Abstract
We describe a catalyzed reported deposition-fluorescence in situ hybridization (CARD-FISH) protocol particularly suited to assess the phagotrophy of mixotrophic protists on prokaryotes, since it maintains cell and plastid integrity, avoids cell loss and egestion of prey, and allows visualization of labeled prey against plastid autofluorescence. This protocol, which includes steps such as Lugol's-formaldehyde-thiosulfate fixation, agarose cell attachment, cell wall permeabilization with lysozyme plus achromopeptidase, and signal amplification with Alexa-Fluor 488, allowed us to detect almost 100% of planktonic prokaryotes (Bacteria and Archaea) and, for the first time, to show archaeal cells ingested by mixotrophic protists.
Mixotrophy, a combination of photoautotrophic and phagoheterotrophic nutrition, is an advantageous life strategy for many microalgae inhabiting environments limited or stressed by light and nutrient availability (11, 22, 24). In such aquatic oligotrophic environments, mixotrophs play a key role in the microbial food webs since they are simultaneously a net source of organic (particulate and dissolved matter forms) and mineral nutrients (24, 31, 39) and a major cause of bacterial losses by bacterivory (17, 18, 24).
Prey surrogates (e.g., fluorescently labeled bacteria, minicells), traditionally used to evaluate phagotrophy in protists, provide unreliable results when uptake rates are low (23, 24). Low uptake is common in mixotrophs because of their nutritional versatility (photosynthesis versus phagotrophy) (22, 30, 31). To increase detection limits, specific and highly sensitive radiotracers have been used as an alternative (24, 25, 37). However, this procedure requires clear segregation of the compartments where the radiotracers can be incorporated (mixotrophs, heterotrophic protists, free bacteria and the dissolved fraction), a shortcoming that is not easily overcome (6).
Over the last decade, fluorescent in situ hybridization (FISH) has been increasingly used to identify microorganisms without previous cultivation in aquatic environments (2, 7, 41). A variant of this method, the catalyzed reported deposition (CARD)-FISH, has improved the detection of small bacteria with low ribosome content by using horseradish peroxidase (HRP)-labeled probes (28, 32). FISH techniques have also been applied in studies on endosymbiontic prokaryotes in protozoans (3, 13, 19) and phytoplankton (1, 5) and to assess protist grazing by quantifying the bacteria in food vacuoles of both ciliates (8, 9, 16) and heterotrophic flagellates (21). Here, we optimized a CARD-FISH protocol to assess the extent of bacterivory in mixotrophic algal species. We examined the maintenance of cell and plastid integrity, the avoidance of cell loss and egestion of prey, the detection of Bacteria and Archaea labeled with generic HRP-labeled probes, and their visualization against plastid autofluorescence. For this purpose, we tested several commonly used procedures for protist fixation (35), cell attachment, permeabilization and stringency conditions to properly hybridize prokaryotes with HRP-labeled probes.
MATERIALS AND METHODS
The plankton assemblages used for the tests were integrate samples collected in two oligotrophic systems: Fuirosos pond and Lake Redon. The former is a small pond located on siliceous (mainly granite) bedrock in a forested catchment of Montnegre-Corredor Natural Park (Barcelona, Spain); Lake Redon is a high-mountain lake located on mainly siliceous bedrock at 2,240 m above sea level in the Central Pyrenees (Spain) and is usually covered by ice and snow for 6 to 7 months of the year (see references 4 and 12 for more-detailed site descriptions, respectively). Samples were prescreened through a 40-μm-pore-size net to remove large zooplankton. Sample aliquots, preserved with Lugol's iodine solution, settled for a minimum of 30 h in Utermöhl settling chambers (50 cm3, 2.5-cm diameter), and examined under an inverted microscope (Wild-Leitz) at ×600 to ×1,000 magnification, were used to identify and quantify algal species (40). Another set of aliquots (500 ml each) were subjected to distinct fixation procedures (35) including (i) 2% buffered (pH 7) 0.2-μm-pore-size-filtered formaldehyde (F); (ii) 1% neutralized, 0.2-μm-pore-size-filtered fresh paraformaldehyde (P); (iii) equal volume of ice-cold 4% glutaraldehyde (G); (iv) a mixture of neutralized and 0.2-μm-pore-size-filtered 1% paraformaldehyde and 0.5% glutaraldehyde (PG); (v) 0.5% (vol/vol) alkaline Lugol solution, followed by 2% buffered (pH 7) 0.2-μm-pore-size-filtered formaldehyde, and several drops of 3% sodium thiosulfate to decolor Lugol's fixation (LFT). After 1 h of fixation at room temperature, portions of each fixed sample (50 ml for protists and 10 ml for bacteria) were gently filtered (<100 mm Hg) onto respective 25-mm-diameter polycarbonate Millipore membrane filters (type RTTP, 1-μm pore size for protists; type GTTP, 0.2-μm pore size for bacteria) to separately collect a reliable representation of prey and consumer assemblages. Filters were then rinsed twice with double-distilled water, allowed to air dry, and stored at −20°C until further processing.
