Abstract
Active members of the bacterial community in the sediment of Lake Washington, with special emphasis on C1 utilizers, were identified by employing two complementary culture-independent approaches: reverse transcription of environmental mRNA and 16S rRNA combined with PCR (RT-PCR) and stable-isotope probing (SIP) of DNA with the 13C-labeled C1 substrates methanol, methylamine, formaldehyde, and formate. Analysis of RT-PCR-amplified fragments of 16S rRNA-encoding genes revealed that gammaproteobacterial methanotrophs belonging to Methylobacter and Methylomonas dominate the active methylotroph population, while only one other known methylotrophic lineage, Methylophilaceae, was detected via this approach. Analysis of RT-PCR-amplified functional genes, pmoA and fae, allowed detection of alphaproteobacterial (Methylosinus) and gammaproteobacterial (Methylobacter, Methylomonas, and Methylomicrobium) methanotrophs, methylotrophs of the genus Methylobacterium, and yet-unidentified proteobacteria. SIP experiments allowed detection of a broad variety of groups actively metabolizing C1 compounds. Comparisons between 16S rRNA gene pools amplified from [13C]DNA and from [12C]DNA revealed that the proportion of Methylophilus-related sequences increased in the presence of [13C]methanol, [13C]methylamine, and [13C]formaldehyde; Novosphingobium-related sequences were enriched in the presence of [13C]methanol; Gemmatimonadaceae-related sequences were enriched in the presence of [13C]formaldehyde and [13C]formate; and Xanthomonadaceae-related sequences were enriched in the presence of [13C]formate. Analysis of fae genes amplified from [13C]DNAs isolated from different microcosms revealed specific shifts in populations in response to a specific C1 compound: Methylosinus sequences dominated the [13C]methanol microcosm pool, and beta- and gammaproteobacterial sequences dominated the [13C]methylamine microcosm pool. The [13C]formaldehyde microcosm was dominated by betaproteobacterial sequences and by sequences of a nonaffiliated group, while the [13C]formate microcosm was dominated by alpha- and betaproteobacterial sequences. Overall, these data point toward the presence of a diverse population of active methylotrophs in Lake Washington sediments and toward the existence of yet-uncultivated organisms.
Methylotrophic bacteria are a group of organisms with the ability to use compounds with no carbon-carbon bonds (C1 compounds) as single sources of carbon and energy (1), thus playing a role in global carbon cycling. A wide range of C1 compounds are consumed by methylotrophs in the environment, including methane, methanol, methylated amines, methylated glycines, halomethanes, and methylated sulfur species (1, 21, 24, 31, 44). Traditional microbial techniques such as enrichment and isolation on defined culture media have revealed that methylotrophic bacteria occur in a variety of environments, such as freshwater, marine, and terrestrial habitats, including habitats characterized by extreme conditions of temperature, salinity, or pH (8, 26, 44). As many methylotrophic bacteria are difficult to isolate, cultivation-independent molecular tools have been developed to characterize natural methylotrophic populations. These tools included oligonucleotide probes and PCR primer sets targeting genes conserved among methylotrophic bacteria, such as 16S RNA genes, or genes encoding specific methylotrophic enzymes, such as particulate and soluble methane monooxygenases, methanol dehydrogenase, corrinoid-linked methyltransferase, or methanesulfonic acid monooxygenase (3, 15, 21, 30, 31). However, none of these probes or primers aimed at detection of C1-oxidizing populations in a broader sense, encompassing their actual diversity. Recently, Kalyuzhnaya et al. (18, 19) have developed a suite of primer sets targeting four genes (fae, mtdB, mch, and fhcD) in the tetrahydromethanopterin (H4MPT)-linked C1 transfer pathway, the pathway for formaldehyde oxidation that is broadly distributed across methylotrophic groups (5, 43). In addition, this pathway has been detected in taxa not characterized as methylotrophs, such as Burkholderia xenovorans and representatives of Planctomycetes (4, 12, 29). These new primer sets allowed identification of a wide variety of methylotrophs but also detected a number of novel, divergent fae, mtdB, mch, and fhcD phylotypes in the sediment of Lake Washington (18, 19), demonstrating their utility in broad detection of C1-oxidizing capacity and contributing to the knowledge of methylotroph presence and diversity in the site.
The recently developed cultivation-independent approaches allow identification of microbial communities active in particular metabolic processes. One of these approaches involves detection and analysis of 16S rRNA or mRNA transcribed from functional genes (i.e., nifH, pmoA, merR, and denitrification genes) via reverse transcription followed by PCR (RT-PCR), cloning, and sequencing (10, 32, 35, 45). Another approach is the use of stable-isotope-labeled substrates (stable-isotope probing [SIP]) (37). One of the most commonly used SIP techniques relies on incorporation of 13C originating from a specific substrate into nucleic acids (DNA and RNA) of microorganisms actively involved in its utilization. The 13C-labeled (heavy) nucleic acids (DNA or RNA) are separated from the 12C-nucleic acids by isopycnic centrifugation and used as templates in PCR amplification to determine the identities of the respective microbes. This approach has been successfully applied to identify members of microbial communities active in utilization of methane, methanol, propionate, and aromatic compounds (11, 17, 27, 28, 37, 38). While it is understood that SIP experiments should be conducted in conditions as closely resembling the conditions in situ as possible in order to obtain a true picture of the activities and processes native to a given environment, the success of SIP inherently depends on altering the in situ conditions to a degree. A higher-than-in-situ concentration of the substrate in question is required for successful labeling (37, 38), and prolonged incubations often are required (37, 38), which may result in enrichment of DNAs of fast-growing microbes. While possessing a potential of uncovering unexpected and unsuspected participants in certain environments (11, 37, 38), SIP results need critical interpretation.
The top layer of the sediment of Lake Washington is an environment in which steep gradients of methane and oxygen are present, and aerobic methane oxidation occurs at high rates (25). The methanotroph population in Lake Washington has been characterized in previous work (2, 6, 7). However, much less is known about metabolism of C1 substrates that are less reduced than methane. While recent tests employing primers targeting genes of the H4MPT-linked formaldehyde oxidation pathway revealed the presence of a diverse population of microbes that do not fall into known methanotrophic groups (18, 19), nothing is known about the respective roles and activities of these organisms in cycling of C1 compounds. The aim of this study was to identify active members of the bacterial community in the sediment of Lake Washington, with emphasis on C1 utilizers, via two complementary culture-independent approaches, analysis of mRNA and rRNA by using RT-PCR-based techniques and DNA-SIP with a variety of C1-labeled substrates.
MATERIALS AND METHODS
Sample collection and storage.
Samples of the Lake Washington sediment were collected at a 60-m-deep study site (Global Positioning System position, 47°38.027′N, 122°15.882′W), using R/V Clifford Barnes on 6 April 2004, as described by Kalyuzhnaya et al. (18). Samples were kept on ice until they were transferred to the laboratory. The samples then were either immediately used for nucleic acid isolation and SIP or stored at 4°C for a few days before use. For the experiments described here, the top 1-cm layer of the sample was used. Lake Washington water used for cultivation was collected from the same core samples, immediately above the sediment.
Stable-isotope probing.
