Abstract
The enzyme lactoperoxidase is part of the innate immune system in vertebrates and owes its antimicrobial activity to the formation of oxidative reaction products from various substrates. In a previous study, we have reported that, with thiocyanate as a substrate, the lactoperoxidase system elicits a distinct stress response in Escherichia coli MG1655. This response is different from but partly overlapping with the stress responses to hydrogen peroxide and to superoxide. In the current work, we constructed knockouts in 10 lactoperoxidase system-inducible genes to investigate their role in the tolerance of E. coli MG1655 to this antimicrobial system. Five mutations resulted in a slightly increased sensitivity, but one mutation (corA) caused hypersensitivity to the lactoperoxidase system. This hypersensitive phenotype was specific to the lactoperoxidase system, since neither the sensitivity to hydrogen peroxide nor to the superoxide generator plumbagin was affected in the corA mutant. Salmonella enterica serovar Typhimurium corA had a similar phenotype. Although corA encodes an Mg2+ transporter and at least three other inducible open reading frames belonged to the Mg2+ regulon, repression of the Mg stimulon by Mg2+ did not change the lactoperoxidase sensitivity of either the wild-type or corA mutant. Prior exposure to 0.3 mM Ni2+, which is also transported by CorA, strongly sensitized MG1655 but not the corA mutant to the lactoperoxidase system. Furthermore, this Ni2+-dependent sensitization was suppressed by the CorA-specific inhibitor Co(III) hexaammine. These results indicate that CorA affects the lactoperoxidase sensitivity of E. coli by modulating the cytoplasmic concentrations of transition metals that enhance the toxicity of the lactoperoxidase system.
The potential of natural antimicrobial systems to be used as biopreservatives in foods, cosmetics, pharmaceuticals, and other industrial products has attracted much attention in recent years. Although they are generally not very powerful when used alone, their antimicrobial efficacy can sometimes be strongly enhanced when they are combined with other treatments, an approach called hurdle technology (28). For example, we have demonstrated earlier that sublethal treatment with high hydrostatic pressure strongly sensitizes a wide range of vegetative food spoilage and pathogenic bacteria to the lactoperoxidase-thiocyanate enzyme system (11, 41). Lactoperoxidase (LP) is a heme-containing enzyme that catalyzes the oxidation by hydrogen peroxide of a wide range of substrates. LP is part of the innate immune system in vertebrates and is found in secretions such as milk, saliva, tears, and airway mucus (16, 26, 44). In view of its occurrence and function in the human body, together with its natural presence in numerous foods, there is little or no toxicological concern for the use of LP in foods, provided that its uptake remains within the range that occurs from normal food exposure (6).
The antimicrobial activity of the LP system is nonspecific and stems from the oxidative power of the enzymatic reaction products formed. The major physiological substrate of LP is thiocyanate (SCN−), which is oxidized to hypothiocyanite (OSCN−). LP can also oxidize bromide and iodide anions to the corresponding hypohalogenous acids but, in contrast to myeloperoxidase and chloroperoxidase, cannot oxidize chloride ions (1, 10, 25). The LP system is believed to induce oxidation of cellular sulfhydryl (—SH) groups into disulfides (———). When the homeostatic capacity of the cell is exceeded, this may result in structural and functional damage, reflected by loss of pH gradient, K+ leakage, inhibition of respiration, and inhibition of protein and nucleic acid synthesis (2, 8, 24, 27, 30). As opposed to oxidants like H2O2 and superoxide (O2−), OSCN− causes no DNA damage and is considered nontoxic for the host cells producing it (43). In fact, the LP system is thought to protect host cells by consuming the potentially harmful H2O2 produced during oxidative burst (19).
Bacteria, like other living organisms, have developed defense systems against oxidative stress. In Escherichia coli and related bacteria, OxyRS and SoxRS are the key regulators of the transcriptional oxidative stress response to (hydrogen) peroxide and superoxide, respectively (37). We recently characterized the stress response in E. coli after challenge with the LP/SCN− system. Thirteen open reading frames (ORFs) were identified that were induced by the enzyme system but not by H2O2 or the superoxide generator plumbagin. In addition, some genes that are inducible by H2O2 (recA) or by O2− (sodA) were also induced by the LP/SCN− system. We concluded that the LP/SCN− system elicits a specific and unique stress response different from but partly overlapping with other oxidative stress responses (29). Similarly, Hansen et al. (13) characterized the stress response in E. coli after challenge with the Curvularia haloperoxidase, using bromide as a substrate, and succeeded in establishing a link between one of the induced genes and bacterial tolerance to the haloperoxidase system. In the current work, we investigated the potential role of 10 of the LP/SCN−-inducible ORFs in tolerance of E. coli against this enzyme system.
MATERIALS AND METHODS
Plasmids, bacterial strains, and culture conditions.
All strains and plasmids used in this work are listed in Table 1. Luria-Bertani (LB; 10 g/liter tryptone, 5 g/liter yeast extract, 5 g/liter NaCl, 10 g/liter agar for solid medium) was used as the standard growth medium. Antibiotics (Sigma-Aldrich, Bornem, Belgium) were added as appropriate at the following concentrations: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; and chloramphenicol, 30 μg/ml. When indicated, medium was supplemented with MgSO4 from a filter-sterilized 1 M stock solution. Tryptone soy agar (30.12 g/liter tryptone soy broth, 10 g/liter agar) was used as the growth medium in sensitivity assays.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| E. coli | ||
| MG1655 | Wild-type strain; rph-1, F−, λ− | 12 |
| QC2411 | MG1655 ΔrecA306 srl::Tn10; Tetr | 7 |
| QC909 | MG1655 sodA::cat and sodB::kan | 3 |
| BW26742 | ΔpstS625::kan | E. coli Genetic Stock Center |
| FB20382 | MG1655 rstA::kan | F. R. Blattner |
| FB21757 | MG1655 intB::kan | F. R. Blattner |
| FB21746 | MG1655 mgtA::kan | F. R. Blattner |
| FB21469 | MG1655 corA::kan | F. R. Blattner |
| LMM-JS03-14 | MG1655 ydjN::kan | This study |
| LMM-JS03-66 | MG1655 ybjG::kan | This study |
| LMM-JS03-64 | MG1655 yhdA::kan | This study |
| LMM-JS03-15 | MG1655 cysJ::cat | This study |
| LMM-JS03-60 | MG1655 fdx::kan | This study |
| S. enterica serovar Typhimurium | ||
| MM2089 | Wild-type strain | M. E. Maguire |
| MM2242 | MM2089 corA52::Tn10d16d17; Tetr | M. E. Maguire |
| Plasmids | ||
| pKD3 | Template plasmid containing FRT-cat-FRT for the one step inactivation protocol; Apr | 5 |
| pKD4 | Template plasmid containing FRT-kan-FRT for the one-step inactivation protocol; Apr | 5 |
| pKD46 | Red recombinase expressing plasmid; Apr | 5 |
| pJS121 | pFPV25 containing E. coli corA; Apr | This study |
| pASV_AOD12 | gfp-mgtA transcriptional fusion | 29 |
| pASV_CIC2 | gfp-corA transcriptional fusion | 29 |
| pASV_ACB1 | gfp-rstA transcriptional fusion | 29 |
| pASV_ATC9 | gfp-ybjG transcriptional fusion | 29 |
| pMAS29 | Contains serovar Typhimurium corA; Apr | M. E. Maguire |
Enzymes and chemicals.