Separate sets of filters were either embedded or not in low-gelling-point agarose (Table 2) (33) and were subjected to cell permeabilization with lysozyme and achromopeptidase (LA permeabilization) according to the protocol by Sekar et al. (33). Furthermore, cell permeabilization with proteinase K (K permeabilization) as described by Teira et al. (38) was also tested for a set of LFT-fixed samples addressed to Archaea detection. Thereafter, all filters were exposed to acid inactivation of any potential endogenous peroxidase (Table 2) (33) and of proteinase K if it had been previously added (38), dehydrated in ethanol series (50, 80, and 100%, 3 min each) (5), and allowed to air dry at room temperature.
TABLE 2.
Proposed CARD-FISH protocol for evaluation of mixotrophic protists phagotrophy on Archaea (HRP-ARCH915 probe) and Bacteria (HRP-EUB338 probe)
| Stage | Step | Descriptiona |
|---|---|---|
| Sample preparation | 1 | Take aliquot (150 ml) from sample and fix it with Lugol's solution. Let it settle onto a Uthermöl chamber and visualize it by using an inverted microscope. |
| 2 | Fix sample with 0.5% (vol/vol) alkaline Lugol's solution, followed by 2% buffered formaldehyde, and within a 2- to 3-min interval add several drops of 3% sodium thiosulfate to decolorate Lugol (fixation time, 1 h maximum). | |
| 3 | Filter using a vacuum pump (<100 mm Hg) separate aliquots (volume for a suitable number of cells homogeneously distributed throughout filter depends on their initial abundance) onto 1- to 2-μm and 0.2-μm-pore-size polycarbonate filters, respectively. | |
| 4 | Wash twice with 10 ml of sterile ddH2O. | |
| 5 | Let air dry.b | |
| Embedding | 6 | Dip filters in 0.1 to 0.2% (wt/vol) low-gelling-point agarose at 35 to 40 °C. |
| 7 | Place filters face down onto parafilm or glass plate, let them dry at 35 to 40°C (10 to 20 min). | |
| 8 | Remove filters by soaking them in 80 to 96% ethanol (RT). | |
| 9 | Let air dry.b | |
| Permeabilization | 10 | Incubate filters in fresh lysozyme solution (10 mg ml−1 FC, dissolved in 0.05 M EDTA [pH 8.0], 0.1 M Tris-HCl [pH 7.4]) for 60 min at 37°C. |
| 11 | Wash with ddH2O (1 min, RT). | |
| 12 | Incubate filters in fresh achromopeptidase solution (60 U ml−1 FC, dissolved in 0.01 M NaCl, 0.01 M Tris-HCl [pH 8.0]) for 30 min at 37°C. | |
| 13 | Wash with ddH2O. | |
| 14 | Incubate filters in 0.01 M HCl for 10 min at RT to inactivate endogenous peroxidase. | |
| 15 | Wash thoroughly with ddH2O. | |
| 16 | Dehydrate in ethanol series (50, 80, 100%, 3 min each at RT). | |
| 17 | Let air dry.b | |
| Hybridization | 18 | Place filters in an adapted Ecostep syringe. |
| 19 | Mix (100:1 [vol/vol]) hybridization buffer (0.9 M NaCl, 20 mM Tris-HCl [pH 7.4], 10% [wt/vol] dextran sulfate, 0.02% [wt/vol] sodium dodecyl sulfate, 55% [vol/vol] formamide, 1% blocking reagent prepared in maleic acid buffer [100 mM maleic acid, 150 mM NaCl; pH 7.5]) and probe solution (50 ng μl−1 sterile ddH2O) at a suitable quantity to dip filters after adjusting the piston. | |
| 20 | Incubate filters at 35°C for 4 h on a rotation shaker. | |
| 21 | Wash filters in prewarmed washing buffer (20 mM NaCl, 5 mM EDTA [pH 8.0], 20 mM Tris-HCl [pH 7.4], 0.02% [wt/vol] sodium dodecyl sulfate) for 5 to 10 min at 37°C. Do not air dry filters after washing. | |
| CARD | 22 | Incubate in 1× PBS (0.2-μm filtered) amended with 0.05% of Triton X-100 (15 min at RT). |