Microcosms were set up, consisting of 15 ml of sediment slurry mixed with an equal volume of 0.2 μm-pore-filtered Lake Washington water placed into sterile 50-ml screw-cap plastic tubes and supplemented with one of following (final concentrations): [13C]methanol (0.05%, vol/vol), [13C]methylamine (20 mM), [13C]formaldehyde (1 mM), or [13C]formate (10 mM). All substrates were 99 atom% 13C and were purchased from Sigma-Aldrich, with the exception of [13C]methanol, which was provided by the National Stable Isotope Resource at Los Alamos National Laboratory. Microcosms were incubated at room temperature (20 to 25°C) with agitation (150 rpm) for up to 10 days. The incubation temperature was chosen based on preliminary experiments in which [13C]DNA fraction complexities for microcosms incubated at in situ temperature (8°C) and microcosms incubated at higher temperatures were compared by restriction fragment length polymorphism (RFLP) analysis. The DNA complexities of the samples were similar, with microcosms incubated at higher temperatures accumulating the [13C]DNA fraction more rapidly (data not shown).
Nucleic acid extraction.
Total nucleic acids (RNA and DNA) were isolated from the sediment samples and from each microcosm according to the protocol described by Griffiths et al. (14), with some modifications. Briefly, 0.5 g (wet weight) of sample and 0.5 g of 0.1-mm zirconia-silica beads (Biospec Products) were suspended in 750 μl of extraction buffer (a mixture of equal volumes of 10% CTAB [cetyltrimethylammonium bromide] in 1.6 M NaCl and 0.2 M phosphate buffer, pH 8.0), to which 75 μl each of 10% sodium dodecyl sulfate and 10% lauroyl sarcosine were added. After addition of 750 μl of phenol-chloroform-isoamyl alcohol (25:24:1), the mixtures were homogenized in a minibeater (Biospec Products) for 30 to 60 s at 4°C (75% of the maximum power) and thencentrifuged at 16,000 × g for 5 min at 4°C. The aqueous phase was mixed withan equal volume of chloroform-isoamyl alcohol (24:1) and centrifuged at 16,000 × g for 5 min at 4°C. Nucleic acids were precipitated for 1 to 2 h at room temperature by adding either MgCl2 (final concentration, 2 mM), 0.1 volume of 3 M sodium acetate and 0.7 volume of isopropanol, or 2 volumes of 30% PEG 6000-1.6 M NaCl. Nucleic acids were recovered by centrifugation at 18,000 × g for 10 min at 4°C, washed with 75% ethanol, and resuspended in 50 μl of sterile nuclease-free water. Nucleic acids isolated from four to five replicates were pooled. Nucleic acids were checked for quality and quantity by electrophoresis in agarose gels containing ethidium bromide. DNAs isolated from the microcosm experiments were purified from the coextracted RNA by incubating preparations for 1 h at 37°C with 1 U of DNase-free RNase (QIAGEN). RNAs isolated from sediment samples were purified from the coextracted DNA by incubating twice for 30 min at 37°C with 4 U of DNase I (Ambion). Total RNA was diluted 50 times for use in RT-PCRs.
Ultracentrifugation and DNA recovery.
DNA extracted from the microcosms was prepared for CsCl-ethidium bromide density gradient ultracentrifugation as described previously (37) and centrifuged at 265,000 × g (Beckman VTi 65 rotor) for 16 h at 20°C. [13C]DNA and [12C]DNA fractions from each microcosm were collected using 19-gauge needles. DNA from each fraction was purified following standard procedures (40) and used in a second CsCl-ethidium bromide density gradient ultracentrifugation, as described above. After centrifugation, discrete fractions of the gradients (<500 μl) were collected and processed as described above. DNA samples were stored at −20°C.
PCR amplifications.
Aliquots (1 μl) of the [13C]DNA or [12C]DNA isolated from each microcosm were used as templates in PCRs employing either 16S rRNA gene-specific (8F/1407R [34, 40]) or fae-specific (18) primers. PCR mixtures (final volume, 20 μl) contained: 1× buffer, 1.5 mM MgCl2, 0.2 μM of each deoxynucleoside triphosphate, 0.2 μM of the primers, and 0.5 U of DNA polymerase (Invitrogen). Cycling was performed as described previously (18, 33). Thirty cycles were used to obtain 16S rRNA gene fragments (approximately 1.4 kb). fae fragments were amplified in two steps, as described by Kalyuzhnaya et al. (18), to increase amplification specificity. After 30 cycles as described above, 1 μl of the amplification mixture was used as a template in a new PCR mixture, as described above, and 25 additional cycles were performed, to amplify a product of approximately 300 bp.
RT-PCR amplifications.
Aliquots (2 μl) of RNA isolated from the sediment were used as templates in reverse transcription reactions followed by amplification by PCR, according to the manufacturer's instructions (QIAGEN One-Step RT-PCR kit). The primer sets 8F/1407R, pmo189/mb661 (7), fae1F/fae1R, and fae2F/fae2R were used to detect, respectively, eubacterial 16S rRNA, pmoA, and fae gene transcripts. In addition, a group-specific pair of primers was used to detect transcripts from a divergent fae clone previously detected in Lake Washington, clone L1N9 (18): primer L1N9fae-f (5′-ATGGAGGCACCCATGGCAG-3′) and primer L1N9fae-r (5′-CTCAGACGCCTTCGACACC-3′). Twenty-five cycles were used to amplify 16S rRNA gene products, and 35 cycles were used to amplify pmoA products. fae products were obtained using a two-step amplification protocol essentially as described above, except that 35 cycles were used in the first step, followed by 20 additional cycles.
Clone library construction.
Amplicons of the expected size were purified using the QIAGEN gel extraction kit and cloned into the pCR2.1 Topo TA cloning vector, according to the manufacturer's instructions.
RFLP analysis and sequencing.
PCR products obtained from single-colony PCR or using purified plasmids as templates were categorized into operational taxonomic units (OTUs), based on their restriction patterns obtained by digestion with the following restriction enzymes: RsaI for 16S rRNA and pmoA genes and either BclI/SacII/NcoI/HincII/BstUI or HaeIII for fae genes. DNA fragments were resolved in 2 to 2.5% agarose gels. The sampling effort in each library was evaluated by calculating the coverage (C) (13) according to the equation C= 1 − (n/N), where n is the number of OTUs containing unique sequences and N is the number of clones analyzed in the library. For 16S rRNA gene libraries, OTUs represented by at least two clones and at least 10% of the OTUs represented by a single clone were analyzed by sequencing using the primer 515-537R (5′-CCGTMTTACCGCGCTGCTGGCA-3′) or the M13F primer (5′-GTAAAACGACGGCCAG-3′) and the Big Dye v3.1 sequencing kit (Applied Biosystems). Reaction analysis was performed using an ABI3790XL high-throughput capillary DNA analyzer (Applied Biosystems) by the Department of Biochemistry DNA sequencing facility at the University of Washington. To sequence representatives of OTUs from pmoA and fae libraries, the M13F primer was used. Sequences of the 16S rRNA gene (∼400 bp), fae, and pmoA were aligned using the FastAligner v3.0 program (ARB software package [http://www.arb-home.de]) against their closest relatives in the GenBank databases as determined using BLASTN and TBLASTX searches (http://www.ncbi.nih.nlm.edu/BLAST). Similarity matrixes were constructed, and threshold values of 97% for 16S rRNA gene sequences (41) and 94% for pmoA and fae sequences (23, 42) were used to group the sequences into phylotypes. One representative of each phylotype was completely sequenced using primers M13F and M13R (5′-CAGGAAACAGCTATGAC-3′), with the exception of a few phylotypes related to Beta-/Gammaproteobacteria that were partial sequences. Affiliations with phylogenetic groups mentioned were defined according to the Ribosomal Database Project (RDP) Classifier (http://rdp.cme.msu.edu/classifier.jsp).
