Abstract
To develop new approaches for the treatment of invasive infections caused by Scedosporium prolificans, the in vitro interaction between amphotericin B and pentamidine against 30 clinical isolates was evaluated using a checkerboard microdilution method based on the National Committee for Clinical Laboratory Standards M38-P guidelines. The interaction between the drugs was analyzed using fractional inhibitory concentration index (FICI) analysis and response surface modeling. Amphotericin B alone was inactive against all the isolates. The geometric mean MIC for pentamidine was 57 μg/ml (range, 8 to 256 μg/ml; MIC at which 50% of the isolates tested were inhibited [MIC50], 64 μg/ml; MIC90, 128 μg/ml). The combination was synergistic against 28 of 30 isolates (93.3%) by FICI analysis and 30 of 30 (100%) by response surface modeling analysis. Antagonism was not observed.
The in vitro susceptibility of Scedosporium prolificans to antifungal agents has been tested in several studies (5, 7, 22), and although the methodological conditions differed in the various studies, in general, their results correlated with the observed poor clinical outcomes. The new azoles, such as ravuconazole, voriconazole, and posaconazole, showed poor in vitro activity (5, 7), with the exception of the experimental azole UR-9825, which showed some activity against S. prolificans (5). Pentamidine (PN) displayed good in vitro and in vivo activity against Pneumocystis carinii, a microorganism that now is believed to belong to the fungal kingdom (12, 24, 28). Also, in combination with amphotericin B (AMB), the drug displayed in vivo and in vitro synergistic activity against other eukaryotic microorganisms such as Leishmania donovani (21, 28). To develop new therapeutic strategies to treat invasive scedosporiosis, we investigated the in vitro activity of AMB and PN, alone or in combination, using two different criteria, the fractional inhibitory concentration index (FICI) (10) and response surface modeling (13).
Thirty clinical isolates (3) of S. prolificans were tested. The isolates were subcultured on potato dextrose agar (PDA) for 5 to 7 days at 30°C. Candida parapsilosis (ATCC 22019) and Candida krusei (ATCC 6258) were used as quality control strains. All isolates were tested in duplicate on two different days. Conidia were obtained from fresh cultures each time. All solutions were prepared ex novo with powders from the same lot.
MICs were determined by a broth microdilution method according to the National Committee for Clinical Laboratory Standards guidelines (M38-P) (25).
Conidia were collected with a cotton stick and suspended in sterile water. After the heavy particles were allowed to settle, the turbidity of the supernatants was measured spectrophotometrically (Spectronic 20D; Milton Roy, Rochester, N.Y.) at 530 nm and the transmission was adjusted to 68 to 70% and diluted 1:50 in RPMI medium to obtain two times the desired inoculum concentration. The inoculum size was verified by determination of the number of viable CFU after plating serial dilutions of the inoculum onto Sabouraud dextrose agar. These cultures showed that the final inoculum concentrations ranged between 1.5 × 104 and 5 × 104 CFU/ml, which is within the recommended upper and lower limits. The drugs used in this study were AMB (Bristol-Myers Squibb, Woerden, The Netherlands) and PN (Sigma-Aldrich Chemie GmbH, Steinheim, Germany). The final concentrations of the drugs ranged from 0.03 to 16 μg/ml for AMB and from 1 to 128 μg/ml for PN. AMB was dissolved in dimethyl sulfoxide (Merck, Darmstadt, Germany), and PN was dissolved in water.