Stock solutions of LP (Sigma-Aldrich) (10 mg/ml) and glucose oxidase (Sigma-Aldrich) (100 U/ml) were prepared in a 50% glycerol solution in phosphate-buffered saline (2.87 mM KH2PO4, 7.12 mM K2HPO4, 0.151 M NaCl, pH 6.0). Stock solutions of plumbagin (5-hydroxy-2-methyl-1,4-naphtoquinone) (Sigma-Aldrich) (200 mM) and of Co(III) hexaammine (Sigma-Aldrich) (200 mM) were prepared in dimethyl sulfoxide and in ultrapure deionized water, respectively. All these stock solutions were stored at −18°C. Every week, fresh stock solutions of potassium thiocyanate (KSCN; 25 mM), hydrogen peroxide (H2O2; 25 mM), and NiCl2 (1 M) were prepared in ultrapure deionized water and stored at 4°C.
Construction of knockout mutants.
Knockout mutants in our laboratory strain MG1655 background were constructed either by P1 transduction of mutant alleles from E. coli strains obtained from elsewhere (Table 1) or by using the standard one-step inactivation protocol of Datsenko and Wanner (5). In the latter procedure, a chloramphenicol (cat) or kanamycin (kan) resistance gene was amplified using platinum pfx polymerase (Invitrogen, Merelbeke, Belgium) from template plasmids pKD3 or pKD4, respectively, using the mutagenesis primers (Isogen Biosciences, Maarsen, The Netherlands) specified in Table 2. These primers contained at their 5′ ends 45-bp regions homologous to the gene of interest, which allows homologous recombination between the PCR fragment containing the resistance gene and the genomic region of interest. Amplified fragments were transferred to MG1655 by electroporation, and recombination was promoted by inducing the λ red genes provided on plasmid pKD46 with 1 mM arabinose. Transformants were isolated and grown at 42°C to cure plasmid pKD46. To avoid any secondary mutations, all mutant alleles were transferred again to MG1655 by P1 transduction. Purified mutants were verified by PCR (Taq polymerase; Fermentas, St. Leon-Rot, Germany) using combinations of control primers (Table 2) (Eurogentec, Seraing, Belgium) upstream and downstream of the genomic region of interest and resistance gene-specific primers C1 and C2 for cat and K1 and K2 for kan (5).
TABLE 2.
Primers used in this study
| Primer | Sequence (5′→3′) |
|---|---|
| Mutagenesis primersa | |
| PcysJmutUP | GACACAGGTCCCACCTTCCGCGTTGCTTCCGTTGAACCCGGAGCAAgtgtaggctggagctgcttc |
| PcysJmutDWN | CTCACTTAAAAATTCATCCGCCGCTTCGGTGTCCATGCCACCAAATTCGcatatgaatatcctcctta |
| PydjNmutUP | ATGAACTTTCCATTAATTGCGAACATCGTGGTGTTCGTTGTACTGCgtgtaggctggagctgcttc |
| PydjNmutDWN | GGTCAGCGTGCCAGCTGTCATCGAGCCACTAACGTTTAACGCCGcatatgaatatcctcctta |
| PfdxmutUP | ACGAGGTTTAATATGCCAAAGATTGTTATTTTGCCTCATCAGGATgtgtaggctggagctgcttc |
| PfdxmutDWN | AATTTCGCGGCTATCGGTCCACTTAAGTCCCATACTAACTCTGTcatatgaatatcctcctta |
| PyhdAmutUP | TTTCGGCCTTTGTTACGCTGCTCACCGGGTTAACAATTTTTGTGAgtgtaggctggagctgcttc |
| PyhdAmutDWN | ATCTTTGTGAATATTTTTTCACGTTAGTATCAAGTGGCTGTGAGGcatatgaatatcctcctta |
| PybjGmutUP | TTTGAATCTCTCTCTATTCTCTCTTATTAACGCGACGCCAGACTCgtgtaggctggagctgcttc |
| PybjGmutDWN | GCGGATCGGTAATGCAAAACAGACGCGATACCACGATTGCAGACGcatatgaatatcctcctta |
| Control primers | |
| PcysJctrUP | GACGATAAAACCGCCGTAGA |
| PcysJctrDWN | GGTTCGAGGTGCAGAGTACG |
| PydjNctrUP | GTTCGTTGGTCATCGCAAAT |
| PydjNctrDWN | GAATGGGAAGTGGTGTGCTT |
| PfdxctrUP | GGTCTGACCGATAGCGAAAT |
| PfdxctrDWN | TATGTGACTGGGGTGAACGA |
| PyhdActrUP | CGCTAATGGAAAAGCAAAGC |
| PyhdActrDWN | ACAAGGCGCAAAAATCATCT |
| PybjGctrUP | GCGGTCATCGAAGTAGGAAC |
| PybjGctrDWN | TAACAAACGGCGGTAAAAGG |
| C1b | TTATACGCAAGGCGACAAGG |
| C2b | GATCTTCCGTCACAGGTAGG |
| K1b | CAGTCATAGCCGAATAGCCT |
| K2b | CGGTGCCCTGAATGAACTGC |
| PgfpFWD | AAAAATAGGCGTATCACGAGG |
| Cloning primers | |
| PcorAclUP | TCGATCTAGACACCTTCTGCTGACCGTTTTc |
| PcorAclDWN | CAGTAAGCTTGGGGGCAACACCAGAATATAAd |
Sequence in uppercase, 45-bp homologous fragment; sequence in lowercase, template plasmid specific primer.
Datsenko and Wanner (5).
TCTAGA, XbaI recognition site.
AAGCTT, HindIII recognition site.
Cloning of corA for complementation experiments.