| 23 | Remove excess liquid with blotting paper, but do not let filters dry. | |
| 24 | Incubate in substrate mix (1 part Alexa488-labeled tyramide [1 mg ml−1 FC dissolved in dimethylformamide containing 20 mg ml−1p-iodophenylboronic acid] and 100 parts amplification buffer [10% {wt/vol} dextran sulfate, 2 M NaCl, 0.1% {wt/vol} blocking reagent, and freshly prepared 0.0015% H2O2 in 1× PBS pH 7.6]) for 15 min at 37°C in the dark. | |
| 25 | Dab filters in blotting paper. | |
| 26 | Wash in 1× PBS amended with 0.05% Triton X-100 (15 min, RT). | |
| 27 | Wash in ddH2O (1 min, RT). | |
| 28 | Wash in 96% ethanol (1 min, RT). | |
| 29 | Let air dry.b | |
| Mounting | 30 | Counterstain filters by soaking them with DAPI solution (1 μg ml−1 FC) and mount filters on slides using Citifluor AF1. |
ddH2O, double-distilled water; RT, room temperature; FC, final concentration.
Preparations may be stored at −20°C for several weeks without loss in signal.
Filters were subjected to hybridization with HRP-labeled probes (Table 1; Biomers.net, Germany) and tyramide signal amplification according to the CARD-FISH standard protocol (27, 33), except that hybridization was performed for 4 h inside 37-ml syringes (Ecostep, Socorex, Switzerland), adapted as incubation chambers, where entire filters fitted without folds. Thus, a reliable representation of protist assemblages collected on the filter was maintained and properly visualized (entire optic fields were well focused). After the syringe piston was adjusted to the appropriate position and gently shaking the syringes during the incubation, a complete, continuous, and homogeneous contact of the probes and the targets was achieved with a minimal waste of hybridization-probe mixture, since hybridized cells were observed throughout the entire filter surface. For the amplification step, tyramide-Alexa-Fluor 488 (Molecular Probes) and a substrate mix containing 2 M NaCl and 10 μg of p-iodophenylboronic acid ml−1 as the enhancer method (20, 27) were used. Finally, filters were allowed to air dry, counterstained with DAPI (4′,6′-diamidino-2-phenylindole; 1 μg ml−1 final concentration), and mounted on glass slides by using Citifluor (Citifluor, Ltd., London, United Kingdom). Slides stored at −20°C in the dark for several weeks did not show a loss of fluorescence intensity.
TABLE 1.
Oligonucleotide probes used in this study
| Probe | Specificity | Sequence (5′-3′) | % FAa | Reference(s) |
|---|---|---|---|---|
| ARCH915 | Archaea | GTGCTCCCCCGCCAATTCCT | 40 | 36 |
| 50 | 14 | |||
| 55 | 36 | |||
| EUB338 | Bacteria | GCTGCCTCCCGTAGGAGT | 55 | 28, 33 |
Formamide (FA) concentration in the hybridization buffer.
Slides were examined at ×100 under a Zeiss Axioplan epifluorescence microscope equipped with a 50-W Hg bulb, appropriate filter sets for DAPI (Zeiss filter set 01, BP365/12 FT396 LP397) and Alexa-Fluor 488 (Zeiss filter sets 09 BP450-490 FT510 LP515, or 24 DBP485/20 DFT500/600 BP515-540 + LP610), a coupled camera- and a PC-based image acquisition software (Spot, Diagnostic Instruments, Inc.). The Mann-Whitney U test (Statistica 6.0; StatSoft, Inc.) was applied for testing differences between treatments on a fraction of positive cells per filter surface unit screened (1,488 μm2 for Bacteria and 14,884 μm2 for Archaea, each unit screened containing >25 and >250 total DAPI counts on average, respectively; n [number of filter surface units screened] = 50).