Drug dilutions were made in RPMI 1640 medium (with l-glutamine and without bicarbonate) (GIBCO BRL, Life Technologies, Woerden, The Netherlands) buffered to pH 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS) (Sigma-Aldrich Chemie). Susceptibility testing was performed in 96-well flat-bottom microtitration plates, which were kept at −70°C until the day of testing. After inoculation and agitation, the plates were incubated at 35°C for 72 h and the MICs were read visually and spectrophotometrically. Growth was graded on a scale of 0 to 4 as follows: 4 indicated no reduction in growth, 3 indicated a 25% reduction of growth, 2 indicated a 50% reduction of growth, 1 indicated a 75% reduction of growth, and 0 indicated an optically clear well. The MIC endpoint was defined as the lowest concentration showing an optically clear well or absence of growth (MIC 0, ≥95% inhibition) for the drugs alone as well as for the combination. The optical density (OD) was measured with a spectrophotometer (MS2 reader, Titertek-plus; ICN Biomedical Ltd., Basingstoke, United Kingdom) at 405 nm. The OD of the blank, to which a conidium-free inoculum had been added, was subtracted from the OD values. The percentage of growth for each well was calculated by comparing the OD of the well with that of the drug-free control.
The in vitro fungicidal activity (minimal fungicidal concentration [MFC]) of each agent was determined by streaking 100 μl from each well that showed complete inhibition (≥95% inhibition or an optically clear well) onto Sabouraud dextrose agar plates. The plates were incubated at 35°C for 72 h, the MFC was the lowest drug concentration at which there was either no growth or only a single colony, which corresponds with 99.9% killing. The drug was considered fungicidal if the ratio of MFC to MIC did not exceed a value of 4. If the ratio was greater than 4, the activity was considered to be fungistatic (15).
A two-dimensional, two-agent broth microdilution checkerboard technique was used to study the interaction between both drugs. Drug interaction was analyzed by two different methods, the FICI and the response surface model of Greco et al. (13, 14).
FICI values were calculated as follows: MIC of AMB-PN/MIC of AMB + MIC of AMB-PN/MIC of PN. The interpretation of the FICI was determined as follows: ≤0.5, synergistic effect; >0.5 but ≤1, additive effect; >1 but ≤4, indifferent effect; and >4, antagonistic effect (10). In practice, synergism or antagonism calculated in this way is equivalent to a reduction or increase of at least two dilution steps in the MICs of both drugs when they are combined compared to the MICs for the drugs alone.
Because there is no definition of PN MIC endpoint for fungi, alone or in combination with AMB, we used the response surface modeling described by Greco et al. (13, 14). The model is described by the formula below and was used previously to characterize the interaction of antiviral, antifungal, and antineoplastic agents (11, 14, 20, 28a):
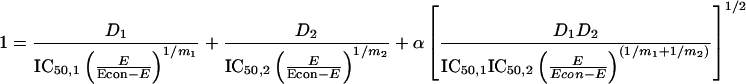 |
where D1 and D2 are the concentrations of drug 1 and drug 2 (AMB and PN), IC50,1 and IC50,2 are the concentrations of drug 1 and drug 2 resulting in 50% inhibition, E is the measured response, Econ is the control response, m1 and m2 are the slope parameters for drugs 1 and 2 in constant ratios, and α is the synergism-antagonism interaction parameter (ICα). If α is zero, the combination is additive; if α is positive, the interaction is synergistic. A negative α value indicates antagonism. The estimate of α has an associated 95% confidence interval; if the confidence interval does not overlap zero, this provides the statistical significance for the estimate of interaction. A computer program (ModLab; Medimatics, Maastricht, The Netherlands) was used to fit the data to this model (28a). The program also determined the 95% confidence interval for each parameter.
The MICs of AMB and PN, based on 95% reduction of growth for C. krusei (ATCC 6258), were 0.5 and 16 μg/ml, respectively, and for C. parapsilosis (ATCC 22019) were 0.25 and 4 μg/ml, respectively. The MICs for the quality control strains were within the reference ranges for AMB, but there is no reference MIC described for PN.
AMB was inactive in vitro against most isolates: MIC at which 50% of the isolates were inhibited (MIC50) and MIC90 were 32 μg/ml, and the geometric mean MIC was 22.62 μg/ml (range, 4 to 32 μg/ml). The geometric mean MIC for PN was 57 μg/ml (range, 8 to 256 μg/ml; MIC50, 64 μg/ml; MIC90, 128 μg/ml). The geometric means of the MFCs of AMB and PN were 30.55 μg/ml (range, 16 to 32 μg/ml) and 165 μg/ml (range, 16 to 256 μg/ml), respectively. The MFC/MIC ratios were more than 4 for all the strains, indicating fungistatic activity.