The corA gene of MG1655 was amplified by PCR using Platinum pfx polymerase with cloning primers PcorAclUP and PcorAclDWN (Eurogentec) containing XbaI and HindIII restriction sites, respectively (Table 2). Plasmid pFPV25 (40) was prepared by removing the 700-bp XbaI-HindIII fragment containing gfp, followed by dephosphorylation with calf intestinal alkaline phosphatase (Epicenter, Landgraaf, The Netherlands) and purification from agarose gel. Subsequently, the amplified wild-type corA fragment was cloned as an XbaI-HindIII fragment in pFPV25, resulting in plasmid pJS121. This construct was verified by PCR using a vector-specific primer PgfpFWD and primer PcorAclDWN.
Sensitivity assays for LP system, H2O2, or plumbagin.
Overnight cultures were diluted to 106 CFU/ml in 10 mM HEPES-KOH, pH 7.0, containing 10 μg/ml LP, 0.5 U/ml of glucose oxidase, 0.4% glucose, and 0.75 mM KSCN. Control samples contained cells together with glucose oxidase, glucose, and KSCN, and blank samples contained cells in HEPES-KOH buffer only. After incubation at 30°C for different times, survivors were counted by a rapid agar spot method: 10-fold dilutions of the bacterial suspensions in 10 mM HEPES-KOH buffer were spotted (5 μl/spot) on tryptone soy agar trays, which were then incubated at 37°C for 22 h. The reduction in CFU/ml could be determined with about 0.5-log unit accuracy by assessing the formation of colonies in the spots corresponding to the subsequent dilutions for each treatment and for the control and blank samples. All experiments were repeated at least two times with three replicates per treatment. Results obtained with replicates did not differ by more than 0.5 log units. To determine inactivation by H2O2 or the O2−-generating agent plumbagin, the same method was used, but the enzyme systems were replaced by 0.5 mM H2O2 or 0.5 mM plumbagin and 0.4% glucose, respectively.
Promoter activity assay for genes belonging to the Mg2+ regulon.
Strains carrying transcriptional gfp fusions to mgtA, corA, rstA, and ybjG (Table 1) were grown overnight in LB with or without the extra addition of 10 mM MgSO4. After 20 h of growth, cells were harvested by centrifugation (6,000 rpm for 5 min) and resuspended in 10 mM HEPES-KOH (pH 7.0), and 200 μl from each sample was transferred to a 96-well microplate. Gfp production was then quantified by measuring fluorescence at 520 nm with a Fluoroskan Ascent FL microplate fluorescence reader (Thermo Labsystems, Brussels, Belgium) using an excitation wavelength of 485 nm. The results were expressed as relative fluorescence units per unit of optical density at 600 nm.
RESULTS
Construction and LP sensitivity of knockout mutants in LP-inducible genes.
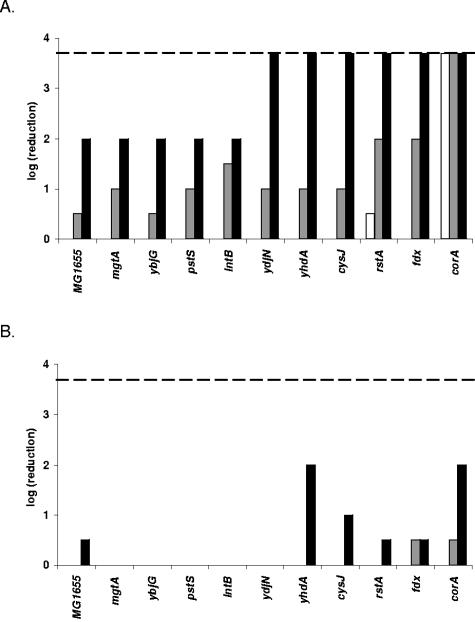
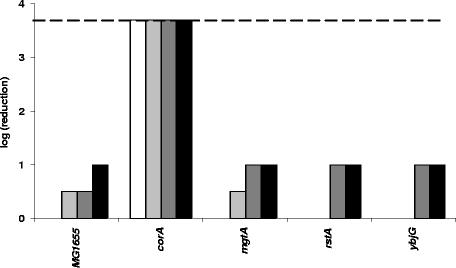
In an earlier study we identified promoters specifically induced upon exposure to the antimicrobial LP/SCN− system (29). The first objective of the present work was to evaluate the possible contribution of the corresponding genes in the protection of E. coli against the LP system. Strains with knockouts in some of the corresponding genes were obtained from F.R. Blattner (rstA, intB, mgtA, and corA) and B. L. Wanner (pstS). These mutant alleles were transferred to our own laboratory E. coli MG1655 strain using P1 transduction. ydjN, ybjG, yhdA, cysJ, and fdx knockout mutants were constructed using the standard gene inactivation protocol from Datsenko and Wanner (5). All these mutants were then tested for sensitivity to the LP system (Fig. 1A). Only the corA mutant was severely affected by the LP treatment, showing complete inactivation (>3.7 log CFU/ml) after 120 min of exposure. In contrast, the other mutants and the wild-type strain were still at their initial level of CFU/ml after 120 min, except for a small reduction of 0.5 log CFU/ml for the rstA mutant. During further exposure all other mutants and the wild-type strain became gradually inactivated by the LP/SCN− system but not all to the same extent. After 210 min, only small differences in inactivation existed among the strains, but after 300 min, when the inactivation of MG1655 was 2.0 log CFU/ml, the rstA, ydjN, yhdA, cysJ, and fdx mutants were also completely inactivated. In contrast, the pstS, intB, ybjG, and mgtA mutants showed the same reduction as MG1655.
FIG. 1.
Susceptibility of knockout mutants to the complete LP/SCN− system (A) and to the control treatment (B). Samples were diluted and spotted after 120 min (white bars), 210 min (gray bars), and 300 min (black bars). The detection limit (dashed line) was 200 CFU/ml. All strains are gradually killed by the antibacterial LP/SCN− system, but corA shows a specific hypersensitivity toward the LP/SCN− system and only slight sensitivity to H2O2 produced by the glucose/glucose oxidase system in the control treatment.
The increased sensitivity of the corA mutant was not caused by a trivial growth retardation since the growth curve of the mutant was indistinguishable from that of the wild-type strain under the same growth conditions as used for the sensitivity experiments (results not shown). When the same experiment was conducted with omission of the LP enzyme (Fig. 1B), much lower levels of inactivation were observed. This indicates that bacterial inactivation in the presence of the complete LP/SCN− system was caused by the antibacterial effect of this enzyme system and not, or only in small part, by the H2O2 generated by the glucose/glucose oxidase system. The true contribution of H2O2 to bacterial inactivation by the LP/SCN− system is likely to be even smaller than what is suggested by Fig. 1B, since the presence of the LP enzyme will reduce the effective concentration of H2O2. Nevertheless, the results suggest a somewhat increased H2O2 sensitivity of the corA and yhdA mutants.
Sensitivity to H2O2 or plumbagin of knockout mutants in LP-inducible genes.