Phylogenetic analyses.
Nearly-full-length 16S rRNA gene sequences were submitted to CHECK-CHIMERA, available on the Ribosomal Database Project release 8.1 (http://rdp8.cme.msu.edu/html/analyses.html), in order to identify chimeras. Phylogenetic analyses were performed using the ARB software package (http://www.arb-home.de). The 16S rRNA gene phylogenetic analyses were performed by the maximum-likelihood method (39), using 1,285 to 1,392 nucleotide positions. The functional genes were translated into amino acid sequences, and these were included in phylogenetic analyses using the neighbor-joining method (39) (Dayhoff PAM model). Ninety-two positions for Fae and 130 positions for PmoA were used. The lists of sequences included in the phylogenetic analyses are available upon request.
Nucleotide sequence accession numbers.
The 16S rRNA gene, fae, and pmoA sequences obtained in this study were deposited in GenBank under accession numbers DQ066940 to DQ067043, DQ067044 to DQ067063, and DQ067064to DQ067087, respectively.
RESULTS
Diversity of 16S rRNA, pmoA, and fae genes in the Lake Washington sediment as assessed by RT-PCR.
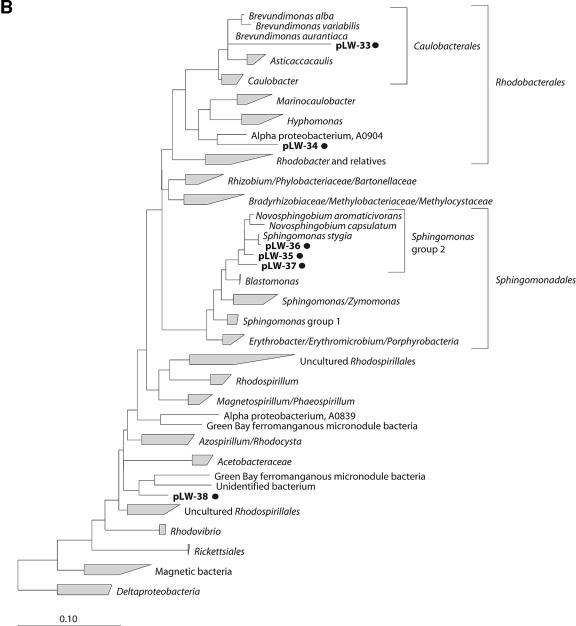
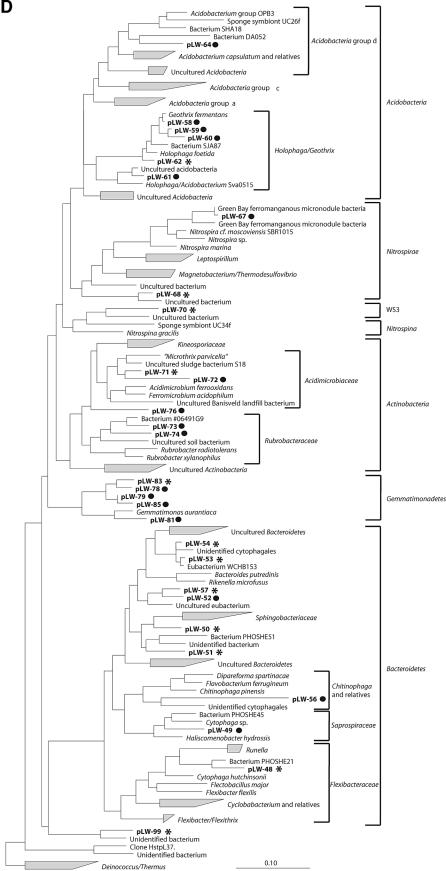
RNA isolated from the Lake Washington sediment was used as a template in RT-PCRs to amplify eubacterial 16S rRNA gene fragments, as well as fae and pmoA gene fragments. PCR products of the expected sizes (∼1.4 kb, ∼300 bp, and ∼500 bp, respectively) were obtained and cloned to generate specific gene libraries. Libraries of 151 clones containing 16S rRNA gene fragments, 98 clones containing fae gene fragments, and 85 clones containing pmoA gene fragments were generated, and these were each screened by RFLP. Based on RFLP patterns, the 151 clones of the 16S rRNA library were assigned to 91 OTUs, the 98 clones of the fae library were assigned to 8 OTUs, and the 85 clones of the pmoA library were assigned to 19 OTUs. Partial sequences were determined for each OTU identified in the 16S rRNA gene library, and these were submitted to the RDP Classifier using default parameters (confidence threshold, 80%) to determine phylogenetic affiliations of the respective bacteria. Of the 91 OTUs in the 16S rRNA gene library, 52 (83 clones) were assigned to the phylum Proteobacteria, 16 (27 clones) were assigned to the phylum Bacteroidetes, 2 (4 clones) were assigned to the phylum Nitrospira, 1 (3 clones) was assigned to the phylum Actinobacteria, and 1 (3 clones) was assigned to the candidate division WS3 (9). Nineteen OTUs (31 clones) were categorized as “unclassified bacteria” by the RDP Classifier. Nearly complete 16S rRNA gene sequences (∼1.3 to 1.4 kb) were determined for OTUs that contained at least two clones and for at least 10% of the OTUs that contained a single clone (a total of 50 sequences), and these were included into a similarity matrix to sort the sequences into phylotypes. A total of 40 phylotypes were identified (Table 1; Fig. 1). Of these, 22 were assigned to the phylum Proteobacteria (Fig. 1A, B, and C). These were further classified as follows: 9 phylotypes (29 clones) were identified as Betaproteobacteria and were assigned to uncultured organisms within the families Commamonadaceae, Oxalobacteraceae, Nitrosomonadaceae, and Methylophilaceae; 10 phylotypes (25 clones) were identified as Gammaproteobacteria, of which 6 phylotypes (16 clones) were related to methanotrophic bacteria of the genera Methylobacter and Methylomonas, while the remaining phylotypes were assigned to organisms belonging to the genera Achromatium and Thioploca/Beggiatoa and to uncultured Gammaproteobacteria; and 3 phylotypes (5 clones) were identified as Deltaproteobacteria and were related to uncultured organisms of the order Myxococcales and Syntrophus. Seven phylotypes (22 clones) were assigned to the phylum Bacteroidetes, within which some were related to uncultured organisms of the order Bacteroidales of the families of Sphingobacteriaceae, Flexibacteraceae, and Saprospiraceae (Table 1; Fig. 1D). The remaining phylotypes were assigned to uncultured representatives of the phyla Actinobacteria, Gemmatimonadetes, Acidobacteria, candidate division WS3, Verrumicrobium, and Spirochaetes (Table 1; Fig. 1D and E).
TABLE 1.
