Abstract
Analysis of levels of hydrogenase processing and activity in Rhizobium leguminosarum biovar viciae bacteroids from pea (Pisum sativum) plants showed that the oxidation of nitrogenase-evolved hydrogen is limited by the availability of nickel in agricultural soils. This limitation was overcome by using an inoculant strain engineered for higher hydrogenase expression.
The efficiency of nitrogen fixation by the Rhizobium-legume symbiosis is affected by the hydrogen released by nitrogenase during this process (14). In bacteroids induced in pea (Pisum sativum L.) nodules by Rhizobium leguminosarum biovar viciae UPM791 the hydrogen generated in the nitrogen fixation process is recycled by a [NiFe] hydrogenase with two structural subunits (HupL and HupS). The biosynthesis of this hydrogenase is a complex process that involves at least 15 accessory proteins, some of which participate in the insertion of an essential nickel atom in the active site of the enzyme (16). This hydrogen recycling has been associated with significant increases of plant productivity in certain symbiotic systems such as soybean (2, 8).
Ni availability to pea bacteroids limits hydrogenase activity in plants grown under gnotobiotic (i.e., sterile vermiculite) conditions by affecting the Ni-dependent processing of hydrogenase subunits (5). Further work has demonstrated that this limitation also affects other symbiotic systems (4). In this work we analyze whether the available Ni levels in agricultural soils are sufficient for synthesis of adequate levels of hydrogenase for efficient hydrogen recycling.
Ni concentration in the soil depends on the parent material, the degree of contamination and the pedogenic process. The soil content of Ni is variable; values reported for total nickel typically range from 1 mg kg−1 to over 400 mg kg−1 in normal agricultural soils (1), with lower values usually associated with acid soil conditions. Ni is essential for some biological functions, such as urease synthesis in soybean leaves (11), and a critical deficiency of nickel has been defined for the growth of plants in urea nitrogen (9).
In order to ascertain whether Ni content in soils could indeed affect hydrogen oxidation by the Rhizobium-legume symbiosis, we decided to study hydrogen metabolism in pea bacteroids from plants grown in different soils. To this aim, six soils representative of the main agricultural areas in Madrid Province were collected and analyzed for relevant physicochemical traits by standard methods (15). The concentration of metallic elements Ni, Co, and Mn was determined by inductively coupled plasma-mass spectrometry (MS) following extraction with nitric acid (total content) or with ammonium oxalate (available content; 17). Inductively coupled plasma-MS analysis was performed using an Elan 6000 instrument (Perkin Elmer Sciex) at the Universidad Autónoma de Madrid core facility. The levels of Ni found in soils were variable (Table 1): the lowest value was associated with acid soil 3 (0.08 mg kg−1 of total nickel), whereas the highest total nickel level was 3 mg kg−1 (soil 5). The levels of available Ni ranged from 0.03 to 0.68 mg kg−1 (soils 3 and 2, respectively). The concentration of Co and Mn showed essentially the same pattern as Ni in the soils analyzed.
TABLE 1.
Physicochemical characteristics of soils
| Soila | Texture | OMb (%) | pH | ECc (μs) | Ni concn (mg kg−1)
|
Co concn (mg kg−1)
|
Mn concn (mg kg−1)
|
|||
|---|---|---|---|---|---|---|---|---|---|---|
| Total | Available | Total | Available | Total | Available | |||||
| 1 | Loamy | 1.9 | 7.51 | 263 | 1.15 | 0.48 | 1.97 | 0.99 | 123 | 49 |
| 2 | Loamy-sandy | 1.4 | 7.25 | 316 | 1.94 | 0.68 | 2.51 | 1.36 | 130 | 92 |
| 3 | Loamy-sandy | 0.68 | 5.83 | 30 | 0.08 | 0.03 | 0.31 | 0.20 | 99 | 64 |
| 4 | Loamy-clayey | 1.00 | 7.02 | 80 | 1.44 | 0.27 | 2.90 | 0.73 | 109 | 25 |
| 5 | Loamy | 1.54 | 7.74 | 312 | 3.00 | 0.53 | 3.20 | 0.75 | 247 | 55 |
| 6 | Loamy-sandy | 0.69 | 8.03 | 151 | 1.93 | 0.28 | 1.68 | 0.15 | 172 | 12 |
Three topsoil subsamples (0 to 15 cm) per site were collected from uniform areas of the field, mixed, and sieved. Collection sites: 1 and 2, El Encin experimental station (40°30′N 3°15′W); 3, Soto del Real (40°46,063′N 3°44,227′W); 4, Torrejón del Rey (40°35,608′N 3°21,940′W); 5, Aranjuez (40°04,657′N 3°36,860′W); 6, Pinto (40°14,184′N 3°39,220′W).