Synergism was found for 28 of 30 isolates (93.3%), according to the FICI. The remaining two isolates showed an additive effect (Table 1). According to the Greco model, AMB and PN showed synergistic interaction against all S. prolificans isolates. The 95% confidence interval of the α values did not overlap zero, indicating significant synergism (Table 1).
TABLE 1.
MIC, FICI, and α values of AMB and PN against S. prolificans after 72 h of incubation
| Strain (AZN number) | FICI model
|
Greco model
|
||||||
|---|---|---|---|---|---|---|---|---|
| AMB MIC | PN MIC | MICs of antifungal agent combination (AMB-PN)a | FICIb | INTc | α | 95% CId | INTc | |
| 7307 | 8 | 64 | 2/16 | 0.5 | SYN | 1.9 | 1.10-3.29 | SYN |
| 7404 | 32 | 64 | 4/8 | 0.25 | SYN | 25.93 | 17.43-38.57 | SYN |
| 7886 | 32 | 16 | 4/4 | 0.37 | SYN | 8.76 | 5.54-13.8 | SYN |
| 7889 | 32 | 16 | 4/2 | 0.37 | SYN | 3.09 | 1.57-6 | SYN |
| 7891 | 32 | 128 | 2/8 | 0.18 | SYN | 15.94 | 9.27-27.37 | SYN |
| 7892 | 16 | 128 | 4/8 | 0.31 | SYN | 7.40 | 4.57-11.96 | SYN |
| 7893 | 32 | 64 | 4/8 | 0.37 | SYN | 24 | 15.11-38.37 | SYN |
| 7894 | 32 | 64 | 4/8 | 0.37 | SYN | 17.5 | 10.35-29.58 | SYN |
| 7895 | 32 | 8 | 2/2 | 0.31 | SYN | 25.28 | 14.7-43.26 | SYN |
| 7897 | 32 | 16 | 4/4 | 0.37 | SYN | 7.97 | 4.21-15.11 | SYN |
| 7898 | 32 | 16 | 2/2 | 0.37 | SYN | 2.82 | 1.44-5.52 | SYN |
| 7900 | 32 | 16 | 4/2 | 0.25 | SYN | 2 | 1-4.25 | SYN |
| 7901 | 32 | 128 | 4/16 | 0.25 | SYN | 89.12 | 51.6-155.3 | SYN |
| 7902 | 4 | 64 | 2/16 | 0.75 | ADD | 1.70 | 0.43-6.6 | SYN |
| 7904 | 32 | 64 | 4/32 | 0.62 | ADD | 39.68 | 26.6-59.21 | SYN |
| 7906 | 32 | 16 | 4/2 | 0.37 | SYN | 2.58 | 1.42-4.68 | SYN |
| 7908 | 32 | 128 | 2/16 | 0.18 | SYN | 4.37 | 2.34-8.15 | SYN |
| 7909 | 32 | 16 | 4/4 | 0.37 | SYN | 6.86 | 3.8-12.37 | SYN |
| 7910 | 16 | 256 | 2/16 | 0.18 | SYN | 11.4 | 7.17-18.37 | SYN |
| 7912 | 16 | 256 | 2/32 | 0.25 | SYN | 6.80 | 4.16-11.14 | SYN |
| 7915 | 32 | 64 | 2/16 | 0.31 | SYN | 5.77 | 3-10.8 | SYN |
| 7917 | 16 | 128 | 2/16 | 0.25 | SYN | 3.82 | 2.11-6.9 | SYN |
| 7918 | 32 | 64 | 4/16 | 0.37 | SYN | 44.08 | 2.89-67.1 | SYN |
| 7919 | 32 | 128 | 4/8 | 0.18 | SYN | 5.8 | 3.54-9.48 | SYN |
| 7920 | 32 | 128 | 2/8 | 0.18 | SYN | 20.24 | 12.2-33.5 | SYN |
| 7921 | 4 | 64 | 1/16 | 0.5 | SYN | 1.27 | 0.61-2.66 | SYN |
| 7924 | 8 | 128 | 2/8 | 0.31 | SYN | 5.81 | 2.5-13.5 | SYN |
| 7927 | 32 | 128 | 2/16 | 0.18 | SYN | 13.84 | 8.69-22 | SYN |
| 7928 | 16 | 32 | 2/4 | 0.37 | SYN | 2.82 | 1.4-5.67 | SYN |
| 7930 | 32 | 64 | 4/4 | 0.18 | SYN | 1.13 | 0.38-3.35 | SYN |
Lowest MIC in combination.