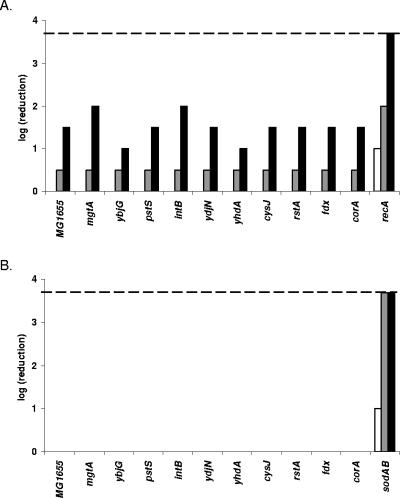
To further examine the specificity of the bactericidal effect of the LP/SCN− system, we studied the sensitivity of the 10 knockout strains toward a direct challenge with H2O2 or to the O2−-generating agent plumbagin. A recA and a sodAB mutant were included in the experiment as an H2O2-sensitive and O2−-sensitive strain, respectively. The recA strain clearly suffered from an attenuated resistance towards H2O2, but all other mutants showed a level of sensitivity to H2O2 similar to that of the wild-type strain (Fig. 2A). Thus, the somewhat increased sensitivity to the glucose/glucose oxidase system noticed above for the corA and yhdA knockout strains could not be reproduced in a direct challenge with 0.5 mM H2O2.
FIG. 2.
Susceptibility of knockout mutants to H2O2 (A) and to plumbagin (B). After treatment with H2O2, samples were diluted and spotted after 120 min (white bars), 210 min (gray bars), and 300 min (black bars). The positive control strain recA was severely inactivated, but none of the other tested mutants was hypersensitive to H2O2. For the plumbagin-treated samples, samples were taken after 210 min (white bars), 300 min (gray bars), and 420 min (black bars). Only the positive control strain sodAB was killed by the plumbagin treatment. The detection limit (dashed line) for both treatments was 200 CFU/ml.
As expected, the O2−-sensitive sodAB strain was rapidly inactivated by plumbagin treatment. However, none of the other strains showed any inactivation under the conditions of the treatment (Fig. 2B). Although this result precludes the observation of possible small differences in sensitivity toward O2−, it can be concluded that none of the mutants was hypersensitive to O2−.
These results and the results of the LP sensitivity assay described above indicate that among all the mutants tested, only the corA mutant is hypersensitive toward the LP system. This hypersensitivity is specific to the LP/SCN− system, since it is not linked to hypersensitivity to H2O2 or O2−. The specificity of the antibacterial effect of the LP system is further illustrated by the finding that recA and sodAB mutants, which are hypersensitive to H2O2 and O2−, respectively, did not exhibit altered sensitivity to the LP system (results not shown). The other mutants in which an LP-inducible gene was knocked out did not show altered sensitivity or showed only moderately increased sensitivity to the LP system and no altered sensitivity to other oxidants.
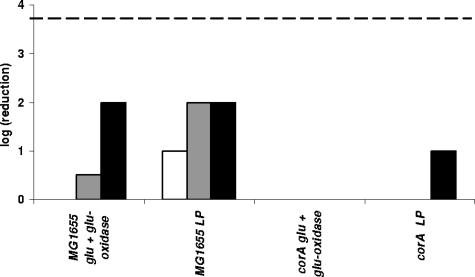
Sensitivity of the Salmonella enterica serovar Typhimurium corA mutant to the LP/SCN− system and complementation of E. coli and S. enterica serovar Typhimurium corA mutants with a cloned corA gene.
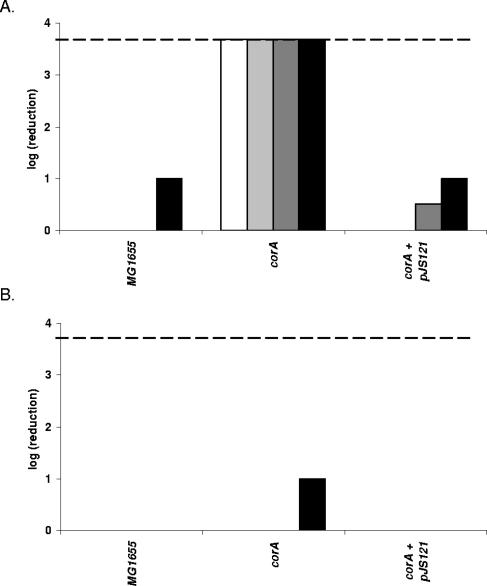
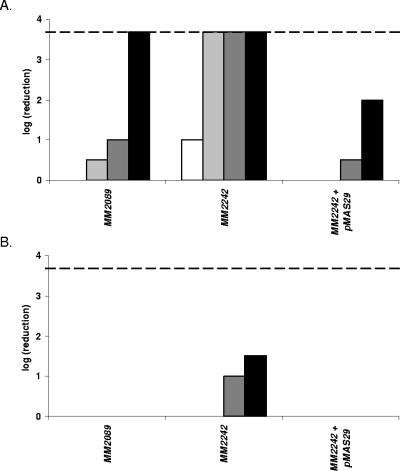
Contrary to what we found in E. coli, a corA knockout mutant of S. enterica serovar Typhimurium was earlier reported to be sensitive to H2O2 (K. M. Papp, J. Lin, D. G. Kehres, L. M. Kucharski, M. E. Maguire, Abstr. 103th Gen. Meet. Am. Soc. Microbiol., abstr. I-068, 2003). It cannot be excluded that this discrepancy is due to different assay methods. Therefore, to sort out whether CorA indeed affects H2O2 sensitivity differently in both bacteria and to determine its contribution to tolerance against the LP/SCN− system in S. enterica serovar Typhimurium, we compared a wild-type strain, a corA knockout, and a corA knockout strain carrying a cloned intact corA gene of both species for resistance to the LP/SCN− system and to the control treatment containing only the H2O2-generating glucose oxidase/glucose system and KSCN. The results for E. coli (Fig. 3) and S. enterica serovar Typhimurium (Fig. 4) were very similar. In both bacteria, knockout of corA caused hypersensitivity to the LP/SCN− system but no hypersensitivity or only very slight hypersensitivity to H2O2. Complementation with an intact corA gene restored the resistance to the wild-type level or even a slightly higher level in both organisms.
FIG. 3.
Sensitivity of E. coli MG1655, corA, and corA containing plasmid pJS121 to the LP/SCN− system (A) and the control treatment (B). When a wild-type copy of the E. coli corA gene is provided on a plasmid, the hypersensitivity of corA to the LP/SCN− system is completely counteracted. Samples were taken 120 min (white bars), 165 min (light gray bars), 210 min (dark gray bars), and 255 min (black bars) after treatment. The detection limit (dashed line) was 200 CFU/ml.
FIG. 4.