Organic matter content (OM) is expressed in %.
The electric conductivity (EC) is expressed in μs.
The soil material was mixed (1:1) with quartz sand and used as the substrate for growing pea plants (Pisum sativum cv. Frisson) in a growth chamber (12). Seeds were surface sterilized and seedlings were inoculated with Rhizobium cultures as described previously (12). Plants were watered with a standard N-free nutrient solution (5) or with the same solution supplemented with different amounts of nickel, added as nickel chloride. Symbiotic H2 metabolism was studied by determining the levels of H2 evolution and acetylene reduction of nodules, and hydrogenase activity of bacteroids (4). In order to monitor the prevalence of the inoculated strain in the nonsterile substrate where plants were grown, we used R. leguminosarum biovar viciae strain UPM1156, a UPM791 (12) derivative carrying a constitutively expressed gusA gene inserted downstream of hydrogen oxidation genes obtained in our lab (C. Fernández et al., unpublished data), as inoculum. The percentage of nodule occupancy by the inoculated strain was determined by scoring for β-glucuronidase activity in intact nodules attached to root systems (19). In this analysis we found that most nodules (72 to 88%) stained blue (Table 2), indicating that the inoculated strain was highly prevalent. No blue nodules were found in plants from uninoculated pots.
TABLE 2.
Hydrogen metabolism in pea bacteroids and nodules induced by R. leguminosarum biovar viciae in plants grown under different Ni addition treatments
| Soil | Strain | Ni addeda | Hydrogenase activityb | Acetylene reductionc | H2 productiond | Implantatione |
|---|---|---|---|---|---|---|
| 1 | UPM1156 | 0 | 1,890 ± 696 | 32 ± 3.8 | <0.25 | 72 |
| UPM1156 | 10 | 2,580 ± 229 | 24.0 ± 0.4 | <0.25 | ||
| UPM1156 | 100 | 7,570 ± 2,368 | 11.8 ± 0.1 | <0.25 | ||
| SPF25 | 0 | 2,559 ± 727 | 29.9 ± 3.6 | <0.25 | ||
| n/if | 0 | <50 | 32.0 ± 6.3 | 7.50 ± 2.80 | ||
| 2 | UPM1156 | 0 | 610 ± 42 | 28.4 ± 1.0 | 5.75 ± 0.01 | 80 |
| UPM1156 | 10 | 4,250 ± 1,956 | 19.4 ± 0.6 | <0.25 | ||
| UPM1156 | 100 | 5,230 ± 158 | 16.5 ± 0.4 | <0.25 | ||
| SPF25 | 0 | 1,336 ± 180 | 17.2 ± 1.8 | <0.25 | ||
| n/i | 0 | <50 | 18.0 ± 1.4 | 15 ± 1.20 | ||
| 3 | UPM1156 | 0 | 670 ± 143 | 38.9 ± 0.1 | 6.23 ± 0.31 | 81 |
| UPM1156 | 10 | 4,950 ± 692 | 39.6 ± 1.9 | <0.25 | ||
| UPM1156 | 100 | 6,210 ± 720 | 37.8 ± 0.3 | <0.25 | ||
| SPF25 | 0 | 1,151 ± 41 | 12.0 ± 0.4 | <0.25 | ||
| n/i | 0 | <50 | 22.0 ± 2.0 | 6.22 ± 1.48 | ||
| 4 | UPM1156 | 0 | 1,500 ± 169 | 30.0 ± 0.9 | <0.25 | 85 |
| UPM1156 | 10 | 2,940 ± 11 | 19.2 ± 2.4 | <0.25 | ||
| UPM1156 | 100 | 4,370 ± 1,820 | 29.8 ± 0.4 | <0.25 | ||
| SPF25 | 0 | 1,834 ± 124 | 41.2 ± 3.5 | <0.25 | ||
| n/i | 0 | <50 | 27.0 ± 3.0 | 15.00 ± 1.80 | ||
| 5 | UPM1156 | 0 | 1,230 ± 366 | 42.2 ± 0.7 | <0.25 | 79 |
| UPM1156 | 10 | 4,830 ± 435 | 44.0 ± 2.8 | <0.25 | ||
| UPM1156 | 100 | 6,800 ± 380 | 39.0 ± 4.9 | <0.25 | ||
| SPF25 | 0 | 2,255 ± 427 | 45.0 ± 5.0 | <0.25 | ||
| n/i | 0 | <50 | 22.0 ± 3.0 | 11.50 ± 0.27 | ||
| 6 | UPM1156 | 0 | 2,460 ± 59 | 20.5 ± 0.4 | <0.25 | 88 |
| UPM1156 | 10 | 3,960 ± 465 | 22.2 ± 0.6 | <0.25 | ||
| UPM1156 | 100 | 5,130 ± 254 | 24.5 ± 0.3 | <0.25 | ||
| SPF25 | 0 | 2,321 ± 55 | 23.0 ± 4.6 | <0.25 | ||
| n/i | 0 | <50 | 15.5 ± 3.3 | 8.00 ± 0.50 |
Ni added is expressed in mg per liter.