16S rRNA gene phylotype distribution in libraries obtained from total RNA isolated from Lake Washington sediment and from [13C]DNA and [12C]DNA fractions obtained from the microcosm experimentsa
| Phylogenetic group | Phylotype(s) | No. of clones in library
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RNA | Methanol
|
Methylamine
|
Formaldehyde
|
Formate
|
||||||
| [13C] DNA | [12C] DNA | [13C] DNA | [12C] DNA | [13C] DNA | [12C] DNA | [13C] DNA | [12C] DNA | |||
| Proteobacteria | ||||||||||
| Betaproteobacteria | ||||||||||
| Comamonadaceae | pLW-1, pLW-2, pLW-3, pLW-4 | 17 | 3 | 3 | 1 | |||||
| Oxalobacteraceae | pLW-5, pLW-6 | 2 | 1 | 2 | ||||||
| Nitrosomonadaceae | pLW-7, pLW-8, pLW-9, pLW-10 | 9 | ||||||||
| Methylophylaceae | pLW-11, pLW-12, pLW-13 | 1 | 22 | 10 | 43 | 3 | 18 | 9 | ||
| Unclassified Betaproteobacteria | pLW-16 | 1 | ||||||||
| Gammaproteobacteria | ||||||||||
| Methylobacter/Methylomonas | pLW-17, pLW-18, pLW-19, pLW-20, pLW-21, pLW-22 | 16 | ||||||||
| Pseudomonas | pLW-23, pLW-24 | 3 | 4 | 1 | 1 | |||||
| Achromatium | pLW-25 | 2 | ||||||||
| Xanthomonadaceae | pLW-26, pLW-27 | 1 | 5 | |||||||
| Thioploca/Beggiatoa | pLW-28, pLW-29 | 5 | ||||||||
| Unclassified Gammaproteobacteria | pLW-30, pLW-31, pLW-32 | 2 | 1 | 4 | ||||||
| Alphaproteobacteria | ||||||||||
| Caulobacterales | pLW-33 | 1 | ||||||||
| Rhodobacterales | pLW-34 | 1 | ||||||||
| Sphingomonadales | pLW-35, pLW-36, pLW-37 | 17 | 2 | |||||||
| Unclassified Alphaproteobacteria | pLW-38, pLW-39, pLW-40 | 1 | 2 | |||||||
| Deltaproteobacteria | ||||||||||
| Syntrophus | pLW-41 | 1 | ||||||||
| Polyangiaceae | pLW-42 | 1 | ||||||||
| Myxococcaceae | pLW-43 | 3 | ||||||||
| Geobacteraceae | pLW-44 | 1 | ||||||||
| Unclassified Deltaproteobacteria | pLW-45, pLW-46, pLW-47 | 3 | 3 | 1 | ||||||
| Bacteroidetes | ||||||||||
| Flexibacteraceae | pLW-48 | 2 | ||||||||
| Saprospiraceae | pLW-49 | 2 | ||||||||
| Sphingobacteriaceae | pLW-50, pLW-51 | 3 | ||||||||
| Unclassified Bacteroidetes | pLW-52, pLW-53, pLW-54, pLW-55, pLW-56, pLW-57 | 15 | 3 | 1 | 1 | |||||
| Acidobacteria | ||||||||||
| Holophaga/Geothrix | pLW-58, pLW-59, pLW-60, pLW-61, pLW-62, pLW-63 | 6 | 1 | 1 | 31 | 33 | 1 | |||
| Unclassified Acidobacteria | pLW-64, pLW-65 | 2 | 1 | |||||||
| Nitrospirae (unclassified) | pLW-66, pLW-67, pLW-68 | 2 | 1 | 4 | 1 | 2 | ||||
| WS3 | pLW-69, pLW-70 | 3 | 3 | |||||||
| Actinobacteria | ||||||||||
| Acidimicrobiaceae | pLW-71, pLW-72 | 3 | 1 | 1 | ||||||
| Rubrobacteraceae | pLW-73, pLW-74, pLW-75 | 2 | 2 | |||||||
| Unclassified Actinobacteria | pLW-76, pLW-77 | 1 | 1 | 1 | ||||||
| Gemmatimonadetes (Gemmatimonadaceae) | pLW-78, pLW-79, pLW-80, pLW-81, pLW-82, pLW-83, pLW-84, pLW-85, pLW-86, pLW-87 | 3 | 2 | 4 | 1 | 8 | 4 | |||
| Verrumicrobium (unclassified) | pLW-88, pLW-89 | 5 | ||||||||
| Planctomycetes (unclassified) | pLW-90, pLW-91 | 1 | 2 | |||||||
| Chlorobi (unclassified) | pLW-92, pLW-93 | 1 | 4 | |||||||
| Spirochaetes (Leptospiraceae) | pLW-94 | 1 | ||||||||
| Chloroflexi (unclassified) | pLW-95, pLW-96, pLW-97 | 1 | 1 | 3 | 3 | |||||
| Fibrobacteres (unclassified) | pLW-98 | 1 | ||||||||
| Unclassified Bacteria | pLW-99 | 3 | ||||||||
| pLW-100 | 3 | 1 | ||||||||
| pLW-101 | 2 | |||||||||
| pLW-102 | 2 | |||||||||
| pLW-103 | 1 | |||||||||
| pLW-104 | 2 | |||||||||
| pLW-105 | 1 | |||||||||
| pLW-106 | 1 | |||||||||
| Total | 107 | 42 | 26 | 43 | 14 | 67 | 66 | 26 | 28 | |
Classification is according to the Ribosomal Database Project-II (http://rdp.cme.msu.edu/classifier.jsp). Phylotypes were defined as sequences less than 97% similar (41). Unclassified phylotypes could not be assigned with a bootstrap confidence estimate above the default threshold value of the RDP Classifier (80%).
FIG.1.
Phylogenetic trees obtained by maximum-likelihood analysis, reflecting the relationships of 16S rRNA gene sequences amplified from RNA isolated from Lake Washington sediment (*) and from [13C]DNA fractions (•). Sequences determined in this study are in boldface. The scale bars indicate the number of expected nucleic acid substitutions per site per unit of branch length. (A) Beta- and Gammaproteobacteria phylogenetic tree. Alphaproteobacteria were used to root the tree; 1,389 characters were used to infer the tree. Partial sequences (<600 bp) were added to the tree by using a maximum-parsimony option within ARB; 433 characters were used. (B) Alphaproteobacteria phylogenetic tree. Deltaproteobacteria were used to root the tree; 1,365 characters were used to infer the tree. (C) Deltaproteobacteria phylogenetic tree; 1,392 characters were used to infer the tree. (D) Acidobacteria, Nitrospirae, Nitrospina, Actinobacteria, Gemmatimonadetes, and Bacteroidetes phylogenetic tree. Deionoccus and Thermus were used to root the tree; 1,287 characters were used to infer the tree. (E) Verrumicrobium, Planctomycetes, Chlorobi, Spirochaetes, and Chloroflexi phylogenetic tree. Deionoccus and Thermus were used to root the tree; 1,285 characters were used to infer the tree.
Representatives of the eight OTUs identified in the fae clone library were sequenced and grouped into five phylotypes (Table 2; Fig. 2). One phylotype (52 clones) was assigned to Methylosinus spp., two phylotypes (3 clones) were assigned to Methylobacterium spp., and the two remaining phylotypes (43 clones) grouped with the cluster formed by fae belonging to Gamma- and Betaproteobacteria but distantly related to cultivated methylotrophic bacteria. In addition to the general-use fae primers (18), we employed a set of fae primers targeting a divergent fae clone (L1N9) previously identified in Lake Washington (18). Specific PCR products were obtained, and the 10 sequences analyzed fell into two phylotypes.
TABLE 2.