Sensitivity of S. enterica serovar Typhimurium MM2089, MM2242 (corA), and MM2242 containing plasmid pMAS29 to the LP/SCN− system (A) and the control treatment (B). The S. enterica serovar Typhimurium corA gene provided on plasmid pMAS29 renders corA more resistant to the LP system than the corresponding wild-type strain. Samples were taken 120 min (white bar), 165 min (light gray bars), 210 min (dark gray bars), and 255 min (black bars) after treatment. The detection limit (dashed line) was 200 CFU/ml.
Involvement of the Mg stimulon in sensitivity of E. coli to the LP/SCN− system.
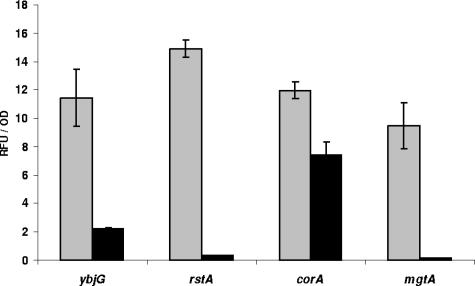
CorA forms a homotetrameric inner membrane transporter of Mg2+ and, to a lesser extent, some other bivalent cations (15, 42). Interestingly, our screen for LP-inducible promoters also yielded three other genes (mgtA, ybjG, and rstA) belonging to the Mg stimulon of E. coli (18, 29). This suggested a possible link between Mg2+ concentration and resistance to the LP/SCN− system. In contrast to the constitutively expressed corA, these genes are inducible by Mg2+ shortage. Unexpectedly, we found that the clones containing the mgtA, ybjG, and rstA promoters fused to the gfp reporter gene became induced during overnight growth (20 h) in normal LB but not in LB supplemented with 10 mM Mg2+ (Fig. 5). Therefore, to investigate the role of the Mg stimulon in resistance toward the LP system, the wild-type strain and the corA, mgtA, ybjG, and rstA mutants were subjected to treatment with the LP system after growth in LB containing 10 mM Mg2+. As was the case for cultures grown in unsupplemented LB, only the corA mutant was hypersensitive to the LP system and no differences in sensitivity were observed compared to results obtained earlier (Fig. 6). Also, the addition of 10 mM Mg2+ during treatment with the LP system did not significantly change these results (data not shown).
FIG. 5.
Promoter activity measured using ybjG, rstA, corA, and mgtA gfp-fusions. After 20 h of growth in normal LB (gray bars) or LB supplemented with 10 mM MgSO4 (black bars), cells were harvested to measure fluorescence (for Gfp production) and optical density (for bacterial growth) as described in Materials and Methods. Results shown are means ± standard deviations of three replicates.
FIG. 6.
Sensitivity of MG1655, corA, mgtA, rstA, and ybjG to the LP/SCN− system after growth in LB supplemented with 10 mM Mg2+. As seen before, only corA is hypersensitive to the antibacterial LP/SCN− system. Samples were taken 120 min (white bars), 165 min (light gray bars), 210 min (dark gray bars), and 255 min (black bars) after treatment. The detection limit (dashed line) was 200 CFU/ml.
Effect of pharmaceutical inhibition of CorA on sensitivity of E. coli to the LP/SCN− system.
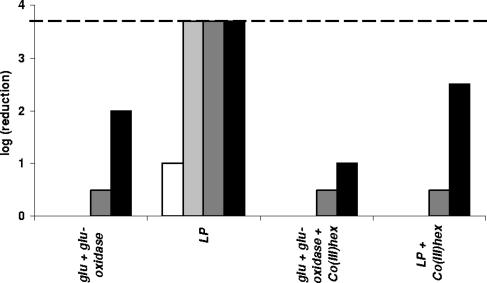
Co(III) hexaammine is a compound reported to specifically inhibit CorA-mediated cation transport (17). Initial experiments in which we added 0.03 to 3 mM of this compound during growth of the bacterial cultures or during exposure to the LP system showed no effect. This concentration of Co(III) hexaammine was sufficient to block CorA since it could completely inhibit the growth of an mgtA knockout strain (data not shown). However, with a modified experimental procedure, a distinct influence of Co(III) hexaammine on LP sensitivity could be demonstrated. In this modified procedure, 0.3 mM Ni2+ was added to the cell suspensions in HEPES-KOH buffer 1 h before the bacteria were exposed to the LP treatment. This concentration of Ni2+ did not cause any inactivation of the strains in itself (data not shown). The addition of Co(III) hexaammine (0.3 mM), when applicable, was done 1 h before the Ni2+ addition. Comparison of the results shown in Fig. 7 with those of the former experiments in the absence of Ni2+ (Fig. 1 and 3) reveals that the addition of Ni2+ severely increased the lethality of the LP system. After 120 min, MG1655 was completely inactivated by the LP treatment, whereas without Ni2+ after 300 min inactivation was only 2 log CFU/ml (Fig. 1). Inactivation due to H2O2 produced by the glucose/glucose oxidase system was also slightly increased in the presence of Ni2+. However, when 0.3 mM Co(III) hexaammine was added before the Ni2+ treatment, the enhancing effect of Ni2+ on the LP lethality was completely suppressed. The enhancing effect of Ni2+ on the lethality of the glucose/glucose oxidase treatment was reduced to a lesser extent.
FIG. 7.
Influence of 0.3 mM Co(III) hexaammine on sensitivity of E. coli MG1655 to the LP system in the presence of 0.3 mM Ni2+. Prior exposure to 0.3 mM Ni2+ severely increased sensitivity of E. coli to the LP system and to the control treatment with the glucose/glucose oxidase system. Inhibition of CorA by addition of 0.3 mM Co(III) hexaammine before addition of Ni2+ almost completely suppressed this sensitization. Samples were taken 60 min (white bars), 120 min (light gray bars), 180 min (dark gray bars), and 240 min (black bars) after LP treatment. The detection limit (dashed line) was 200 CFU/ml.
These results suggest that Ni2+ uptake sensitizes the cells for the LP system. Consequently, one would predict a corA mutant to be less sensitive to this Ni2+ enhancing effect because it has a reduced Ni2+ uptake capacity. To confirm this, we reduced the dosage of KSCN substrate of the LP enzyme system from 0.75 mM to 0.15 mM for practical reasons, because otherwise the corA mutant is inactivated too rapidly (Fig. 1 and 3). Under these conditions, MG1655 was inactivated by 2 log CFU/ml after 240 min in the presence of Ni2+, while inactivation of the corA mutant was only 1 log CFU/ml after 300 min (Fig. 8). Also, for treatment with the glucose/glucose oxidase system only, the corA mutant was more resistant to the LP system in the presence of Ni2+ than MG1655 in this experiment.