Lowest FICI.
INT, interpretation; SYN, synergistic effect; ADD, additive effect.
CI, confidence interval.
Disseminated infection by S. prolificans most commonly occurs in neutropenic patients with hematologic malignancies. It is a rapidly fatal infection characterized by fever and multiorgan failure. Many patients have been treated with AMB and occasionally with other antifungals but commonly with unsuccessful outcomes (3, 5, 18).
PN is an aromatic diamidine that displays multiple effects and is active in vitro against a number of different bacteria, protozoa, and fungi, such as Blastomyces dermatitidis, Saccharomyces cerevisiae, Candida species, and Cryptococcus neoformans (2, 8, 19, 23, 27, 29). Patients who receive 4 mg/kg of body weight daily by slow intravenous infusion can achieve a blood concentration of 0.5 to 3.2 μg/ml. However, much higher levels are found in tissue, with concentrations of up to 56 μg/g in lung, 35 to 300 μg/g in liver, 40 to 368 μg/g in spleen, and 8.5 to 123 μg/g in kidney tissue (4, 9). When drug levels were related to MICs, 20 of 30 of the S. prolificans isolates were considered susceptible to this drug in vitro. Considering the MIC/MFC ratios, fungistatic activity was observed for PN.
A promising approach to treatment of invasive scedosporiosis might be that of combining antifungal drugs with different mechanisms of action. PN in combination with AMB showed synergistic interaction in most of the strains, using either the FICI or the Greco model. Several different mechanisms of antimicrobial activity have been proposed for PN, such as inhibition of DNA, RNA, phospholipid, and protein synthesis (8, 28). However, because the mechanism of action is not fully understood, it is difficult to characterize the synergistic interaction with AMB.
AMB in combination with tetracyclines, azithromycin, or rifampin was synergistic in vitro against Aspergillus spp. (6, 16, 17, 26). As with PN, the above-mentioned antibacterial drugs inhibit the protein synthesis, and this could be an explanation of their positive interaction. A disadvantage of the combination of AMB and PN was that it caused acute reversible renal failure in vivo, and therefore, caution should be used when these agents are given concomitantly (1). However, since the most frequent portal of entry of the fungus appears to be the respiratory tract, administration of aerosolized PN, combined with systemic administration of AMB, could reduce toxicity.
In conclusion, this is the first description of activity of PN alone or in combination with AMB against S. prolificans in vitro.
Further studies with this and other combinations in appropriate animal models are required to develop therapeutic strategies for treatment of invasive scedosporiosis.
(This work was presented in part at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 16 to 19 December 2001.)
Acknowledgments
This work was supported by a grant from the EC-TMR-EUROFUNG network (ERBFMXR-CT970145) and by the Mycology Research Center of Nijmegen.