Fae phylotype distribution in libraries obtained from total RNA isolated from Lake Washington sediment and from [13C]DNA fractions obtained from the microcosm experiments
| Phylogenetic group | Phylotypea | No. of clones in library
|
||||
|---|---|---|---|---|---|---|
| RNA | [13C]methanol | [13C]methylamine | [13C]formaldehyde | [13C]formate | ||
| Alphaproteobacteria | ||||||
| Methylosinus spp. | pLWFae-1 | 52 | 24 | 1 | ||
| Methylobacterium spp. | pLWFae-2 | 2 | ||||
| Methylobacterium spp. | pLWFae-3 | 1 | ||||
| Unknown Alphaproteobacteria | pLWFae-4 | 9 | ||||
| Unknown Alphaproteobacteria | pLWFae-5 | 12 | ||||
| Unknown Alphaproteobacteria | pLWFae-6 | 1 | ||||
| Beta/Gammaproteobacteria | ||||||
| Unknown Beta/Gammaproteobacteria | pLWFae-7 | 2 | ||||
| Unknown Beta/Gammaproteobacteria | pLWFae-8 | 41 | 1 | |||
| Rubrivivax related | pLWFae-9 | 1 | ||||
| Unknown Beta/Gammaproteobacteria | pLWFae-10 | 22 | ||||
| Rubrivivax related | pLWFae-11 | 14 | 3 | 20 | ||
| Rubrivivax related | pLWFae-12 | 17 | ||||
| Burkholderia related | pLWFae-13 | 4 | ||||
| Unknown Beta/Gammaproteobacteria | pLWFae-14 | 3 | ||||
| Unaffiliated groups | pLWFae-15 | 6 | ||||
| pLWFae-16 | 14 | |||||
| pLWFae-17 | —b | |||||
| pLWFae-18 | — | |||||
| pLWFae-19 | 9 | |||||
| pLWFae-20 | 1 | |||||
| Total | 98 | 31 | 47 | 41 | 43 | |
FIG. 2.
Phylogenetic tree reflecting the relationships of fae gene sequences amplified from RNA isolated from Lake Washington sediment (*) and from [13C]DNA fractions (•). The tree topology was obtained from inferred amino acid sequences (92 positions) by using neighbor-joining analysis. Sequences determined in this study are in boldface. The scale bar indicates the number of expected amino acid substitutions per site per unit of branch length.
The sequenced representatives of the pmoA clone library fell into 24 phylotypes (Fig. 3). Of these, 19 (64 clones) were closely related to the sequences of known methanotrophs belonging to Methylobacter spp., Methylomonas spp., and Methylomicrobium spp.; 1 phylotype (9 clones) was closely related to highly divergent pmoA sequences from uncharacterized alphaproteobacterial methanotrophs (36); and the 4 remaining phylotypes (12 clones) clustered with divergent sequences from unclassified, uncultured bacteria (Fig. 3).
FIG. 3.
Phylogenetic tree reflecting the relationships of pmoA gene sequences amplified from RNA isolated from Lake Washington sediment. The tree topology was obtained from inferred amino acid sequences (130 positions) by using neighbor-joining analysis. Sequences determined in this study are in boldface. The scale bar indicates the number of expected amino acid substitutions per site per unit of branch length.
Stable-isotope probing with 13C-labeled C1 compounds.
Preliminary experiments were conducted in which the model laboratory strains Methylomonas sp. strain LW13 and Methylobacillus flagellatus were grown in the presence of [13C]methane and [13C]methanol, respectively. Control cultures were grown on [12C]methane and [12C]methanol, respectively. DNA was isolated from cultures, and [13C]DNA was mixed with unlabeled DNA from the respective strains and subjected to ultracentrifugation as described in Materials and Methods. [13C]DNA was separated from [12C]DNA by approximately 1 cm (data not shown), as previously observed for DNAs isolated from pure cultures of methylotrophs or from natural communities exposed to 13C1-labeled substrates (37). DNA was then isolated from microcosms incubated in the presence of [13C]methanol, [13C]methylamine, [13C]formaldehyde, or [13C]formate, as described in Materials and Methods, and subjected to ultracentrifugation. Distinct [13C]DNA fractions were detected in methanol and methylamine microcosms after 2 to 4 days of incubation, while only faint [13C]DNA bands were observed in DNA isolated from formaldehyde and formate microcosms after 7 to 10 days of incubation (data not shown). The labeled and unlabeled DNA fractions were separated by approximately 1 cm, as observed in our experiments with pure cultures and as described by Radajewski et al. (37). [13C]DNA and [12C]DNA from the methanol and methylamine microcosms incubated for 4 days and from the formaldehyde and formate microcosms incubated for 10 days were purified and used as templates in PCR amplifications.
Diversity of bacterial 16S rRNA in [13C]DNA versus [12C]DNA fractions isolated from microcosms.
Two 16S rRNA gene libraries were constructed from each microcosm, using, respectively, the [13C]DNA fraction and the [12C]DNA fraction as templates for PCR. The libraries were analyzed by RFLP and categorized into OTUs as described above. The 45 clones of the library originating from the [13C]DNA fraction of the methanol microcosm were assigned to 10 OTUs, 3 of which were represented by 32 clones. The 43 clones of the library originating from the [13C]DNA fraction of the methylamine microcosm were assigned to four OTUs, of which two were represented by 41 clones. The 88 clones of the library originating from the [13C]DNA fraction of the formaldehyde microcosm were assigned to 31 OTUs, of which 4 were represented by 52 clones. The 41 clones of the library originating from the [13C]DNA fraction of the formate microcosm were assigned to 29 OTUs. Coverage values were calculated as described in Materials and Methods. For methanol and methylamine microcosm libraries the values were above 90%, indicating that the sampling effort was likely sufficient to describe the 16S rRNA gene diversity represented in these libraries. Lower coverage values were obtained for formaldehyde and formate microcosms (50% and 75%, respectively), indicating that these libraries were only partially sampled.
The 16S rRNA gene diversity in the libraries originating from [13C]DNA was compared to the diversity in libraries originating from the [12C]DNA microcosm fractions. The 48 clones of the library originating from the [12C]DNA fraction of the methanol microcosm were assigned to 31 OTUs, the 45 clones of the library originating from the [12C]DNA fraction of the methylamine microcosm were assigned to 38 OTUs, the 94 clones of the library originating from the [12C]DNA fraction of the formaldehyde microcosm were assigned to 43 OTUs, and the 46 clones of the library originating from the [12C]DNA fraction of the formate microcosm were assigned to 30 OTUs. The coverage values calculated for these libraries (29% to 69%) were lower than the values for the [13C]DNA-based libraries, reflecting a higher diversity of the 16S rRNA gene in these libraries than in the [13C]DNA-derived libraries.
Partial sequences were obtained for OTUs in each library containing at least two clones and for 10% of the OTUs containing a single clone, and these were included into a similarity matrix in order to categorize the sequences into phylotypes. Of the 90 OTUs sequenced, a total of 68 phylotypes were identified (Table 1). The phylogenetic analysis of the sequences obtained in [13C]DNA fractions revealed that these phylotypes were associated with diverse bacterial lineages, mainly in the phyla Proteobacteria, Bacteroidetes, Gemmatimonadetes, Acidobacteria, and Actinobacteria (Table 1; Fig. 1A, B, C, D, and E).