RESULTS AND DISCUSSION
The optimal combination of procedures as a right protocol for detecting prokaryotes ingested by individual mixotrophic algal species is summarized in Table 2.
Microscopic observations evidenced that embedding of filters in low-gelling-point agarose prevented cell loss; otherwise, a large fraction of protist cells was lost during the CARD-FISH protocol, even after the permeabilization step. Cell attachment, therefore, is a crucial step to assess the specific phagotrophy of protists on prokaryotes, minimizing bias as much as possible.
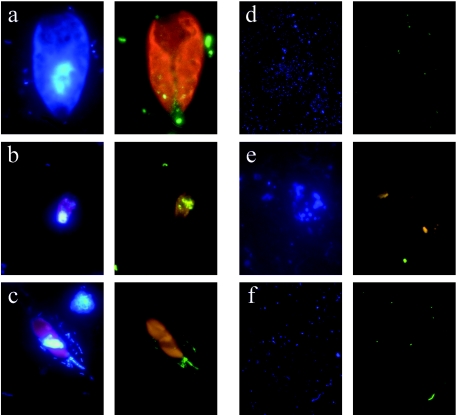
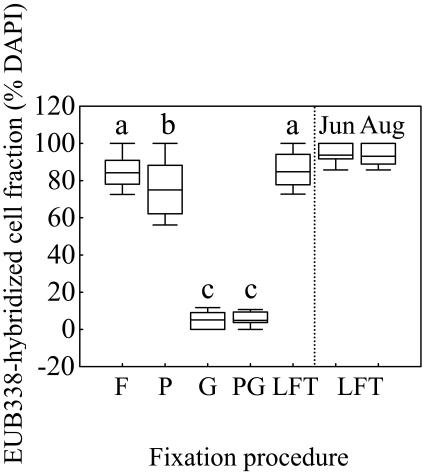
For most mixotrophic algal species, all of the fixation procedures, followed by LA permeabilization, sufficiently preserved the integrity of the main cell features, such as cell and nucleus shape (observed under UV excitation) and plastid shape and autofluorescence (observed under blue excitation) (Fig. 1a to c). Consequently, mixotrophs were directly identified up to low taxonomic levels (genus or species) under an epifluorescence microscope. The identification was facilitated and confirmed by parallel observations on Lugol-stained subsamples under an inverted light microscope. Whereas main cell features were not affected by fixation procedure, probe signal was. Thus, G and PG fixation procedures weakened the intensity of the signal (Fig. 1d), yielding lower percentages of well-contrasted EUB338-hybridized cells than the other treatments (Fig. 2). The highest percentages of EUB338-hybridized cells on the 0.2-μm-pore-size filters were obtained with F and LFT fixations, yielding values (median range, 84.2 to 93.7% of DAPI-stained cells, Fig. 2) within the range reported by Sekar et al. (33). No trend in the amount of hybridized prey (EUB338 probe) inside the food vacuoles of the mixotrophs was observed when F, P, and LFT fixation procedures were compared, despite the fact that, among these, only LFT has been reported to prevent prey egestion of protists food vacuole content (35). Therefore, LFT was chosen as the most appropriate fixation procedure for further studies, whereas G and PG fixation were discarded because of the lower fluorescence intensity provided.
FIG. 1.
Epifluorescence microscope photographs. Within each lettered panel, the left image depicts DAPI staining, and the right one depicts CARD-FISH staining with EUB338 probe on Cryptomonas ovata (right image using filter set 24) (a), EUB338 probe on Rhodomonas minuta (b), ARCH915 probe on Dinobryon cylindricum (c), EUB338 probe after G fixation (d), ARCH915 after LFT fixation and K permeabilization showing cell fragments of unidentified phytoplankton (e), and ARCH915 probe after LFT fixation and LA permeabilization (f).
FIG. 2.