Hydrogenase activity is expressed as nanomoles of hydrogen per hour per milligram of protein, and are the averages of two independent bacteroid preparations± S.E.
Acetylene reduction is expressed as micromoles of acetylene reduced per hour per gram of nodule fresh weight and are the averages of two independent determinations ± S.E.
Values of H2 production are expressed as micromoles of H2 produced per hour per gram of nodule fresh weight and are the averages of two independent determinations ± S.E.
Values of implantation are expressed as % of blue nodules.
n/i, plants not inoculated.
Analysis of the acetylene reduction activity revealed normal levels of nitrogenase activity in all cases (Table 2), indicating that the conditions tested were compatible with the normal development of the symbiosis. In soils with no nickel addition, the levels of hydrogenase activity were very low in soils 2 and 3 (ca. 650 nmol H2 h−1 mg protein−1). These low values resulted in the inability to completely recycle the hydrogen evolved by nitrogenase. Higher levels of hydrogenase activity, similar to those observed in unamended vermiculite (13), were observed for the remaining soils. With uninoculated plants no hydrogenase activity was detected, and the H2 evolution values indicated that a large fraction (25 to 50%) of the electron flux through nitrogenase, estimated as the ratio between H2 production and acetylene reduction activities, was diverted to proton reduction. We conclude from these results that most, if not all, R. leguminosarum biovar viciae strains resident in the soils tested were hydrogenase-negative, a situation that has been described previously in this species (16).
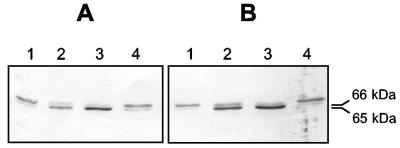
Addition of Ni to the nutrient solution caused a significant increase of H2 uptake in all soils tested (Table 2) that was more evident for the highest level of nickel added (100 mg liter−1). This increase led to the suppression of H2 evolution where it was present. Since the Ni-dependent limitation of hydrogenase activity affects the processing of the hydrogenase large subunit HupL (5), we performed an immunoblot analysis of bacteroid crude extracts using an anti-HupL antiserum (a gift from R. J. Maier). Figure 1 shows representative results obtained for two of the soils tested. In this analysis we observed the presence of two bands (of 66 and 65 kDa) in crude extracts from UPM1156 (Fig. 1, lanes A1 and B1), with most of the specific signal associated with the larger unprocessed form of HupL (5). Addition of increasing concentrations of Ni2+ correlated with an increase in the relative amount of the fast-migrating band (Fig. 1, lanes 2 and 3). Similar results were obtained with the remaining soils (data not shown).
FIG. 1.
Immunodetection of the hydrogenase large subunit (HupL) in bacteroids from pea plants grown in soil 1 (panel A) and soil 2 (panel B) with different Ni addition treatments. The immunoblot shows bands immunoreactive with an antiserum raised against B. japonicum HupL in bacteroid crude extracts resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (10% acrylamide) and transferred to polyvinylidene difluoride membranes. Numbers on the right side indicate the molecular masses of HupL protein in the unprocessed form (66 kDa) and the processed form (65 kDa) as deduced from comparison to molecular weight standards. Ni2+ addition treatments: lanes 1 and 4, no nickel added; lanes 2, 10 mg liter−1 Ni2+ added; lanes 3, 100 mg liter−1 Ni2+ added. Strains: lanes 1 to 3, UPM1156; lanes 4, SPF25.