REFERENCES
- 1.Antoniskis, D., and R. A. Larsen. 1990. Acute, rapidly progressive renal failure with simultaneous use of amphotericin B and pentamidine. Antimicrob. Agents Chemother. 34:470-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barchiesi, F., M. Del Poeta, V. Morbiducci, F. Ancarani, and G. Scalise. 1994. Effect of pentamidine on the growth of Cryptococcus neoformans. J. Antimicrob. Chemother. 33:1229-1232. [DOI] [PubMed] [Google Scholar]
- 3.Berenguer, J., J. L. Rodriguez-Tudela, C. Richard, M. Alvarez, M. A. Sanz, L. Gaztelurrutia, J. Ayats, J. V. Martinez-Suarez, et al. 1997. Deep infections caused by Scedosporium prolificans. A report on 16 cases in Spain and a review of the literature. Medicine (Baltimore) 76:256-265. [DOI] [PubMed] [Google Scholar]
- 4.Bernard, E. M., H. J. Donnelly, M. P. Maher, and D. Armstrong. 1985. Use of a new bioassay to study pentamidine pharmacokinetics. J. Infect. Dis. 152:750-754. [DOI] [PubMed] [Google Scholar]
- 5.Carrillo, A. J., and J. Guarro. 2001. In vitro activities of four novel triazoles against Scedosporium spp. Antimicrob. Agents Chemother. 45:2151-2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clancy, C. J., Y. C. Yu, A. Lewin, and M. H. Nguyen. 1998. Inhibition of RNA synthesis as a therapeutic strategy against Aspergillus and Fusarium: demonstration of in vitro synergy between rifabutin and amphotericin B. Antimicrob. Agents Chemother. 42:509-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cuenca-Estrella, M., B. Ruiz-Diez, J. V. Martinez-Suarez, A. Monzon, and J. L. Rodriguez-Tudela. 1999. Comparative in-vitro activity of voriconazole (UK-109,496) and six other antifungal agents against clinical isolates of Scedosporium prolificans and Scedosporium apiospermum. J. Antimicrob. Chemother. 43:149-151. [DOI] [PubMed] [Google Scholar]
- 8.Donkor, I. O., and A. M. Clark. 1999. In vitro antimicrobial activity of aromatic diamidines and diimidazolines related to pentamidine. Eur. J. Med. Chem. 34:639-643. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly, H., E. M. Bernard, H. Rothkotter, J. W. Gold, and D. Armstrong. 1988. Distribution of pentamidine in patients with AIDS. J. Infect. Dis. 157:985-989. [DOI] [PubMed] [Google Scholar]
- 10.Eliopoulos, G. M., and R. C. Moellering. 1996. Antimicrobial combinations, p. 330-396. In V. Lorain (ed.), Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, Md.
- 11.Faessel, H. M., H. K. Slocum, R. C. Jackson, T. J. Boritzki, Y. M. Rustum, M. G. Nair, and W. R. Greco. 1998. Super in vitro synergy between inhibitors of dihydrofolate reductase and inhibitors of other folate-requiring enzymes: the critical role of polyglutamylation. Cancer Res. 58:3036-3050. [PubMed] [Google Scholar]
- 12.Fishman, J. A. 1998. Prevention of infection due to Pneumocystis carinii. Antimicrob. Agents Chemother. 42:995-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greco, W. R., G. Bravo, and J. C. Parsons. 1995. The search for synergy: a critical review from a response surface perspective. Pharmacol. Rev. 47:331-385. [PubMed] [Google Scholar]
- 14.Greco, W. R., H. S. Park, and Y. M. Rustum. 1990. Application of a new approach for the quantitation of drug synergism to the combination of cis-diamminedichloroplatinum and 1-beta-d-arabinofuranosylcytosine. Cancer Res. 50:5318-5327. [PubMed] [Google Scholar]
- 15.Hazen, K. C. 1998. Fungicidal versus fungistatic activity of terbinafine and itraconazole: an in vitro comparison. J. Am. Acad. Dermatol. 38:S37-S41. [DOI] [PubMed] [Google Scholar]
