Abstract
Among five potentially probiotic lactobacilli investigated, Lactobacillus plantarum MF1298 and Lactobacillus salivarius DC5 showed the highest increase in the transepithelial electrical resistance (TER) of polarized monolayers of Caco-2 cells, and this increase was shown to be dose dependent. Furthermore, preincubation with MF1298 attenuated a decrease in TER induced by Listeria monocytogenes.
Unregulated inflammation of the gastrointestinal tract (GIT), e.g., during active periods of inflammatory bowel diseases, causes an increased permeability of the epithelium, leading to deterioration of the epithelial barrier function (4). Consequently, luminal bacteria and luminal substances (e.g., bile salts and gastric or pancreatic enzymes) are allowed access into the underlying tissue and bloodstream of the host. Several studies have focused on the molecular mechanisms responsible for increased epithelial permeability (6, 7, 10) and have observed that it is linked to weakening of the tight junctions. Tight junctions establish a polarity of the epithelial cell layer by forming a seal between adjacent epithelial cells, thereby separating the luminal compartment from the basolateral surface (11). Treatment with probiotic bacteria may prevent or reverse increased permeability of the epithelium and act antagonistically towards pathogens (7, 10). A method for attaining the permeability in vitro includes the measurement of electrical physical resistance to determine the transepithelial electrical resistance (TER).
TER was applied in the present study to provide further support for the probiotic properties of five Lactobacillus spp. which previously demonstrated desirable probiotic properties in vitro, such as good adhesion capacity, antimicrobial activity, and tolerance to low pH (2.5) and bile (0.3%) (3). These strains include Lactobacillus plantarum MF1291 (DSM 17320), L. plantarum MF1298 (DSM 16997), and Lactobacillus pentosus MF1300 (DSM 17321), which are all dominant nonstarter lactic acid bacteria of fermented meats isolated by Klingberg et al. (3). Furthermore, the strains include Lactobacillus salivarius DC5 (isolated from poultry salami) and L. plantarum DC13 (isolated from raw ham) provided by Danisco Innovation A/S, Copenhagen, Denmark. The possibility that the strains modulate the TER was determined by the addition of the strains to polarized monolayers of Caco-2 cells seeded on Transwell filter inserts (0.4-μm pore size, 12-mm inside diameter; Corning Incorporated, Corning, NY) at a density of 1 × 105 cells/cm2. Functional polarity was developed when electrical resistance between the apical and basolateral surfaces of the monolayers was >450 Ω/cm2 as measured by the Millicell electrical resistance system (Millipore, Bedford, MA). Bacteria were suspended in cell growth medium without antibiotics. The bacterial suspension (500 μl) was added to the apical compartment at the various concentrations examined (from 105 to 108 CFU/ml) and incubated at 37°C. Following aerobic exposure to cell growth media for 48 h, the bacteria were still viable, as determined by the number of CFU. TER was measured before the addition of the bacteria (time zero) and then at various time intervals and expressed as the ratio of TER at time t in relation to the initial value (at time zero) for each series. The TER of monolayers without bacteria added represented the control for each experiment. Significance was determined using the two-tailed Student t test, and P values of <0.05 were considered significant.
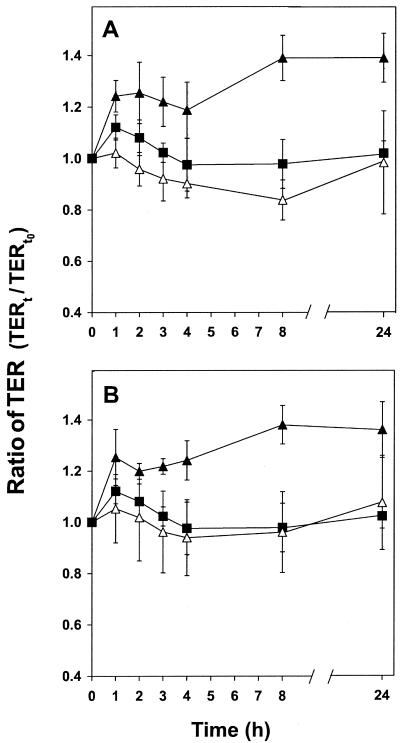
The five examined potential probiotics, used at a concentration of 108 CFU/cm2, increased the TER of polarized Caco-2 monolayers (results not shown). The two strains that showed the highest increase in TER, MF1298 and DC5, were selected for further experiments. The increase in TER was dependent on the concentration of the bacteria added. As shown in Fig. 1, addition of MF1298 and DC5 at a concentration of 107 cells/cm2 had no effect on the TER, whereas a 10-fold-higher bacterial concentration (108 cells/cm2) significantly (P <0.05) increased TER (∼40%) within 1 to 2 h of incubation. However, the dose needed to obtain an effect on the host is not well known and in vivo GIT permeability tests are needed. In addition, the survival rate of the ingested probiotics in the GIT may depend on how they are distributed, e.g., as freeze-dried cultures or in a food matrix.