Detection of Bacteria (probe EUB338) by CARD-FISH at different fixation procedures. The boxes represent the median and the 25 and 75% percentiles. The bars indicate the 10 and 90% percentiles. The vertical dashed line segregates values for Fuirosos pond (left) from Lake Redon (right, June and August samples). Different letters above each box indicate treatments that differed significantly (P < 0.05, n = 50). See the text for abbreviations.
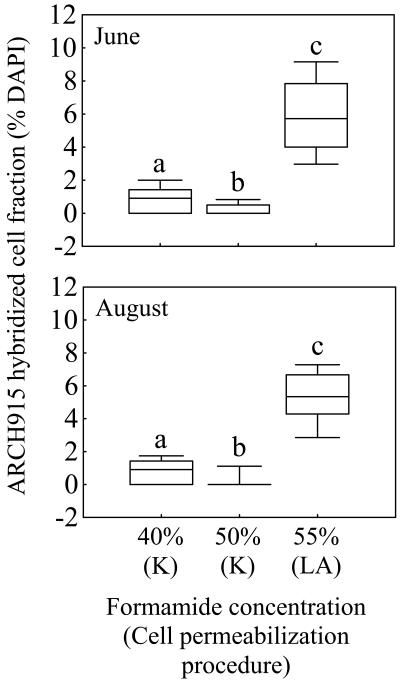
Conversely, K permeabilization broke mixotrophic cells and their plastids (Fig. 1e) and caused a detectable disruption of bacterial cells under DAPI observation, as reported by Teira et al. (38). Moreover, this procedure yielded a lower detection of Archaea than that obtained after LA-permeabilization under higher stringency conditions (Fig. 3). Using the latter procedure, Ishii et al. (20) also detected Archaea, and we obtained a similar percentage of Archaea (median, 5.3 to 5.7% of DAPI-stained cells; Fig. 3) to that reported for another high-mountain lake (1 to 6% in Lake Gossenköllesee, Austria) using the FISH protocol and the ARCH915 “universal” archaeal probe (15, 29). Despite the theoretical considerations and results reported by Teira et al. (38) in support of K permeabilization as an improved procedure for the detection of Archaea by CARD-FISH, our comparative results indicate that LA permeabilization allowed HRP-labeled probes to access the targets (Fig. 1f) and caused minimal disturbance of the eukaryotic cells (Fig. 1c).
FIG. 3.
Detection of Archaea (probe ARCH915) by CARD-FISH at different cell permeabilization procedures and formamide concentrations in Lake Redon. The boxes represent the median and the 25 and 75% percentiles. The bars indicate the 10 and 90% percentiles. Different letters above each box indicate treatments that differed significantly (P < 0.05, n = 50). Abbreviations: K, K permeabilization; LA, LA permeabilization (see the text).
The use of Alexa-Fluor 488 combined with the enhancer method in the amplification step (see above) yielded a strong, photostable, and high-fading-time green fluorescence of the hybridized cell. These features make Alexa-Fluor a more useful stain than other dyes with the same spectra of emission (e.g., fluorescein and its derivatives) (26). This green fluorescence contrasted the red chlorophyll autofluorescence in the same optical image under the commonly used filter set for blue excitation light, thus facilitating the visualization of the labeled prey inside the mixotrophic cell. The use of Zeiss filter set 24 improved the contrast between Alexa-Fluor 488 and chlorophyll fluorescence (Fig. 1a), but visualization of cell contour was slightly worse for some species. For all of the samples examined, the EUB338 probe did not target the plastids of eukaryotic cells, which always showed red fluorescence. Although consistent with a survey of the literature, our results are, however, in disagreement with those reported by Biegala et al. (5) on plastid hybridization with the EUB338 probe for several phytoplankton species. We attribute this discrepancy to the difficulty in discriminating the contribution of Cy3 versus chlorophyll to red fluorescence in the merged images at 488- and 568-nm excitations that those authors reported. In our study, the use of the green fluorescent Alexa-Fluor 488 prevented the potential overlapping of fluorescence between plastids and Cy3-labeled prey.
