Abstract
Although the relationships between trophic conditions and viral dynamics have been widely explored in different pelagic environments, there have been few attempts at independent estimates of both viral production and decay. In this study, we investigated factors controlling the balance between viral production and decay along a trophic gradient in the north Adriatic basin, providing independent estimates of these variables and determining the relative importance of nanoflagellate grazing and viral life strategies. Increasing trophic conditions induced an increase of bacterioplankton growth rates and of the burst sizes. As a result, eutrophic waters displayed highest rates of viral production, which considerably exceeded observed rates of viral decay (up to 2.9 × 109 VLP liter−1 h−1). Viral decay was also higher in eutrophic waters, where it accounted for ca. 40% of viral production, and dropped significantly to 1.3 to 10.7% in oligotrophic waters. These results suggest that viral production and decay rates may not necessarily be balanced in the short term, resulting in a net increase of viruses in the system. In eutrophic waters nanoflagellate grazing, dissolved-colloidal substances, and lysogenic infection were responsible together for the removal of ca. 66% of viral production versus 17% in oligotrophic waters. Our results suggest that different causative agents are primarily responsible for the removal of viruses from the water column in different trophic conditions. Factors other than those considered in the past might shed light on processes responsible for the removal and/or decay of viral particles from the water column.
Viruses have been recognized as responsible for a relevant fraction of bacterial mortality (see references 5, 36, and 41 and references therein). Viral lysis, by causing the release of new viruses and host cell contents, can lead to a significant increase of dissolved organic carbon (DOC) in the environment, which in turn can affect bacterial community structure (30) and have a large impact on bacterial carbon cycling (17, 24).
Virus-induced bacterial mortality has been shown to be strongly dependent on local trophic conditions (20, 33, 34). Several authors have found that eutrophic environments support a higher standing stock of bacteria and consequently of bacteriophages than oligotrophic systems. Trophic conditions can influence the production of new viral particles by changing the metabolism and size of the host cells (3, 9, 23).
Since the maintenance of viral assemblages and the host-virus relationships are controlled by the decay and replenishment (production) of viral particles, estimates of viral turnover times are crucial to evaluate the potential of viruses to change in space and time (20). In order to test the steady-state assumption, often utilized as a basic assumption in viral ecology studies (4), a correct balance of viral production versus viral decay rates has to be evaluated. A correct analysis of viral dynamics should require an independent measurement of both viral production and decay rates. This, together with the analysis of viral life strategies, can provide a complete view of the actual fate of virioplankton, parameters, and factors influencing the removal of viral particles.
The fate of viral production can be greatly influenced by the presence of heterotrophic nanoflagellates, which can graze upon viruses exerting a direct control or can act indirectly through grazing on infected bacteria (2, 7, 34). The relative importance of these processes may vary depending on the environmental conditions (8, 27, 31, 34).
In order to test the effect of different trophic conditions upon viral dynamics and to test whether the steady-state assumption reflects the in situ conditions under different ecological constraints, we carried out independent measurements of viral production and decay rates along a trophic gradient of the north Adriatic basin. The relative importance of nanoflagellates grazing in controlling bacterial and indirectly viral production was also investigated, together with virus life strategies (lysogenic infections). In addition, we attempted to estimate the relative importance of factors responsible for viral decay in different environmental conditions.
MATERIALS AND METHODS
Study site.
Sampling was carried out in the north basin of the Adriatic Sea. Due to shallow water depth (on average ca. 35 m for the entire north basin) and the presence of the Po River outflows (1,450 m3 s−1) (13), the north Adriatic Sea typically displays a decreasing trophic gradient moving southward. This gradient is associated with an increase in surface salinity (1, 3) and a clear spatial pattern of key variables such as DOC, DON, DOP, and chlorophyll a. DOC concentrations ranged from 140 to 172 μM in the northern area, from 97 to 112 μM in the mesotrophic region, and from 81 to 92 μM in the southern station. DON and DOP concentrations followed a similar pattern. DON concentrations ranged from 7.8 to 8.3 μM in the northern region to 1.2 to 2.5 μM in the southernmost station, whereas DOP concentrations ranged from 0.2 to 0.3 μM in eutrophic waters to 0.05 to 0.1 μM in oligotrophic waters. Analyses of seasonal changes in chlorophyll a concentrations provided further evidence of such a gradient: chlorophyll a concentrations ranged from 0.2 to 12.0 μg liter−1 at the northern station, from 0.1 to 4.0 μg liter−1 at the intermediate station, and from 0.1 to 0.4 μg liter−1 at the southern station. Nutrient concentrations (DIN and PO4−) followed a similar gradient: DIN ranged from lows of 0.8 to 2.5 to highs of 0.9 to 50 μmol liter−1, whereas phosphates ranged from lows of 0.05 to 0.25 to highs of 0.1 to 1.3 μmol liter−1 in the southern and northern areas, respectively. Also, heavy metal content, typically very low, and distribution are controlled by the amount of suspended solids (1, 3, 25).