- 16.Hughes, C. E., C. Harris, J. A. Moody, L. R. Peterson, and D. N. Gerding. 1984. In vitro activities of amphotericin B in combination with four antifungal agents and rifampin against Aspergillus spp. Antimicrob. Agents Chemother. 25:560-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hughes, C. E., C. Harris, L. R. Peterson, and D. N. Gerding. 1984. Enhancement of the in vitro activity of amphotericin B against Aspergillus spp. by tetracycline analogs. Antimicrob. Agents Chemother. 26:837-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Idigoras, P., E. Perez-Trallero, L. Pineiro, J. Larruskain, M. C. Lopez-Lopategui, N. Rodriguez, and J. M. Gonzalez. 2001. Disseminated infection and colonization by Scedosporium prolificans: a review of 18 cases, 1990-1999. Clin. Infect. Dis. 32:E158-E165. [DOI] [PubMed] [Google Scholar]
- 19.Ludewig, G., J. M. Williams, Y. Li, and C. Staben. 1994. Effects of pentamidine isethionate on Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 38:1123-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maga, G., U. Hübscher, M. Pregnolato, D. Ubiali, G. Gosselin, and S. Spadari. 2001. Potentiation of inhibition of wild-type and mutant human immunodeficiency virus type 1 reverse transcriptases by combinations of nonnucleoside inhibitors and d- and l-(β)-dideoxynucleoside triphosphate analogs. Antimicrob. Agents Chemother. 45:1192-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mbongo, N., P. M. Loiseau, D. G. Craciunescu, and M. Robert-Gero. 1998. Synergistic effect of Ir-(COT)-pentamidine alizarin red and pentamidine, amphotericin B, and paromomycin on Leishmania donovani. Acta Trop. 70:239-245. [DOI] [PubMed] [Google Scholar]
- 22.Meletiadis, J., J. W. Mouton, J. L. Rodriguez-Tudela, J. F. Meis, and P. E. Verweij. 2000. In vitro interaction of terbinafine with itraconazole against clinical isolates of Scedosporium prolificans. Antimicrob. Agents Chemother. 44:470-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miletti, K. E., and M. J. Leibowitz. 2000. Pentamidine inhibition of group I intron splicing in Candida albicans correlates with growth inhibition. Antimicrob. Agents Chemother. 44:958-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris-Jones, S. D., and P. J. Easterbrook. 1997. Current issues in the treatment and prophylaxis of Pneumocystis carinii pneumonia in HIV infection. J. Antimicrob. Chemother. 40:315-318. [DOI] [PubMed] [Google Scholar]
- 25.National Committee for Clinical Laboratory Standards. 1998. Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi. Proposed standard M38-P. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 26.Nguyen, M. H., C. J. Clancy, Y. C. Yu, and A. S. Lewin. 1997. Potentiation of antifungal activity of amphotericin B by azithromycin against Aspergillus species. Eur. J. Clin. Microbiol. Infect. Dis. 16:846-848. [DOI] [PubMed] [Google Scholar]
- 27.Nolan, A., P. J. Lamey, T. W. MacFarlane, T. C. Aitchison, J. Shaw, and J. Y. Sirel. 1994. The effect of nebulised pentamidine on the concentration of intra-oral Candida albicans in HIV-infected patients. J. Med. Microbiol. 41:95-97. [DOI] [PubMed] [Google Scholar]
- 28.Sands, M., M. A. Kron, and R. B. Brown. 1985. Pentamidine: a review. Rev. Infect. Dis. 7:625-634. [DOI] [PubMed] [Google Scholar]
- 28a.Te Dorsthorst, D. T. A., P. E. Verweij, J. F. G. M. Meis, N. C. Punt, and J. W. Mouton. 2002. Comparison of fractional inhibitory concentration index with response surface modeling for characterization of in vitro interaction of antifungals against intraconazole-susceptible and -resistant Aspergillus fumigatus isolates. Antimicrob. Agents Chemother. 46:702-707. [DOI] [PMC free article] [PubMed]
- 29.Tracy, J. A., and L. T. Webster. 1996. Drugs used in the chemotherapy of protozoal infections: malaria, p. 965-985. In J. Hardman, A. Gilman, and L. Limbird (ed.), Goodman and Gilman's the pharmacological basis of therapeutics, 9th ed. McGraw-Hill Companies, New York, N.Y.


