Scholar]
- 15.Junkins, A. D., and M. P. Dole. 1992. Demonstration of exopolysaccharide production by enterohemorrhagic Escherichia coli. Curr. Microbiol. 25:9-17. [DOI] [PubMed] [Google Scholar]
- 16.Kolter, R., and R. Losick. 1998. Enhanced: one for all and all for one. Science 280:226-227. [DOI] [PubMed] [Google Scholar]
- 17.Langille, S. E., G. G. Geesey, and R. M. Weiner. 2000. Polysaccharide-specific probes inhibit adhesion of Hyphomonas rosenbergii strain VP-6 to hydrophilic surfaces. J. Ind. Microbiol. Biotechnol. 25:81-85. [Google Scholar]
- 18.Moxon, E. R., and J. S. Kroll. 1990. The role of bacterial polysaccharide capsules as virulence factors. Curr. Top. Microbiol. Immunol. 150:65-85. [DOI] [PubMed] [Google Scholar]
- 19.Murray, R. G. E., R. N. Doetsch, and C. F. Robinow. 1994. Determinative and cytological light microscopy, p. 21-41. In R. Gerhardt (ed.), Methods for general and molecular bacteriology. American Society for Microbiology, Washington, D.C.
- 20.Nikaido, H., and M. Vaara. 1987. Outer membrane, p. 7-22. In J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, vol. 1. American Society for Microbiology, Washington, D.C. [Google Scholar]
- 21.O'Toole, G., H. B. Kaplan, and R. Kolter. 2000. Biofilm formation as microbial development. Annu. Rev. Microbiol. 54:49-79. [DOI] [PubMed] [Google Scholar]
- 22.Poppe, C., K. Ziebell, L. Martin, and K. Allen. 2002. Diversity in antimicrobial resistance and other characteristics among Salmonella typhimurium DT104 isolates. Microb. Drug Resist. 8:107-122. [DOI] [PubMed] [Google Scholar]
- 23.Potera, C. 1999. Forging a link between biofilms and disease. Science 283:1837-1839. [DOI] [PubMed] [Google Scholar]
- 24.Prigent-Combaret, C., G. Prensier, T. T. Le Thi, O. Vidal, P. Lejeune, and C. Dorel. 2000. Developmental pathway for biofilm formation in curli-producing Escherichia coli strains: role of flagella, curli and colonic acid. Environ. Microbiol. 2:450-464. [DOI] [PubMed] [Google Scholar]
- 25.Quintero, E. J., and R. M. Weiner. 1995. Evidence for the adhesive function of the exopolysaccharide of Hyphomonas strain MHS-3 in its attachment to surfaces. Appl. Environ. Microbiol. 61:1897-1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Read, R. R., and J. W. Costerton. 1987. Purification and characterization of adhesive exopolysaccharides from Pseudomonas putida and Pseudomonas fluorescens. Can. J. Microbiol. 33:1080-1090. [DOI] [PubMed] [Google Scholar]
- 27.Romling, U., and M. Rohde. 1999. Flagella modulate the multicellular behavior of Salmonella typhimurium on the community level. FEMS Microbiol. Lett. 180:91-102. [DOI] [PubMed] [Google Scholar]
- 28.Romling, U., W. D. Sierralta, K. Eriksson, and S. Normark. 1998. Multicellular and aggregative behavior of Salmonella typhimurium strains is controlled by mutations in the agfD promoter. Mol. Microbiol. 28:249-264. [DOI] [PubMed] [Google Scholar]
- 29.Sauer, K. 2003. The genomics and proteomics of biofilm formation. Genome Biol. 4:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solano, C., B. Garcia, J. Valle, C. Berasain, J. M. Ghigo, C. Gamazo, and I. Lasa. 2002. Genetic analysis of Salmonella enteritidis biofilm formation: critical role of cellulose. Mol. Microbiol. 43:793-808. [DOI] [PubMed] [Google Scholar]
- 31.Starr, C. M., R. I. Masada, C. Hague, E. Skop, and J. C. Klock. 1996. Fluorophore-assisted carbohydrate electrophoresis in the separation, analysis, and sequencing of carbohydrates. J. Chromatogr. 720:295-321. [DOI] [PubMed] [Google Scholar]
- 32.van Duijkeren, E., W. J. B. Wannet, D. J. Houwers, and W. van Pelt. 2002. Serotype and phage type distribution of Salmonella strains isolated from humans, cattle, pigs, and chickens in The Netherlands from 1984 to 2001. J. Clin. Microbiol. 40:3980-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wai, S. N., Y. Mizunoe, A. Takade, S. I. Kawabata, and S. I. Yoshida. 1998. Vibrio cholerae O1 strain TSI-4 produces the exopolysaccharide materials that determine colony morphology, stress resistance, and biofilm formation. Appl. Environ. Microbiol. 64:3648-3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiner, R., S. Langille, and E. Quintero. 1995. Structure, function and immunochemistry of bacterial exopolysaccharides. J. Indust. Microbiol. 15:339-346. [DOI] [PubMed] [Google Scholar]
- 35.White, A. P., D. L. Gibson, S. K. Collinson, P. A. Banser, and W. W. Kay. 2003. Extracellular polysaccharides associated with thin aggregative fimbriae of Salmonella enterica serovar Enteritidis. J. Bacteriol. 185:5398-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yildiz, F. H., and G. K. Schoolnik. 1999. Vibrio cholerae O1 El Tor: identification of a gene cluster required for rugose colony type, exopolysaccharide production, chlorine resistance, and biofilm formation. Proc. Natl. Acad. Sci. USA 96:4028-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zogaj, X., M. Nimtz, M. Rohde, W. Bokranz, and U. Romling. 2001. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol. Microbiol. 39:1452-1463. [DOI] [PubMed] [Google Scholar]