The largest amount of processed form was observed in extracts from bacteroids obtained from plants exposed to 100 mg liter−1 Ni2+, and this correlated with the highest level of hydrogenase activity (Table 2). These data indicate that the level of hydrogenase activity is limited in agricultural soils due to low availability of Ni, and that this low availability of Ni affects the processing of the hydrogenase large subunit, thus extending to natural conditions the conclusions previously obtained in gnotobiotic systems (5). In our previous work the level of hydrogenase activity increased with higher amounts of Ni2+ added up to 10 mg liter−1, but with 100 mg liter−1 Ni2+ the level of hydrogenase activity was lower because this was a toxic concentration for the plants. In the current soil-based assays the overall pattern was similar, but the addition of 100 mg liter−1 of Ni2+ resulted in higher levels of hydrogenase activity compared to those from 10 mg liter−1 Ni2+. This difference is probably due to a lower Ni2+ availability for the plants in soils because Ni2+ forms stronger complexes with the soil particles that may act as buffer for this element (1).
The results presented in this paper contrast with previous work with soybean (7), where it was concluded that soil nickel deficiency seemed unlikely to be agronomically important. These differences might be explained on the basis of the different abilities to provide nickel to bacteroids in pea versus soybean symbioses (5). In our case, a direct correlation between available Ni and hydrogenase activity cannot be deduced from the data obtained in this analysis. For instance in soil 2 we detected the maximal value of available Ni (0.68 mg liter−1) but, at the same time, hydrogenase activity was very low and this situation could be reversed by nickel addition. This can be taken as another example of the fact that current chemical methods to predict the availability of nickel to the plants are unsatisfactory (1) and overestimate in most cases the amount of bioavailable nickel (3).
The need for efficient biological systems to determine the bioavailability of heavy metals has been pointed out (10), and a whole-cell biosensor for bioavailable Ni2+ has been developed (18). However this biosensor detects nickel available to bacteria in the free-living state, not to plants. The results presented in this paper indicate that bacteroid hydrogenase processing and activity could be used as a biosensor for soil Ni bioavailability to legume plants.
Toxicity associated with nickel implies that addition of this element to soil is not a feasible option to optimize the hydrogenase activity in nodules. Instead, we decided to use a modified R. leguminosarum strain (SPF25) engineered for higher hydrogenase expression by modification of the HupSL promoter (6). Pea plants inoculated with R. leguminosarum biovar viciae SPF25 were grown in the different soils without Ni addition, and hydrogen metabolism of nodules and bacteroids was analyzed (Table 2). In all cases, except for soil 6, SPF25 bacteroids exhibited significantly higher levels of hydrogenase activity. The increase compared to UPM1156 ranged between 6% (soil 4) and 117% (soil 2). Such activities were high enough to allow full recycling of the hydrogen evolved by nitrogenase. Immunoblot analysis of bacteroid crude extracts also revealed an increase in the levels of the processed form of the hydrogenase large subunit (Fig. 1, lanes A4 and B4). These results indicate that the promoter modification introduced into SPF25 has a beneficial impact on the energy efficiency of R. leguminosarum inoculants used in normal agricultural soils containing low nickel levels.
Acknowledgments
We are grateful to J. Torrent for valuable advice on chemical nickel analysis. This work has been supported by funds from Spain's Ministry of Education and Science (projects BIO2004-0251 to J.P. and AGL01-2295 to T.R.A.) and from Comunidad Autónoma de Madrid (project P-AMB-321-505 to T.R.A.).
REFERENCES
- 1.Adriano, D. C. 2001. Trace elements in terrestrial environments. Biogeochemistry, bioavailability and risks of metals. Springer-Verlag, New York, N.Y.