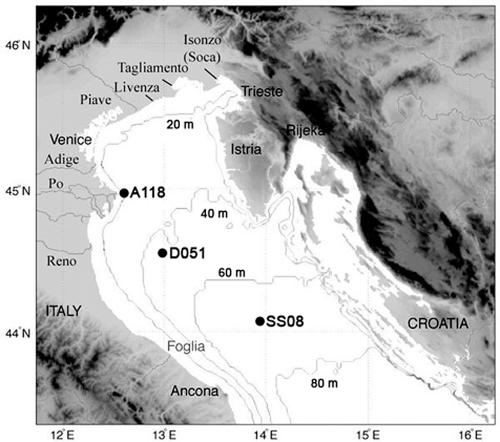
Sampling was carried out from 28 April to 3 May 2003 on board the R/V Alliance. Water samples were collected at three stations along a southeast to northeast transect (SS08, D051, and A118; Fig. 1, Table 1). At each station, samples were collected from the surface (∼2-m depth) by using a carousel water sampler carrying 12 Niskin bottles (12 liters). Additional water samples were collected at station SS08 (64-m depth) at the sediment-water interface. A CTD Sea Bird Electronics SBE 911 was used to obtain values of dissolved oxygen, temperature, salinity, transmittance, fluorescence, and turbidity at all stations (Table 1).
FIG. 1.
Sampling area and stations location along the transect in the north Adriatic basin (Mediterranean Sea).
TABLE 1.
Environmental features and hydrological variables in the four sampling stations along a transect in the northern basin of the Adriatic Sea
| Station | Latitude (N) | Longitude (E) | Sampling depth (m) | Bottom depth (m) | Temp (°C) | Salinity (‰) | Fluorescence (AU)a | Oxygen (ml liter−1) | Transmittance (%) | Turbidity (NFU)b |
|---|---|---|---|---|---|---|---|---|---|---|
| A118 | 44°96 | 12°59 | 2.0 | 20.7 | 13.08 | 36.04 | 0.62 | 6.64 | 67.15 | 0.93 |
| D051 | 44°61 | 12°84 | 2.9 | 38 | 15.61 | 35.50 | 0.32 | 6.00 | 73.28 | 0.69 |
| SS08 | 44°25 | 13°90 | 1.4 | 64.5 | 14.72 | 38.50 | 0.13 | 5.87 | 88.80 | 0.14 |
| SS08 | 44°25 | 13°90 | 64.5 | 64.5 | 12.20 | 38.52 | 0.04 | 5.90 | 80.70 | 0.45 |
AU, autofluorescence unit.
NFU, nephelometric formazine unit.
Virus-like particles (VLP) and bacterial direct counts.
Bacterial and viral counts were carried out as described by Noble and Fuhrman (19) with some modifications. In order to avoid underestimation of VLP counts determined by samples fixed with formalin (35; data not shown), all seawater samples were immediately processed without any fixative. After collection, subsamples (200 μl) were diluted 1:10 in prefiltered MilliQ, filtered through a 0.02-μm-pore-size filter (Anodisc; diameter, 25 mm; Al2O3) and immediately stained with 20 μl of SYBR Green I (stock solution diluted 1:20). Filters were incubated in the dark for 15 min and mounted on glass slides with a drop of 50% phosphate buffer (6.7 mM, pH 7.8) and 50% glycerol containing 0.5% ascorbic acid. When counts were not carried out immediately, the slides were stored at −20°C until analysis. Viral and bacterial counts were obtained by epifluorescence microscopy (Zeiss Axioplan; magnification, ×1,000) by examining at least 10 fields, i.e., at least 200 cells or particles per replicate. Bacterial biovolume was estimated by assigning each fluorescent bacterial cell to a dimensional size class on the basis of cell length and shape. Bacterial biovolume was then converted to biomass by assuming an organic carbon content of 310 fg μm−3 (6). Recognition of the bacterial active fraction is based on the presence of a visible nucleoid region (42). To enumerate the number of nucleoid-containing cells (NuCC), we applied a staining/destaining procedure that was adapted for sediment samples by Luna et al. (12). After the living/dead counting (as described above), the filters were placed in a filtration setup and rinsed with 10 ml of 2-propanol over 10 min (to remove excess stain). The NuCC counting was carried out under epifluorescence microscopy (magnification, ×1,000; Zeiss Axioskop 2), where the nucleoid of living bacteria appeared green due to the excitation of the SYBR Green I dye with which the cells were stained.