FIG. 1.
TER of polarized Caco-2 monolayers exposed to Lactobacillus plantarum MF1298 (A) or Lactobacillus salivarius DC5 (B) at a concentration of 108 (▴) and 107 (▵) CFU/cm2 or without bacteria added (▪). TER (Ω/cm2) is expressed as the ratio of TER at time t in relation to the initial value (at time zero [t0]) for each series. The error bars indicate the standard deviations for four independent experiments. Significant differences (P < 0.05) from values for the polarized monolayer without bacteria added (control) were observed after 1 to 2 h.
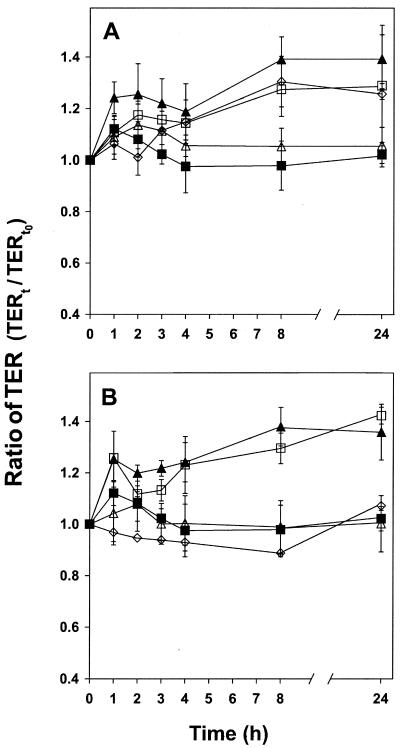
It is commonly presumed that the probiotic bacteria should be alive to exert their beneficial effects (2, 4, 8). The effect of inactivated bacteria was examined in the same way as described above. A concentration of 108 CFU/ml of MF1298 and DC5 was inactivated by exposure to gentamicin (1 mg/ml for 1 h at 37°C; Life Technologies, Gibco, Rockville, MD), heat treatment (1 h at 100°C), or irradiation (15 kGy, 0.2 Gy/s, cobolt-60 gamma cell; Risø, Roskilde, Denmark). Heat-treated bacteria showed no effect on the epithelial barrier function (Fig. 2) as determined by the level of TER, whereas the gentamicin-treated bacteria and the γ-irradiated cells of MF1298 increased TER even though they were not able to form colonies on MRS agar. Previously, a similar lack of effect on TER was observed for the heat-treated probiotic mixture of Streptococcus thermophilus and Lactobacillus acidophilus (10). It could be speculated that heat treatment, in contrast to gentamicin treatment, may cause denaturation of the surface proteins of lactobacilli, which are known to be involved in their adhesion to epithelial cells (12). Furthermore, the supernatant including secreted metabolic components from MF1298 and DC5 did not increase TER (results not shown), suggesting that the presence of bacteria is required to modulate TER.
FIG. 2.
TER of polarized Caco-2 monolayers exposed to Lactobacillus plantarum MF1298 (A) or Lactobacillus salivarius DC5 (B) initially treated with gentamicin (1 mg/ml) for 1 h at 37°C (□), heat for 1 h at 100°C (▵), or γ-irradiation (15 kGy, 0.2 Gy/s) (⋄) compared to that of untreated bacteria (▴) and nonexposed polarized monolayers (▪). Bacteria were added at a concentration of 108 CFU/cm2. TER (Ω/cm2) is expressed as the ratio of TER at time t in relation to the initial value (at time zero [t0]) for each series. The error bars indicate the standard deviations for four independent experiments, except for the γ-irradiated strains, with which two independent experiments were performed. Significant differences from values for the polarized monolayer without bacteria added were observed for γ-irradiated and antibiotic-treated MF1298, as well as for antibiotic-treated DC5.