FIG. 8.
Sensitivity of E. coli MG1655 and corA to the LP system (10 μg/ml LP, 0.5 u glucose oxidase, 0.4% glucose, and 0.15 mM KSCN) in the presence of 0.3 mM Ni2+. Prior exposure to 0.3 mM Ni2+ increased the sensitivity of E. coli MG1655 to the LP system and to the control treatment with the glucose oxidase system. The corA mutant was much less sensitized to the LP system by Ni2+. Samples were taken 180 min (white bars), 240 min (gray bars), and 300 min (black bars) after treatment. The detection limit (dashed line) was 200 CFU/ml.
DISCUSSION
In this work we constructed 10 E. coli MG1655 knockout mutants in genes or ORFs identified earlier to be specifically inducible by the LP/SCN− system (29) and tested their susceptibility to the antibacterial activity of this enzyme system. Several mutants showed slightly to moderately increased sensitivity (ydjN, yhdA, cysJ, rstA, and fdx), but only one mutant (corA) exhibited hypersensitivity to the LP/SCN− system. The genes that were most strongly induced (cysJ and ydjN) had only a small contribution to the resistance against the LP/SCN− system. Similar conclusions were reached by Hansen et al. (13), who identified 25 upregulated ORFs (including cpxARP) upon exposure of E. coli to the Curvularia haloperoxidase system with bromide as an electron donor. Only one of the constructed corresponding knockout mutants (a cpxARP triple mutant) was found to be sensitive to the enzyme system. Apparently, the majority of the induced genes are not required for specific defense against these enzyme systems. This can be either because they are simply not involved or because the cellular defense systems against these stresses are redundant (13, 39). Interestingly, corA mutants of both E. coli and S. enterica serovar Typhimurium were specifically sensitive to the LP/SCN− system, and not, or to a much lower degree, to H2O2 and the superoxide-generating agent plumbagin (Fig. 1 and 2). In an earlier study, S. enterica serovar Typhimurium was reported to be peroxide sensitive (Papp et. al, Abstr. 103th Gen. Meeting ASM, 2003). However, to our knowledge experimental details of this study were not published, and it is therefore difficult to compare our results with those of the earlier study. It is quite possible that the apparent discrepancy stems from the use of different experimental procedures.
The specific sensitivity of corA mutants to an LP challenge, together with the specificity of the LP-induced stress response that we reported earlier (29), strongly suggests that OSCN−, the primary antimicrobial reaction product of the LP enzyme system, targets other cellular constituents than H2O2 and O2− target. As a consequence, bacteria may have developed specific defense systems to cope with this form of oxidative stress.
Bacterial magnesium transporters were first identified in E. coli (20, 21, 23, 31, 32) and have also been well characterized in S. enterica serovar Typhimurium (15, 22, 35, 42). CorA is a 37-kDa integral membrane protein that forms the primary constitutive Mg2+ uptake system in many Bacteria and some Archaea (15) and lacks homology to any other known transporter. It has an affinity for Mg2+ of 15 to 20 μM, and Mg2+ uptake is driven by the cellular membrane potential (9, 15, 42). However, there is no straightforward clue for the role of CorA in bacterial sensitivity to the LP system, since there are no indications for a direct involvement of Mg2+ in oxidative stress resistance. On the other hand, CorA can also mediate the influx of other bivalent cations, including some transition metal ions that have been linked to the generation of or protection against oxidative stress. The affinity of CorA for Co2+ and Ni2+ is 20 to 40 μM and 200 to 400 μM, respectively, which is in the toxic concentration range of these cations for bacteria like S. enterica serovar Typhimurium. Nevertheless, in view of the very small cellular requirements for these cations, their leakage through CorA might be physiologically relevant (15). Mn2+, Zn2+, and Ca2+ are probably not transported by CorA and do not interfere with Mg2+ transport via corA (33, 36), but for Fe2+ the situation is less clear. Hantke (14) reported reduced uptake of Fe2+ in corA mutants of E. coli and S. enterica serovar Typhimurium. Concomitantly, an increased resistance of these mutants was found against Fe2+-mediated oxidative stress caused by the Fenton reaction. Similarly, Chamnongpol and Groisman (4) revealed that phoP mutation rendered S. enterica serovar Typhimurium extremely sensitive to Fe2+. This phenotype was rescued in a corA phoP double mutant. In both studies, the increased resistance of a corA mutant was attributed to reduced Fe2+ uptake. However, the increased Fe2+ resistance of S. enterica serovar Typhimurium corA mutants could not be reproduced more recently, and direct measurements with radioisotopes indicated that Fe2+ is not transported by CorA and does not inhibit CorA-mediated Ni2+ transport. In addition, cellular Fe2+ uptake was not affected by Co(III) hexaammine, a selective inhibitor of CorA (22). Therefore, a link between CorA, the Fe2+ status of the cell, and LP resistance remains speculative.
Knockout of the three other Mg2+-related ORFs that are induced by the LP system had different effects: there was no change in LP sensitivity for ybjG and a slight and moderate increase in sensitivity for mgtA and rstA, respectively (Fig. 1A). These ORFs belong to the Mg stimulon and are strongly derepressed under Mg2+ limitation, together with a whole set of other genes under the control of the PhoP/Q two-component regulatory system (18), among which several are also induced after challenge with the LP system (29). Therefore, we investigated whether repression or derepression of these regulons by Mg2+ would affect LP sensitivity. Although the Mg stimulon was reported to remain repressed during growth in standard LB broth (22, 34, 38), expression of mgtA, rstA, and ybjG was clearly derepressed when cells were analyzed after 20 h of growth. Addition of 10 mM Mg2+ was required to maintain the repressed state (Fig. 5) during the complete growth time of the cultures. It is possible that this derepression in LB starts only in an advanced growth phase, when Mg2+ becomes depleted. However, since the purpose of our experiment was only to compare LP resistance of cells with a repressed and a derepressed Mg stimulon, we did not further investigate the causes of this derepression in LB medium. Addition of Mg2+ to the growth medium to repress the Mg stimulon, or even during LP challenge, did not significantly affect the LP sensitivity of wild type or corA, mgtA, rstA, and ybjG mutants of E. coli.