- 2.Albretch, S. L., R. J. Maier, F. J. Hanus, S. A. Russell, D. W. Emerich, and H. J. Evans. 1979. Hydrogenase in Rhizobium japonicum increases nitrogen fixation by nodulated soybeans. Science 203:1255-1257. [DOI] [PubMed] [Google Scholar]
- 3.Boisson, J., M. Mench, V. Sappin-Didier, P. Solda, and J. Vangronsveld. 1998. Short-term in situ immobilisation of Cd and Ni by beringite and steel shots application to long-term sludged plots. Agronomie 18:347-359. [Google Scholar]
- 4.Brito, B., J. Monza, J. Imperial, T. Ruiz-Argüeso, and J. M. Palacios. 2000. Nickel availability and hupSL activation by heterologous regulators limit symbiotic expression of the Rhizobium leguminosarum biovar viciae hydrogenase system in Hup− rhizobia. Appl. Environ Microbiol. 66:937-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brito, B., J. M. Palacios, E. Hidalgo, J. Imperial, and T. Ruiz-Argüeso. 1994. Nickel availability to pea (Pisum sativum L.) plants limits hydrogenase activity of Rhizobium leguminosarum biovar viciae bacteroids by affecting the processing of the hydrogenase structural subunits. J. Bacteriol. 176:5297-5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brito, B., J. M. Palacios, J. Imperial, and T. Ruiz-Argüeso. 2002. Engineering the Rhizobium leguminosarum biovar. viciae hydrogenase system for expression in free-living microaerobic cells and increased symbiotic hydrogenase activity. Appl. Environ. Microbiol. 68:2461-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalton, D., H. J. Evans, and F. J. Hanus. 1985. Stimulation by nickel of soil microbial urease activity and urease and hydrogenase activities in soybeans in a low-nickel soil. Plant Soil 88:245-258. [Google Scholar]
- 8.Evans, H. J., S. A. Russell, F. J. Hanus, and T. Ruiz-Argüeso. 1988. The importance of hydrogen recycling in nitrogen fixation by legumes, p. 777-791. In R. J. Summerfield (ed.), World crops: cool season food legumes. Kluwer Academic, Boston, Mass.
- 9.Gerendas, J., J. C. Polacco, S. K. Freyermuth, and B. Sattelmarcher. 1999. Significance of nickel for plant growth and metabolism. J. Plant Nutr. Soil Sci. 162:241-256. [Google Scholar]
- 10.Kamnev, A. A., and D. van der Lelie. 2000. Chemical and biological parameters as tools to evaluate and improve heavy metal phytoremediation. Biosci. Rep. 20:239-258. [DOI] [PubMed] [Google Scholar]
- 11.Klucas, R. V., F. J. Hanus, A. Sterling, and H. J. Evans. 1983. Nickel: a micronutrient element for hydrogen-dependent growth of Rhizobium japonicum and for expression or urease activity in soybeans leaves. Proc. Natl. Acad. Sci. USA 80:2253-2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leyva, A., J. M. Palacios, T. Mozo, and T. Ruiz-Argüeso. 1987. Cloning and characterization of hydrogen uptake genes from Rhizobium leguminosarum. J. Bacteriol. 169:4929-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leyva, A., J. M. Palacios, J. Murillo, and T. Ruiz-Argüeso. 1990. Genetic organization of the hydrogen uptake (hup) cluster from Rhizobium leguminosarum. J. Bacteriol. 172:1647-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maier, R. J., and E. W. Triplett. 1996. Towards more productive, efficient and competitive nitrogen-fixation symbiotic bacteria. Crit. Rev. Plant Sci. 15:191-234. [Google Scholar]
- 15.Ministerio de Agricultura, Pesca y Alimentación. 1994. Métodos oficiales de análisis. Tomo III. Secretaría General Técnica, Ministerio de Agricultura, Pesca y Alimentación, Madrid, Spain.
- 16.Ruiz-Argüeso, T., J. Imperial, and J. M. Palacios. 2000. Uptake hydrogenases in root nodule bacteria, p. 489-507. In E. W. Triplett (ed.), Prokaryotic nitrogen fixation: a model system for analysis of a biological process. Horizon Scientific Press, Wymondham, United Kingdom.
- 17.Schwertmann, U. 1964. Differenzierung der Eisenoxide des Bodens durch Extraktion mit Ammoniumoxalat-Lösung. Z. Pflanzenernär. 105:194-202. [Google Scholar]
- 18.Tibazarwa, C., P. Corbisier, M. Mench, A. Bossus, P. Solda, M. Mergeay, L. Wyns, and D. van der Lelie. 2001. A microbial biosensor to predict bioavailable nickel in soil and its transfer to plants. Environ. Pollut. 113:19-26. [DOI] [PubMed] [Google Scholar]
- 19.Wilson, K. J., A. Sessitsch, J. C. Corbo, K. E. Giller, A. D. Akkermans, and R. A. Jefferson. 1995. Beta-glucuronidase (GUS) transposons for ecological and genetic studies of rhizobia and other gram-negative bacteria. Microbiology 141:1691-1705. [DOI] [PubMed] [Google Scholar]