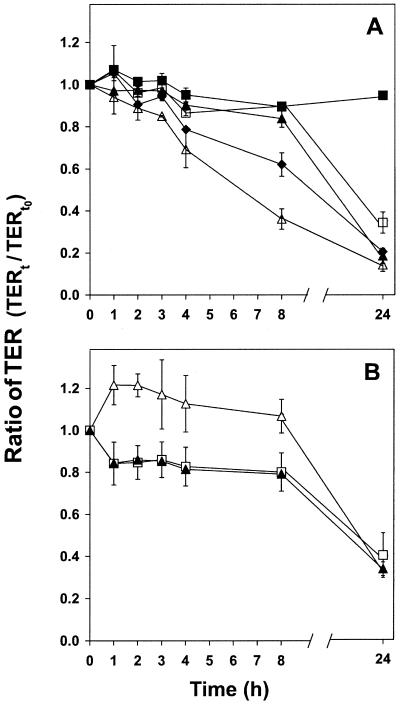
In contrast to probiotic cultures, Listeria monocytogenes 6896 decreased the TER of polarized Caco-2 monolayers when added at concentrations of 105 to 108 cells/cm2. As seen in Fig. 3A, this effect was shown to be time and dose dependent. Previously, a decrease in TER has been observed following the addition of various pathogenic bacteria, as demonstrated for Salmonella enterica subsp. enterica serovar Dublin (7), Salmonella enterica serovar Typhimurium (13), Escherichia coli (enterohemorrhagic and enteroinvasive) (9, 10), Yersinia pseudotuberculosis (5), and Vibrio cholerae (1). Western blot analysis of the protein ZO-1, which is associated with tight junctions of Caco-2 cells, was applied to examine the alterations of TER. The expression of ZO-1 was slightly changed upon the addition of bacteria to Caco-2 cells, with a decrease in cells to which L. monocytogenes was added and an increase in cells to which MF1298 or DC5 was added (results not shown). Furthermore, preincubation with MF1298 for 1 h prior to exposure to L. monocytogenes significantly attenuated the decrease in TER caused by L. monocytogenes (Fig. 3B). However, this effect of MF1298 diminished after 8 h of incubation, and MF1298 could not compensate for the marked decrease in TER caused by L. monocytogenes. Coincubation of MF1298 and DC5 with L. monocytogenes was not sufficient to attenuate the decrease in TER (results not shown). This indicates that only the establishment of MF1298 in the GIT may increase the resistance of the host towards L. monocytogenes.
FIG. 3.
(A) TER of polarized Caco-2 monolayers exposed to Listeria monocytogenes at a concentration of 105 (□), 106 (▴), 107 (⧫), or 108 (▵) CFU/cm2 or without bacteria added (▪); (B) TER of polarized Caco-2 monolayers preincubated with Lactobacillus plantarum MF1298 (▵) or Lactobacillus salivarius DC5 (▴) 1 h prior to the addition of L. monocytogenes or L. monocytogenes alone (□). L. monocytogenes was added at a concentration of 105 CFU/cm2, and MF1298 and DC5 were added at a concentration of 108 CFU/cm2. TER (Ω/cm2) is expressed as the ratio of TER at time t in relation to the initial value (at time zero [t0]) for each series. The error bars indicate the standard deviations for two independent experiments. (A) Significant differences (P < 0.05) from values for the polarized monolayer without bacteria added were observed after 3 h for 108 CFU/cm2, after 4 h for 106 to 107 CFU/cm2, and after 24 h for 105 CFU/cm2. (B) During the first 8 h of incubation, the TER of Caco-2 cells with added MF1298 and L. monocytogenes was significantly higher (P < 0.05) than that of polarized monolayer only with added L. monocytogenes.