Phylotypes that were more represented in the libraries originating from [13C]DNA fractions than in the libraries originating from [12C]DNA fractions were of special interest for this study, as enrichment in [13C]DNA in the respective organisms could be indicative of active metabolism of C1 compounds. Two phylotypes following this pattern were closely related to the sequences of known methylotrophic species belonging to the family Methylophilaceae, and these were enriched in the methanol, methylamine, and formaldehyde microcosms (Table 1). However, other phylotypes following a similar pattern were not related to any known methylotrophs (Table 1). Phylotypes related to Novosphingobium were enriched in the methanol microcosm, phylotypes related to the family Gemmatimonadaceae were enriched in the formaldehyde and formate microcosms, and a phylotype related to Xanthomonanadaceae was enriched in the formate microcosm.
Diversity of fae in [13C]DNA fractions isolated from microcosms.
A single fae clone library was constructed for each microcosm, using the [13C]DNA fraction as a template. The libraries were analyzed by RFLP and categorized into OTUs as described above (Table 2). The 31 clones of the library originating from the methanol microcosm were assigned to seven OTUs, of which three contained 27 clones. The 47 clones of the library originating from the methylamine microcosm were assigned to six OTUs, of which three contained 44 clones. The 41 clones of the library originating from the formaldehyde microcosm were assigned to seven OTUs, of which two contained 30 clones. The 43 clones of the library originating from the formate microcosm were assigned to five OTUs, of which two contained 32 clones. Representatives of all the OTUs identified were sequenced and grouped into a total of 15 phylotypes (Table 2; Fig. 3). Of the three phylotypes identified in the methanol microcosm, one was associated with Methylosinus sequences, the second was closely related to the Rubrivivax sequences, and the third clustered with the sequences with no cultivated representatives (18). The methylamine microcosm also revealed the presence of fae sequences belonging to Methylosinus spp. and sequences related to the Rubrivivax-like sequences, with the rest of the phylotypes affiliated with the unknown organisms belonging to the Alpha- and Beta-/Gammaproteobacteria. Four (27 clones) of the five phylotypes identified in the formaldehyde microcosm were related to the sequences clustering with those of Beta-/Gammaproteobacteria (related to both Rubrivivax and Burkholderia), while the fifth phylotype (14 clones) clustered with sequences previously identified in Lake Washington that are not related to the sequences of Proteobacteria, Planctomycetes, or archaeal homologs of fae (18). The phylotypes identified in the formate microcosm were related to those of unidentified Alphaproteobacteria and Beta-/Gammaproteobacteria (Rubrivivax relatives) and to unaffiliated sequences.
DISCUSSION
The methanotroph population from Lake Washington has been characterized in detail (2, 6, 7). Phospholipid analysis and direct quantitative hybridization experiments all pointed toward the dominance of methanotrophs in the top layer of the sediment, accounting for at least 10% of the total bacterial community, with type I methanotrophs numerically exceeding type II methanotrophs by about one order of magnitude (6). A number of methanotrophs, belonging to the Alpha- and Gammaproteobacteria and representing the well-characterized genera of Methylosinus, Methylocystis, Methylomonas, Methylobacter, and Methylosarcina, have been isolated from this environment (2, 20). In addition, sequences related to Methylomicrobium and Methylococcus have been detected via culture-independent approaches (7).
Much less is known about bacterial C1 metabolism in this site that involves substrates less reduced than methane. However, recent tests employing primers targeting one of the most ubiquitous C1 oxidation pathways, the H4MPT-linked formaldehyde oxidation pathway, revealed the presence of a diverse population of microbes capable of C1 oxidation (18, 19). Further experiments involving incubations of Lake Washington sediment communities in the presence of various C1 substrates have resulted in the enrichment of a number of novel phylotypes, pointing toward the presence of yet-unidentified methylotrophs in Lake Washington (19). In this work, we continued to characterize the active methylotroph population in the Lake Washington sediment via two complementary culture-independent approaches, analysis of rRNA and mRNA by using the RT-PCR-based technique and SIP with a variety of C1 substrates, with emphasis on non-methane utilizers. RT-PCR analysis of both rRNA and mRNA has been previously employed to characterize active microbial populations in a number of environments, including methanotroph populations (10, 15, 32, 35, 45). SIP has been previously successfully applied to detect active microbial populations involved in aerobic methane and methanol oxidation, aerobic aromatic compound oxidation, or anaerobic propionate oxidation in environments such as soil (36, 38), rice field (27), bacterial mat (16), contaminated aquifer (17) and soda lake (26). Here we extended both the range of environments and the range of the C1 substrates used in SIP experiments.
Analysis of 16S rRNA isolated directly from the Lake Washington sediment revealed a high diversity of microorganisms present. The majority of the sequences recovered were affiliated with the Beta-, Delta-, and Gammaproteobacteria, phylogenetic groups that include organisms of very diverse phenotypes, lifestyles, and trophic capabilities (22). In addition, a number of the sequences were associated with phylogenetic groups lacking or with very few cultured representatives (e.g., Bacteroidetes, Actinobacteria, Acidobacteria, Gemmatimonadetes, and candidate division WS3). The presence of such a variety of microorganisms in the sample may reflect the wide range of environmental conditions and metabolic activities that take place in the top layer of Lake Washington sediment, one of these being methane oxidation. The data obtained in this work support the previous observations on the dominant role of the gammaproteobacterial methanotrophs, based on rRNA and functional gene mRNA (pmoA) detection. Another functional gene employed in this study, fae, was used to identify active populations capable of C1 oxidation downstream of formaldehyde. Most of the fae sequences uncovered were affiliated with those of Methylosinus spp. or belonged to uncultivated organisms of the Beta- and Gammaproteobacteria, indicating that the latter may also contribute to the cycling of C1 compounds in Lake Washington. The lack of fae sequences related to known methanotrophs of the Gammaproteobacteria (Methylomonas spp. and Methylobacter spp.) from PCR-amplified clone libraries has been reported before and has been attributed to the toxic effect of this gene on Escherichia coli (18), underscoring the importance of using multiple culture-independent methods for assessing functional diversity in natural habitats.
In a previously conducted inventory of fae genes in Lake Washington, based on PCR amplification from environmental DNA, many of the sequences fell within a novel fae cluster and diverged significantly from known fae or fae homologs characterized for Proteobacteria, Planctomycetes, or Archaea (18). The phylogenetic identities of the respective microbes, their lifestyles, and their roles in elemental cycling in the site remain unknown. In this study, transcripts of the sequences related to this novel fae cluster were identified (phylotypes pLWFae-18 and pLWFae-19), suggesting that these divergent fae genes are expressed in Lake Washington and suggesting a role for this unknown group in C1 cycling.
While the analysis of fae transcripts has revealed the presence of active organisms other than methanotrophs that are capable of C1 metabolism, the range of C1 substrates potentially used by these organisms remained largely unknown. We used the stable-isotope probing technique in order to link the identity of these organisms to their substrate specificity and thus to their potential function. The analysis of 16S rRNA gene libraries implied that Methylophylaceae may be involved not only in utilization of methanol and methylamine but also in the utilization of formaldehyde in the environment. This approach also allowed us to identify novel phyla potentially involved in the use of C1 compounds. Our data suggest that Alphaproteobacteria of the genus Novosphingobium are candidates for methanol utilization, Gemmatimonadaceae are candidates for utilization of both formaldehyde and formate, and Xanthomonadaceae are candidates for utilization of formate. These results now suggest experiments focused on isolation and taxonomic characterization of the candidate organisms to test these predictions. The fae libraries also provided evidence suggesting that Methylosinus may be involved not only in the oxidation of methane but also in methanol and formaldehyde oxidation. In addition, many of the sequences in the fae libraries belonged to unidentified species, suggesting that novel groups of bacteria are involved in C1 cycling in this habitat. As the SIP technique has certain inherent biases, for example, toward fast-growing organisms, these results should be used only as hints for future experiments uncovering the potential roles of these phyla in C1 metabolism in Lake Washington sediment.