Overall, the proposed protocol (Table 2) is suitable to assess the specific phagotrophy of mixotrophic protists on prokaryotes and opens up new possibilities to further study prey preference of mixotrophs at the complete range of taxonomic resolutions using the appropriate bacterial and archaeal probes. The protocol was tested on natural plankton assemblages composed of a high diversity of algae (chlorophytes, cryptophytes, chrysophytes, diatoms, cyanobacteria, etc.) covering a wide trophic spectrum from nonphagotrophic autotrophs to mixotrophs (even strict heterotrophs) from two selected ecosystems. The suitability of the protocol is supported by the absence of prokaryotic prey inside well-known nonphagotropic algae (e.g., diatoms, chlorophytes, cyanobacteria, etc.) but their presence inside most mixotrophic algae, as well as by the proportion of hybridized prokaryotes (Archaea plus Bacteria) found that reached near 100% of DAPI-stained prokaryotic population. This method fulfills the requirements of an ideal tracer, such as vitality, maintenance of microbial community integrity, prevention of cell loss and prey egestion, staining specificity, detection sensitivity, and temporal stability (6, 10). The protocol is nondestructive, is culture independent, and is an a posteriori method and can therefore be easily applied to natural assemblages, thus attending to the requirements of in situ studies of protistan phagotrophy (34) using methods that do not rely on incubation procedures (6). We conclude that this protocol is a useful tool to determine the grazing impact of mixotrophic algae on prokaryotic communities.
Acknowledgments
We thank Jordi Catalan for critical reading of the manuscript and valuable suggestions, Eliseu Badosa and Daniel Díaz de Quijano for assistance in the field, Francesc Xavier Gómez and Fèlix Gómez Agustí (A. Coloma) for providing filter set no. 24, and two anonymous reviewers for constructive criticism.
This research is a contribution of the Limnology Group CEAB-UB and was supported by VIARC projects REN2003-08333 and CGL2004-02989 from the Spanish Ministerio de Ciencia y Tecnología and a postdoctoral contract from the Spanish Junta de Andalucía (to J.M.M.-S.).
REFERENCES
- 1.Alverca, E., I. C. Biegala, G. M. Kennaway, J. Lewis, and S. Franca. 2002. In situ identification and localization of bacteria associated with Gyrodinium instriatum (Gymnodiniales, Dinophyceae) by electron and confocal microscopy. Eur. J. Phycol. 37:523-530. [Google Scholar]
- 2.Amann, R., W. Ludwig. 2000. Ribosomal RNA-targeted nucleic acid probes for studies in microbial ecology. FEMS Microbiol. Rev. 24:555-565. [DOI] [PubMed] [Google Scholar]
- 3.Beier, C. L., M. Horn, R. Michel, M. Schweikert, H.-D. Görtz, and M. Wagner. 2002. The genus Caedibacter comprises endosymbionts of Paramecium spp. related to the Rickettsiales (Alphaproteobacteria) and to Francisella tularensis (Gammaproteobacteria). Appl. Environ. Microbiol. 68:6043-6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernal, S., A. Butturini, J. L. Riera, E. Vázquez, and F. Sabater. 2004. Calibration of the INCA model in a Mediterranean forested catchment: the effect of hydrological inter-annual variability in an intermittent stream. Hydrol. Earth Syst. Sci. 8:729-741. [Google Scholar]
- 5.Biegala, I., G. Kennaway, E. Alverca, J. Lennon, D. Vaulot, and N. Simon. 2002. Identification of bacteria associated with dinoflagellates (Dinophyceae) Alexandrium spp. using tyramide signal amplification-fluorescent in situ hybridization and confocal microscopy. J. Phycol. 38:404-411. [Google Scholar]
- 6.Caron, D. A. 2001. Protistan herbivory and bacterivory. Method. Microbiol. 30:289-315. [Google Scholar]