As opposed to Mg2+, Ni2+ severely sensitized E. coli MG1655 to the LP system. This enhancing effect was clearly mediated by CorA, since it was suppressed by the CorA inhibitor Co(III) hexaammine (Fig. 7) and in a corA mutant (Fig. 8). An enhancing effect of several transition metal cations, particularly Fe2+, on the toxicity of H2O2 is well known and is ascribed to the Fenton reaction. In contrast, the enhancement of LP system toxicity by transition metals has not been described to our knowledge, and its mechanism remains unclear to date. Nevertheless, our results indicate that CorA affects the LP sensitivity of E. coli by its effect on the cytoplasmic concentrations of transition metals that enhance the toxicity of the LP system. Taking into account that CorA can mediate both uptake and efflux of bivalent cations, this allows us to propose the following model. When cells are loaded with a transition metal prior to exposure to the LP system, strain MG1655 will be more sensitive than a corA mutant because of a higher accumulation of the metal. This is the case in the experiment shown in Fig. 8. Conversely, when LB-grown cells are resuspended in HEPES buffer without added metal ions, some release of transition metal ions from the cytoplasm through CorA may take place in MG1655, resulting in an enhanced tolerance of this strain compared to a corA mutant. Of course, for this efflux to occur, at least some of the transition metal cations that can be transported by CorA must occur in a free form in the cytoplasm. Because these metals can bind tightly to proteins and nucleic acids, their free cellular concentrations are normally very low. An alternative explanation is that the loss of CorA results in an increased production or activity of one or more of the other divalent cation uptake systems that exist in E. coli and, thus, in an increased cellular concentration of one or more divalent cations. Further experimentation is necessary to distinguish between these possibilities.
It was already noted previously that although loss of CorA does not produce any significant Mg2+-dependent growth phenotype, it causes a surprising variety of other phenotypes, including altered expression of genes encoded by Salmonella pathogenicity island I; increased sensitivity to heat shock and peroxide; defective invasion, survival, and proliferation within macrophages and epithelial cells; and diminished virulence (unpublished results from M. E. Maguire laboratory, mentioned in references 15 and 22). Although the causes of these effects have not been elucidated precisely, it has been observed that loss of CorA affects transcription of several genes. For example, a number of genes from the PhoP/Q regulon are derepressed while others remain PhoP/Q- and Mg2+-dependent (15, 22). On the other hand, other genes including mgtA could no longer be fully induced by low Mg2+ concentrations in a corA background (34; our preliminary results). These observations suggest a pleiotropic effect of a mutation in corA that could explain the wide range of associated phenotypes. Our work reveals LP sensitivity as an additional phenotype of corA mutants in E. coli and S. enterica serovar Typhimurium. This phenotype may well be the basis for the observed virulence-related defects in the latter organism, since LP and the closely related myeloperoxidase are important components of the vertebrate innate immune system.
Acknowledgments
We acknowledge M. Berlyn, D. Touati, F. R. Blattner, B. L. Wanner and M. E. Maguire for generously providing strains.
This work was conducted in the framework of research projects financed by the K.U. Leuven Research Fund (OT/01/35) and the Fund for Scientific Research Flanders (F.W.O. G.0195.02).
REFERENCES
- 1.Aune, T. M., and E. L. Thomas. 1977. Accumulation of hypothiocyanite ion during peroxidase-catalyzed oxidation of thiocyanate ion. Eur. J. Biochem. 80:209-214. [DOI] [PubMed] [Google Scholar]
- 2.Aune, T. M., and E. L. Thomas. 1978. Oxidation of protein sulfhydryls by products of peroxidase-catalyzed oxidation of thiocyanate ion. Biochemistry 17:1005-1010. [DOI] [PubMed] [Google Scholar]
- 3.Carlioz, A., and D. Touati. 1986. Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life? EMBO J. 5:623-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamnongpol, S., and E. A. Groisman. 2002. Mg2+ homeostasis and avoidance of metal toxicity. Mol. Microbiol. 44:561-571. [DOI] [PubMed] [Google Scholar]
- 5.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Wit, J. N., and A. C. M. Van Hooydonk. 1996. Structure, functions and applications of lactoperoxidase in natural antimicrobial systems. Neth. Milk Diary J. 50:227-244. [Google Scholar]
- 7.Dukan, S., S. Belkin, and D. Touati. 1999. Reactive oxygen species are partially involved in the bacteriocidal action of hypochlorous acid. Arch. Biochem. Biophys. 367:311-316. [DOI] [PubMed] [Google Scholar]
- 8.Ekstrand, B. 1994. Lactoperoxidase and lactoferrin, p. 15-63. In V. M. Dillon and R. G. Board (ed.), Natural antimicrobial systems in food preservation, CAB International, Wallingford, United Kingdom.
- 9.Froschauer, E. M., M. Kolisek, F. Dieterich, M. Schweigel, and R. J. Schweyen. 2004. Fluorescence measurements of free [Mg2+] by use of mag-fura 2 in Salmonella enterica. FEMS Microbiol. Lett. 237:49-55. [DOI] [PubMed] [Google Scholar]
- 10.Furtmüller, P. G., W. Jantschko, G. Regelsberger, C. Jakopitsch, J. Arnhold, and C. Obinger. 2002. Reaction of lactoperoxidase compound I with halides and thiocyanate. Biochemistry 41:11895-11900. [DOI] [PubMed] [Google Scholar]
- 11.García-graells, C., I. Van Opstal, S. C. M Vanmuysen, and C. W. Michiels. 2003. The lactoperoxidase system increases efficacy of high-pressure inactivation of foodborne bacteria. Int. J. Food Microbiol. 81:211-221. [DOI] [PubMed] [Google Scholar]
- 12.Guyer, M. S., R. R. Reed, J. A. Steitz, and K. B. Low. 1981. Identification of a sex-factor-affinity site in E. coli as gamma delta. Cold Spring Harb. Symp. Quant. Biol. 45:135-140. [DOI] [PubMed] [Google Scholar]
- 13.Hansen, E. H., M. A. Schembri, P. Klemm, T. Schäfer, S. Molin, and L. Gram. 2004. Elucidation of the antibacterial mechanism of the Curvularia haloperoxidase system by DNA microarray profiling. Appl. Environ. Microbiol. 70:1749-1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hantke, K. 1997. Ferrous iron uptake by a magnesium transport system is toxic for Escherichia coli and Salmonella typhimurium. J. Bacteriol. 179:6201-6204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kehres, D. G., and M. E. Maguire. 2002. Structure, properties and regulation of magnesium transport proteins. Biometals 15:261-270. [DOI] [PubMed] [Google Scholar]
- 16.Kiser, C., J. Caterina, J. A. Engler, B. Rahemtulla, and F. Rahemtulla. 1996. Cloning and sequence analysis of the human salivary peroxidase-encoding cDNA. Gene 173:261-264. [DOI] [PubMed] [Google Scholar]
- 17.Kucharski, L. M., W. J. Lubbe, and M. E. Maguire. 2000. Cation hexaammines are selective and potent inhibitors of the CorA magnesium transport system. J. Biol. Chem. 275:16767-16773. [DOI] [PubMed] [Google Scholar]
- 18.Minagawa, S., H. Ogasawara, A. Kato, K. Yamamoto, Y. Eguchi, T. Oshima, H. Mori, A. Ishihama, and R. Utsumi. 2003. Identification and molecular characterization of the Mg2+ stimulon of Escherichia coli. J. Bacteriol. 185:3696-3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naidu, A. S. 2000. Lactoperoxidase, p. 103-132. In Naidu (ed.), Natural food antimicrobial systems. CRC Press, Boca Raton, Fla.