In conclusion, we here describe the use of a combination of two culture-independent molecular techniques (RT-PCR and SIP of DNA) to identify active bacterial populations involved in utilization of C1 compounds in the sediment of Lake Washington, a freshwater lake. The diversity of 16S rRNA in our library reflected the high level of complexity of the active microbial community in the sediment. Using functional genes that are signatures of methylotrophy, pmoA and fae, we identified genes from known methanotrophs and methylotrophs such as Methylobacter, Methylomonas, Methylosinus, Methylobacterium and also novel sequences affiliated with uncultivated organisms of the Alpha-, Beta-, and Gammaproteobacteria, as well as divergent lineages possibly representing novel phyla. Using SIP, we were able to identify a few known methylotroph species as being active in utilization of methanol, methyl- amine, formaldehyde, and formate. However, most of the sequences obtained were not affiliated with any cultivated organisms. The combined results of these two approaches suggest that many bacteria involved in C1 cycling in the site remain uncultivated and uncharacterized. These data highlight the existing gaps in the understanding of C1 cycling, especially downstream of methane, in Lake Washington and likely globally and form the basis for culture-dependent investigations to identify these new groups of bacteria.
Acknowledgments
This work was funded by the National Science Foundation's Microbial Observatories program.
We are grateful to the crew of R/V Clifford Barnes and S. Stolyar for help with sample acquisition, to D. Stahl for sharing a UNIX server, and to G. Jacobson for technical assistance. [13C]methanol was provided by the National Stable Isotope Resource at Los Alamos National Laboratory.
REFERENCES
- 1.Anthony, C. 1982. The biochemistry of methylotrophs. Academic Press, New York, N.Y.
- 2.Auman, A. J., S. Stolyar, A. M. Costello, and M. E. Lidstrom. 2000. Molecular characterization of methanotrophic isolates from freshwater lake sediment. Appl. Environ. Microbiol. 66:5259-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodrossy, L., N. Stralis-Pavese, J. C. Murrell, S. Radajewski, A. Weilharter, and A. Sessitsch. 2003. Development and validation of a diagnostic microbial microarray for methanotrophs. Environ. Microbiol. 5:566-582. [DOI] [PubMed] [Google Scholar]
- 4.Chistoserdova, L., C. Jenkins, M. G. Kalyuzhnaya, C. J. Marx, A. Lapidus, J. A. Vorholt, J. T. Staley, and M. E. Lidstrom. 2004. The enigmatic planctomycetes may hold a key to the origins of methanogenesis and methylotrophy. Mol. Biol. Evol. 21:1234-1241. [DOI] [PubMed] [Google Scholar]
- 5.Chistoserdova, L., J. A. Vorholt, R. K. Thauer, and M. E. Lidstrom. 1998. C1 transfer enzymes and coenzymes linking methylotrophic bacteria and methanogenic Archaea. Science 281:99-102. [DOI] [PubMed] [Google Scholar]
- 6.Costello, A. M., A. J. Auman, J. L. Macalady, K. M. Scow, and M. E. Lidstrom. 2002. Estimation of methanotroph abundance in a freshwater lake sediment. Environ. Microbiol. 4:443-450. [DOI] [PubMed] [Google Scholar]
- 7.Costello, A. M., and M. E. Lidstrom. 1999. Molecular characterization of functional and phylogenetic genes from natural populations of methanotrophs in lake sediments. Appl. Environ. Microbiol. 65:5066-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dedysh, S. N., W. Liesack, V. N. Khmelenina, N. E. Suzina, Y. A. Trotsenko, J. D. Semrau, A. M. Bares, N. S. Panikov, and J. M. Tiedje. 2000. Methylocella palustris gen. nov., sp. nov., a new methane-oxidizing acidophilic bacterium from peat bogs, representing a novel subtype of serine-pathway methanotrophs. Int. J. Syst. Evol. Microbiol. 50:955-969. [DOI] [PubMed] [Google Scholar]
- 9.Dojka, M. A., P. Hugenholtz, S. K. Haack, and N. R. Pace. 1998. Microbial diversity in a hydrocarbon- and chlorinated-solvent-contaminated aquifer undergoing intrinsic bioremediation. Appl. Environ. Microbiol. 64:3869-3877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felske, A., H. Rheims, A. Wolterink, E. Stackebrandt, and A. Akkermans. 1997. Ribosome analysis reveals prominent activity of an uncultured member of the class Actinobacteria in grassland soils. Microbiology 143:2983-2989. [DOI] [PubMed] [Google Scholar]
- 11.Ginige, M. P., P. Hugenholtz, H. Daims, M. Wagner, J. Keller, and L. L. Blackall. 2004. Use of stable-isotope probing, full-cycle rRNA analysis, and fluorescence in situ hybridization-microautoradiography to study a methanol-fed denitrifying microbial community. Appl. Environ. Microbiol. 70:588-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glöckner, F. O., M. Kube, M. Bauer, H. Teeling, T. Lombardot, W. Ludwig, D. Gade, A. Beck, K. Borzym, K. Heitmann, R. Rabus, H. Schlesner, R. Amann, and R. Reinhardt. 2003. Complete genome sequence of the marine planctomycete Pirellula sp. strain 1. Proc. Natl. Acad. Sci. USA 100:8298-8303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Good, I. J. 1953. The population frequencies of species and the estimation of population parameters. Biometrika 40:237-262. [Google Scholar]
- 14.Griffiths, R. I., A. S. Whiteley, A. G. O'Donnell, and M. J. Bailey. 2000. Rapid method for coextraction of DNA and RNA from natural environments for analysis of ribosomal DNA- and rRNA-based microbial community composition. Appl. Environ. Microbiol. 66:5488-5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horz, H. P., M. T. Yimga, and W. Liesack. 2001. Detection of methanotroph diversity on roots of submerged rice plants by molecular retrieval of pmoA, mmoX, mxaF, and 16S rRNA and ribosomal DNA, including pmoA-based terminal restriction fragment length polymorphism profiling. Appl. Environ. Microbiol. 67:4177-4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutchens, E., S. Radajewski, M. G. Dumont, I. R. McDonald, and J. C. Murrell. 2004. Analysis of methanotrophic bacteria in Movile Cave by stable isotope probing. Environ. Microbiol. 6:111-120. [DOI] [PubMed] [Google Scholar]
- 17.Jeon, C. O., W. Park, P. Padmanabhan, C. DeRito, J. R. Snape, and E. L. Madsen. 2003. Discovery of a bacterium, with distinctive dioxygenase, that is responsible for in situ biodegradation in contaminated sediment. Proc. Natl. Acad. Sci. USA 100:13591-13596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalyuzhnaya, M. G., M. Lidstrom, and L. Chistoserdova. 2004. Utility of environmental primers targeting ancient enzymes: methylotroph detection in Lake Washington. Microb. Ecol. 48:463-472. [DOI] [PubMed] [Google Scholar]
- 19.Kalyuzhnaya, M. G., O. G. Nercessian, M. E. Lidstrom, and L. Chistoserdova. 2005. Development and application of PCR primers based on fhcD for environmental detection of methanopterin-linked C1-metabolism in bacteria. Environ. Microbiol. 7:1269-1274. [DOI] [PubMed] [Google Scholar]
- 20.Kaluzhnaya, M. G., S. M. Stolyar, A. J. Auman, J. C. Lara, M. E. Lidstrom, and L. Chistoserdova. Methylosarcina lacus sp. nov., a methanotroph from Lake Washington, Seattle, USA. Int. J. Syst. Evol. Microbiol., in press. [DOI] [PubMed]
- 21.Kelly, D. P., and J. C. Murrell. 1999. Microbial metabolism of methanesulfonic acid. Arch. Microbiol. 172:341-348. [DOI] [PubMed] [Google Scholar]
- 22.Kersters, K., P. De Vos, M. Gillis, J. Swings, P. Vandamme, and E. Stackebrandt. 2003. Introduction to the Proteobacteria. In M. Dworkin et al. (ed.), The prokaryotes: an evolving electronic resource for the microbiological community, 3rd ed., release 3.12, 28 March 2003. [Online.] Springer-Verlag, New York, N.Y. http://link.springer-ny.com/service/books/10125.