- 7.Casamayor, E. O., C. Pedrós-Alió, G. Muyzer, and R. Amann. 2002. Microheterogeneity in 16S rDNA-defined bacterial populations from a stratified planktonic environment is related to temporal changes and to ecological adaptations. Appl. Environ. Microbiol. 68:1706-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diederichs, S., C. Beardsley, and E.-J. Cleven. 2003. Detection of ingested bacteria in benthic ciliates using fluorescence in situ hybridization. Syst. Appl. Microbiol. 26:624-630. [DOI] [PubMed] [Google Scholar]
- 9.Eisenmann, H., H. Harms, R. Meckenstock, E. I. Meyer, and A. J. B. Zehnder. 1998. Grazing of a Tetrahymena sp. on adhered bacteria in percolated columns monitored by in situ hybridization with fluorescent oligonucleotide probes. Appl. Environ. Microbiol. 64:1264-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein, S. S., and J. Rossel. 1995. Methodology of in situ grazing experiments: evaluation of a new vital dye for preparation of fluorescently labeled bacteria. Mar. Ecol. Prog. Ser. 128:143-150. [Google Scholar]
- 11.Felip, M., F. Bartumeus, S. Halac, and J. Catalan. 1999. Microbial plankton assemblages, composition, and biomass, during two ice-free periods in a deep high mountain lake (Estany Redó, Pyrenees). J. Limnol. 58:193- 202. [Google Scholar]
- 12.Felip, M., L. Camarero, and J. Catalan. 1999. Temporal changes of microbial assemblages in the ice and snow cover of a high mountain lake. Limnol. Oceanogr. 44:973-987. [Google Scholar]
- 13.Fokin, S. I., and M. Schweikert. 2003. Bacterial endocytobionts in the macronucleus of Frontonia leucas (Ciliophora, Peniculida). Eur. J. Protistol. 39:311-318. [Google Scholar]
- 14.Giovannoni, S. J., E. F. DeLong, G. J. Olsen, and N. R. Pace. 1988. Phylogenetic group-specific oligodeoxynucleotide probes for identification of single microbial cells. J. Bacteriol. 170:720-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glöckner, F. O., B. M. Fuchs, and R. Amann. 1999. Bacterioplankton compositions of lakes and oceans: a first comparison based on fluorescence in situ hybridization. Appl. Environ. Microbiol. 65:3721-3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunderson, J. H., and S. H. Goss. 1997. Fluorescently labeled oligonucleotide probes can be used to identify protistan food vacuole contents. J. Eukaryot. Microbiol. 44:300-304. [DOI] [PubMed] [Google Scholar]
- 17.Havskum, H., and B. Riemann. 1996. Ecological importance of bacterivorous, pigmented flagellates (mixotrophs) in the Bay of Aarhus, Denmark. Mar. Ecol. Prog. Ser. 137:251-263. [Google Scholar]
- 18.Hitchman, R. B., and H. L. J. Jones. 2000. The role of mixotrophic protists in the population dynamics of the microbial food web in a small artificial pond. Freshw. Biol. 43:231-241. [Google Scholar]
- 19.Horn, M., M. Wagner, K.-D. Müller, E. N. Schmid, T. R. Fritsche, K.-H. Schleifer, and R. Michel. 2000. Neochlamydia hartmannellae gen. nov., sp. nov. (Parachlamydiaceae), an endoparasite of the amoeba Hartmannella vermiformis. Microbiology 146:1231-1239. [DOI] [PubMed] [Google Scholar]
- 20.Ishii, K., M. Mussmann, B. J. MacGregor, and R. Amann. 2004. An improved fluorescence in situ hybridization protocol for the identification of bacteria and archaea in marine sediments. FEMS Microbiol. Ecol. 50:203-212. [DOI] [PubMed] [Google Scholar]
- 21.Jezbera, J., K. Hornak, and K. Simek. 2005. Food selection by bacterivorous protists: insight from the analysis of the food vacuole content by means of fluorescence in situ hybridization. FEMS Microbiol. Ecol. 52:351-363. [DOI] [PubMed] [Google Scholar]
- 22.Jones, R. I. 2000. Mixotrophy in planktonic protists: an overview. Freshw. Biol. 45:219-226. [Google Scholar]
- 23.Laybourn-Parry, J., and W. A. Marshal. 2003. Photosynthesis, mixotrophy, and microbial plankton dynamics in two high Arctic lakes during summer. Polar Biol. 26:517-524. [Google Scholar]
- 24.Medina-Sánchez, J. M., M. Villar-Argaiz, and P. Carrillo. 2004. Neither with nor without you: a complex algal control on bacterioplankton in a high mountain lake. Limnol. Oceanogr. 49:1722-1733. [Google Scholar]