- 20.Nelson, D. L., and E. P. Kennedy. 1971. Magnesium transport in Escherichia coli. Inhibition by cobaltous ion. J. Biol. Chem. 246:3042-3049. [PubMed] [Google Scholar]
- 21.Nelson, D. L., and E. P. Kennedy. 1972. Transport of magnesium by a repressible and nonrepressible system in Escherichia coli. Proc. Natl. Acad. Sci. USA 69:1091-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papp, K. M., and M. E. Maguire. 2004. The CorA Mg2+ transporter does not transport Fe2+. J. Bacteriol. 186:7653-7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park, M. H., B. B. Wong, and J. E. Lusk. 1976. Mutants in three genes affecting transport of magnesium in Escherichia coli: genetics and physiology. J. Bacteriol. 126:1096-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pruitt, K. M., and B. Reiter. 1985. Biochemistry of peroxidase system—antimicrobial effects, p. 143-178. In K. M. Pruitt and J. Tenovuo (ed.), The lactoperoxidase system, chemistry and biological significance. Marcel Dekker, New York, N.Y.
- 25.Pruitt, K. M., J. Tenovuo, R. W. Andrews, and T. McKane. 1982. Lactoperoxidase-catalyzed oxidation of thiocyanate: polarographic study of the oxidation products. Biochemistry 21:562-567. [DOI] [PubMed] [Google Scholar]
- 26.Reiter, B., and J. D. Oram. 1967. Bacterial inhibitors in milk and other biological fluids. Nature 216:328-330. [DOI] [PubMed] [Google Scholar]
- 27.Reiter, B., and J. P. Perraudin. 1991. Lactoperoxidase: biological functions, p. 143-180. In J. Everse, M. B. Grisham, K. E. Everse (ed.), Peroxidases in chemistry and biology. CRC Press, Boca Raton, Fla.
- 28.Ross, A. I. V., M. W. Griffiths, G. S. Mittal, and H. C. Deeth. 2003. Combining nonthermal technologies to control foodborne microorganisms. Int. J. Food Microbiol. 89:125-138. [DOI] [PubMed] [Google Scholar]
- 29.Sermon, J., K. Vanoirbeek, P. De Spiegeleer, R. Van Houdt, A. Aertsen, and C. W. Michiels. 2005. Unique stress response to the lactoperoxidase-thiocyanate enzyme system in Escherichia coli. Res. Microbiol. 156:225-232. [DOI] [PubMed] [Google Scholar]
- 30.Shin, K., H. Hayasawa, and B. Lönnerdal. 2001. Inhibition of E. coli respiratory enzymes by the lactoperoxidase-hydrogen peroxide-thiocyanate antimicrobial system. J. Appl. Microbiol. 90:489-493. [DOI] [PubMed] [Google Scholar]
- 31.Silver, S. 1969. Active transport of magnesium in Escherichia coli. Proc. Natl. Acad. Sci. USA 62:764-771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silver, S., and D. Clark. 1971. Magnesium transport in Escherichia coli. Interference by manganese with magnesium metabolism. J. Biol. Chem. 246:569-576. [PubMed] [Google Scholar]
- 33.Smith, R. L., J. L. Banks, M. D. Snavely, and M. E. Maguire. 1993. Sequence and topology of the CorA magnesium transport systems of Salmonella typhimurium and Escherichia coli. J. Biol. Chem. 268:14071-14080. [PubMed] [Google Scholar]
- 34.Smith, R. L., M. T. Kaczmarek, L. M. Kucharski, and M. E. Maguire. 1998. Magnesium transport in Salmonella typhimurium: regulation of mgtA and mgtCB during invasion of epithelial and macrophage cells. Microbiology 144:1835-1843. [DOI] [PubMed] [Google Scholar]
- 35.Smith, R. L., and M. E. Maguire. 1998. Microbial magnesium transport: unusual transporters searching for identity. Mol. Microbiol. 28:217-226. [DOI] [PubMed] [Google Scholar]
- 36.Snavely, M. D., J. B. Florer, C. G. Miller, and M. E. Maguire. 1989. Magnesium transport in Salmonella typhimurium: 28Mg2+ transport by the CorA, MgtA, and MgtB systems. J. Bacteriol. 171:4761-4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Storz, G., and R. Hengge-Aronis (ed.). 2000. Bacterial stress responses. ASM Press, Washington, D.C.
- 38.Tao, T., P. F. Grulich, L. M. Kucharski, R. L. Smith, and M. E. Maguire. 1998. Magnesium transport in Salmonella typhimurium: biphasic magnesium and time dependence of the transcription of the mgtA and mgtCB loci. Microbiology 144:655-664. [DOI] [PubMed] [Google Scholar]
- 39.Thorpe, G. W., C. S. Fong, N. Alic, V. J. Higgins, and I. W. Dawes. 2004. Cells have distinct mechanisms to maintain protection against different reactive oxygen species: oxidative-stress-response genes. Proc. Natl. Acad. Sci. USA 101:6564-6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valdivia, R. H., and S. Falkow. 1996. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid inducible promoters by differential fluorescence induction. Mol. Microbiol. 22:367-378. [DOI] [PubMed] [Google Scholar]
- 41.Van Opstal, I., S. C. M. Vanmuysen, and C. W. Michiels. 2003. High sucrose concentration protects E. coli against high pressure inactivation but not against high pressure sensitization to the lactoperoxidase system. Int. J. Food Microbiol. 88:1-9. [DOI] [PubMed] [Google Scholar]
- 42.Warren, M. A., L. M. Kucharski, A. Veenstra, L. Shi, P. F. Grulich, and M. E. Maguire. 2004. The CorA Mg2+ transporter is a homotetramer. J. Bacteriol. 186:4605-4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White, W. E. Jr., K. M. Pruitt, and B. Mansson-Rahemtulla. Peroxidase-thiocyanate-peroxide antibacterial system does not damage DNA. Antimicrob. Agents Chemother. 23:267-272. [DOI] [PMC free article] [PubMed]
- 44.Wijkstrom-Frei, C., S. El-Chemaly, R. Ali-Rachedi, C. Gerson, M. A. Cobas, R. Forteza, M. Salathe, and G. E. Conner. 2003. Lactoperoxidase and human airway host defense. Am. J. Respir. Cell. Mol. Biol. 29:206-212. [DOI] [PubMed] [Google Scholar]