In conclusion, the presence of MF1298 and DC5 strengthened the epithelial barrier function by increasing the TER in a time- and dose-dependent manner corresponding to the increase in the expression of ZO-1, which is associated with the barrier function. The biological significance of this increase in TER needs to be verified by in vivo GIT permeability tests. Furthermore, the establishment of MF1298 seems to attenuate the L. monocytogenes-induced disruption of the epithelial barrier function. Overall, these results contributed to the identification of MF1298 as a promising probiotic candidate to be selected for further in vivo studies.
Acknowledgments
This work has been supported financially by the Nordic Innovation Centre and is a part of a collaboration project (NI00060) between the Norwegian and Swedish meat industries; Matforsk, Oslo, Norway; Danisco Innovation, Copenhagen, Denmark; and The Royal Veterinary and Agricultural University, Frederiksberg, Denmark.
We thank Arne Miller (Risø, Roskilde, Denmark) for the irradiation of the strains.
REFERENCES
- 1.Fullner, K. J., W. I. Lencer, and J. J. Mekalanos. 2001. Vibrio cholerae-induced cellular responses of polarized T84 intestinal epithelial cells are dependent on production of cholera toxin and the RTX toxin. Infect. Immun. 69:6310-6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gotteland, M., S. Cruchet, and S. Verbeke. 2001. Effect of Lactobacillus on the gastrointestinal mucosal barrier alterations induced by indometacin in humans. Aliment. Pharmacol. Ther. 15:11-17. [DOI] [PubMed] [Google Scholar]
- 3.Klingberg, T. D., L. Axelsson, K. Naterstad, D. Elsser, and B. B. Budde. Identification of potential probiotic starter cultures for Scandinavian-type fermented sausages. Int. J. Food Microbiol., in press. [DOI] [PubMed]
- 4.Madsen, K., A. Cornish, P. Soper, C. McKaigny, H. Jijon, C. Yachimec, J. Doyle, L. Jewell, and C. De Simone. 2001. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology 121:580-591. [DOI] [PubMed] [Google Scholar]
- 5.McCormick, B. A., A. Nusrat, C. A. Parkos, L. D'Andrea, P. M. Hofman, D. Carnes, T. W. Liang, and J. L. Madara. 1997. Unmasking of intestinal epithelial lateral membrane B1 integrin consequent to transepithelial neutrophil migration in vitro facilitates inv-mediated invasion by Yersinia pseudotuberculosis. Infect. Immun. 65:1414-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nusrat, A., C. von Eichel-Streiber, J. R. Turner, P. Verkade, J. L. Madara, and C. A. Parkos. 2001. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect. Immun. 69:1329-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otte, J.-M., and D. K. Podolsky. 2004. Functional modulation of enterocytes by gram-positive and gram-negative microorganisms. Am. J. Physiol. 286:G613-G626. [DOI] [PubMed] [Google Scholar]
- 8.Ouwehand, A. C., and S. J. Salminen. 1998. The health effects of cultured milk products with viable and non-viable bacteria. Int. Dairy J. 8:749-758. [Google Scholar]
- 9.Philpott, D. J., D. M. McKay, W. Mak, M. H. Perdue, and P. M. Sherman. 1998. Signal transduction pathways involved in enterohemorrhagic Escherichia coli-induced alterations in T84 epithelial permeability. Infect. Immun. 66:1680-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Resta-Lenert, S., and K. E. Barrett. 2003. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 52:988-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneeberger, E. E., and R. D. Lynch. 2004. The tight junction: a multifunctional complex. Am. J. Physiol. Cell Physiol. 286:C1213-C1228. [DOI] [PubMed] [Google Scholar]
- 12.Sillanpää, J., B. Martínez, J. Antikainen, T. Toba, N. Kalkkinen, S. Tankka, K. Lounatmaa, J. Keränen, M. Höök, B. Westerlund-Wikstrom, P. H. Pouwels, and T. K. Korhonen. 2000. Characterization of the collagen-binding S-layer protein CbsA of Lactobacillus crispatus. J. Bacteriol. 182:6440-6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tafazoli, F., K.-E. Magnusson, and L. Zheng. 2003. Disruption of epithelial barrier integrity by Salmonella enterica serovar Typhimurium requires geranylgeranylated proteins. Infect. Immun. 71:872-881. [DOI] [PMC free article] [PubMed] [Google Scholar]