- 23.Konstantinidis, K. T., and Tiedje, J. M. 2005. Genomic insights that advance the species definition for prokaryotes. Proc. Natl. Acad. Sci. USA 102:2567-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lidstrom, M. E. 2001. The aerobic methylotrophic bacteria. In M. Dworkin (ed.), The prokaryotes: an evolving electronic resource for the microbiological community, 3rd ed., release 3.7, 2 November 2001. [Online]. Springer-Verlag, New York, N.Y. http://link.springer-ny.com/service/books/10125.
- 25.Lidstrom, M. E., and L. Somers. 1984. Seasonal study of methane oxidation in Lake Washington. Appl. Environ. Microbiol. 47:1255-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin, J. L., S. Radajewski, B. T. Eshinimaev, Y. A. Trotsenko, I. R. McDonald, and J. C. Murrell. 2004. Molecular diversity of methanotrophs in Transbaikal soda lake sediments and identification of potentially active populations by stable isotope probing. Environ. Microbiol. 6:1049-1060. [DOI] [PubMed] [Google Scholar]
- 27.Lueders, T., B. Pommerenke, and M. W. Friedrich. 2004. Stable-isotope probing of microorganisms thriving at thermodynamic limits: syntrophic propionate oxidation in flooded soil. Appl. Environ. Microbiol. 70:5778-5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manefield, M., A. S. Whiteley, R. I. Griffiths, and M. J. Bailey. 2002. RNA isotope probing, a novel means of linking microbial community function to phylogeny. Appl. Environ. Microbiol. 68:5367-5373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marx, C. J., J. A. Miller, L. Chistoserdova, and M. E. Lidstrom. 2004. Multiple formaldehyde oxidation/detoxification pathways in Burkholderia fungorum LB400. J. Bacteriol. 186:2173-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McDonald, I. R., and J. C. Murrell. 1997. The particulate methane monooxygenase gene pmoA andits use as a functional gene probe for methanotrophs. FEMS Microbiol. Lett. 156:205-210. [DOI] [PubMed] [Google Scholar]
- 31.McDonald, I. R., K. L. Warner, C. McAnulla, C. A. Woodall, R. S. Oremland, and J. C. Murrell. 2002. A review of bacterial methyl halide degradation: biochemistry, genetics and molecular ecology. Environ. Microbiol. 4:193-203. [DOI] [PubMed] [Google Scholar]
- 32.Miskin, I. P., P. Farrimond, and I. M. Head. 1999. Identification of novel bacterial lineages as active members of microbial populations in a freshwater sediment using a rapid RNA extraction procedure and RT-PCR. Microbiology 145:1977-1987. [DOI] [PubMed] [Google Scholar]
- 33.Nercessian, O., Y. Fouquet, C. Pierre, D. Prieur, and C. Jeanthon. 2005. Diversity of Bacteria and Archaea associated with carbonate-rich metalliferous sediments at the Rainbow vent field on the Mid-Atlantic Ridge. Environ. Microbiol. 7:698-714. [DOI] [PubMed] [Google Scholar]
- 34.Nercessian, O., A.-L. Reysenbach, D. Prieur, and C. Jeanthon. 2003. Archaeal diversity associated with in situ samplers deployed on hydrothermal vents on the East Pacific Rise (13N). Environ. Microbiol. 5:492-502. [DOI] [PubMed] [Google Scholar]
- 35.Nogales, B., K. N. Timmis, D. B. Nedwell, and A. M. Osborn. 2002. Detection and diversity of expressed denitrification genes in estuarine sediments after reverse transcription-PCR amplification from mRNA. Appl. Environ. Microbiol. 68:5017-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pacheco-Oliver, M., I. R. McDonald, D. Groleau, J. C. Murrell, and C. B. Miguez. 2002. Detection of methanotrophs with highly divergent pmoA genes from Arctic soils. FEMS Microbiol. Lett. 209:313-319. [DOI] [PubMed] [Google Scholar]
- 37.Radajewski, S., P. Ineson, N. R. Parekh, and J. C. Murrell. 2000. Stable-isotope probing as a tool in microbial ecology. Nature 403:646-649. [DOI] [PubMed] [Google Scholar]
- 38.Radajewski, S., G. Webster, D. S. Reay, S. Morris, P. Ineson, D. B. Nedwell, J. I. Prosser, and J. C. Murrell. 2002. Identification of active methylotroph populations in an acidic forest soil by stable-isotope probing. Microbiology 148:2331-2342. [DOI] [PubMed] [Google Scholar]
- 39.Saitou, N., and M. Nei. 1987. The neighbour joining method: a new tool for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 40.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 41.Stackebrandt, E., and B. M. Goebel. 1994. A place for DNA-DNA reassociation and 16S rRNA sequence analysis in the present species definition in bacteriology. Int. J. Syst. Bacteriol. 44:846-849. [Google Scholar]
- 42.Venter, J. C., K. Remington, J. F. Heidelberg, A. L. Halpern, D. Rusch, J. A. Eisen, D. Wu, I. Paulsen, K. E. Nelson, W. C. Nelson, D. E. Fouts, S. Levy, A. H. Knap, M. l. W. Lomas, K. Nealson, O. White, J. Peterson, J. Hoffman, R. Parsons, H. Baden-Tillson, C. Pfannkoch, Y.-H. Rogers, and H. O. Smith. 2004. Environmental genome shotgun sequencing of the Sargasso Sea. Science 304:66-74. [DOI] [PubMed] [Google Scholar]
- 43.Vorholt, J. A., L. Chistoserdova, S. M. Stolyar, R. K. Thauer, and M. E. Lidstrom. 1999. Distribution of tetrahydromethanopterin-dependent enzymes in methylotrophic bacteria and phylogeny of methenyl tetrahydromethanopterin cyclohydrolases. J. Bacteriol. 181:5750-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wackett, L. P. 2003. Aerobic methylotrophs and methanotrophs. An annoted selection of World Wide Web sites relevant to the topics in environmental microbiology. Environ. Microbiol. 5:217-218. [DOI] [PubMed] [Google Scholar]
- 45.Zani, S., M. T. Mellon, J. L. Collier, and J. P. Zehr. 2000. Expression of nifH genes in natural microbial assemblages in Lake George, New York, detected by reverse transcriptase PCR. Appl. Environ. Microbiol. 66:3119-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]