- 25.Nygaard, K., and A. Tobiesen. 1993. Bacterivory in algae: a survival strategy during nutrient limitation. Limnol. Oceanogr. 38:273-279. [Google Scholar]
- 26.Panchuk-Voloshina, N., R. P. Haugland, J. Bishop-Stewart, M. K. Bhalgat, P. J. Millard, F. Mao, W. Y. Leung, and R. P. Haugland. 1999. Alexa dyes, a series of new fluorescent dyes that yield exceptionally bright, photostable conjugates. J. Histochem. Cytochem. 47:1179-1188. [DOI] [PubMed] [Google Scholar]
- 27.Pernthaler, A., J. Pernthaler, and R. Amann. 2004. Sensitive multi-color fluorescence in situ hybridization for the identification of environmental microorganisms, p. 711-726. In A. D. L. Akkermans, F. J. de Bruijn, and J. D. van Elsas (ed.), Molecular microbial ecology manual, 2nd ed. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 28.Pernthaler, A., J. Pernthaler, and R. Amann. 2002. Fluorescence in situ hybridization and catalyzed reporter deposition for the identification of marine bacteria. Appl. Environ. Microbiol. 68:3094-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pernthaler, J., F. O. Glöckner, S. Unterholzner, A. Alfreider, R. Psenner, and R. Amann. 1998. Seasonal community and population dynamics of pelagic Bacteria and Archaea in a high mountain lake. Appl. Environ. Microbiol. 64:4299-4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raven, J. A. 1997. Phagotrophy in phototrophs. Limnol. Oceanogr. 42:198-205. [Google Scholar]
- 31.Rothhaupt, K. O. 1997. Nutrient turnover by freshwater bacterivorous flagellates: differences between a heterotrophic and a mixotrophic chrysophyte. Aquat. Microb. Ecol. 12:65-70. [Google Scholar]
- 32.Schönhuber, W., B. Fuchs, S. Juretschko, and R. Amann. 1997. Improved sensitivity of whole-cell hybridization by the combination of horseradish peroxidase-labeled oligonucleotides and tyramide signal amplification. Appl. Environ. Microbiol. 63:3268-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sekar, R., A. Pernthaler, J. Pernthaler, F. Warnecke, T. Posch, and R. Amann. 2003. An improved protocol for quantification of freshwater actinobacteria by fluorescence in situ hybridization. Appl. Environ. Microbiol. 69:2928-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherr, E. B., and B. F. Sherr. 2002. Significance of predation by protists in aquatic microbial food webs. Antonie Leeuwenhoek Int. J. G. 81:293- 308. [DOI] [PubMed] [Google Scholar]
- 35.Sherr, E. B., and B. F. Sherr. 1993. Protistan grazing rates via uptake of fluorescently labeled prey, p. 695-701. In P. Kemp, B. F. Sherr, E. B. Sherr, and J. Cole (ed.), Handbook of methods in aquatic microbial ecology. Lewis, Boca Raton, Fla.
- 36.Stahl, D. A., and R. Amann. 1991. Development and application of nucleic acid probes, p. 205-248. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Ltd., Chichester, England.
- 37.Stibor, H., and U. Sommer. 2003. Mixotrophy of a photosynthetic flagellate viewed from an optimal foraging perspective. Protist 154:91-98. [DOI] [PubMed] [Google Scholar]
- 38.Teira, E., T. Reinthaler, A. Pernthaler, J. Pernthaler, and G. J. Herndl. 2004. Combining catalyzed reporter deposition-fluorescence in situ hybridization and microautoradiography to detect substrate utilization by bacteria and archaea in the deep ocean. Appl. Environ. Microbiol. 70:4411-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thingstad, T. F., H. Havskum, K. Garde, and B. Riemann. 1996. On the strategy of “eating your competitor’': a mathematical analysis of algal mixotrophy. Ecology 77:2108-2118. [Google Scholar]
- 40.Utermöhl, H. 1958. Zur Vervollkomnung der quantitativen Phytoplankton-Methodik. Mitt. Int. Ver Theor. Angew Limnol. 9:1-38. [Google Scholar]
- 41.Wagner, M., M. Horny, and H. Daimsz. 2003. Fluorescence in situ hybridization for the identification and characterization of prokaryotes. Curr. Opin. Microbiol. 6:302-309. [DOI] [PubMed] [Google Scholar]