Bacterial carbon production.
Bacterial carbon production (BCP) was determined by [3H]leucine incorporation (28). Triplicate subsamples and two blanks (1.7 ml) were added with tritiated leucine (final concentration, 20 nM) and incubated for 1 h in the dark. Incubation was stopped with 100% trichloroacetic acid (TCA; final concentration, 5%) and stored at 4°C. Pellets were washed with 5% TCA and 80% ethanol and supplemented with 1 ml of liquid scintillation cocktail (Ultima Gold MV; Packard). The incorporated radioactivity was measured by determining the counts per minute with a liquid scintillation counter (Packard Tri-Carb 300).
Burst size.
The burst size (number of virus released per cell due to viral lysis) was estimated from time course experiments of viral production in which we performed counting of the active bacterial fraction (NuCC) according to the method of Mei and Danovaro (15). Measured NuCC decrease was assumed to represent the fraction of bacteria lysed by viral infection. Effective decrease of NuCC was calculated as differences between theoretical δNuCC (calculated as the ratio of BCP to bacterial cell biomass) and observed δNuCC (calculated as the difference between NuCC at the beginning and end of the experiment). The ratio of net viral production to the number of lysed bacteria, integrated over the entire time course of the experiment, was used to calculate the burst size in each sample.
Experimental design.
In order to obtain information on bacterial and virus dynamics, water samples were transferred immediately after collection in 1-liter polyethylene Whirl-Pak bags and subjected to different treatments described below. Parallel to each treatment replicated untreated water samples (200 ml) were incubated at in situ temperature, and 1-ml subsamples were taken from each polyethylene bag at time zero and at 24 h for determination of virus and bacterial abundance and bacterial carbon production. All microcosms (n = 3 per treatment) were incubated in the dark and at the in situ temperature. Sampling was performed at the beginning and at different time intervals within 24 h of incubation.
(i) Viral production.
In order to estimate viral production, we used the dilution technique of Wilhelm et al. (38). Water samples (50 ml) were transferred in Whirl-Pak bags, mixed with 100 ml of virus-free (0.02-μm-pore-size prefiltered) seawater, and incubated in the dark. One-milliliter subsamples were collected every 3 h for 12 h. Viral production rates were determined from first-order regressions of viral abundance versus time for triplicate incubations. Viral turnover rates were estimated by dividing viral production rates by viral abundance.
(ii) Viral decay.
The virioplankton decay rates were assessed according to the method of Noble and Furhman (18) by filtering water samples through 0.2-μm-pore-size polycarbonate filters to exclude bacteria and >0.2-μm particles. Filtered water was then dispensed in Whirl-Pak bags and incubated in the dark at in situ temperature. Subsamples (1 ml) were retrieved every 3 h for 12 h and processed for virus counts as described previously.
(iii) Bacterial lysogenic fraction.
The lysogenic fraction was estimated by using one of the most effective inducing agents, mitomycin C (21, 39). After a 6-h incubation with mitomycin C (1 μg ml−1 final concentration in 0.02-μm-pore-size-prefiltered seawater), 1-ml subsamples were collected and analyzed for bacterial and viral abundance.
(iv) Bacterivore-free treatment.
In order to test the influence of bacterivores on bacteria and viruses, 50-ml water samples were prefiltered with 0.8-μm-pore-size MF-Millipore filters (in order to remove all bacterial predators) and incubated in Whirl-Pak bags. One-milliliter subsamples were collected at time zero and 24 h and processed for viral and bacterial abundance and bacterial carbon production.
(v) Estimates of virus removal.
Contributions of different factors causing virus decay and/or removal were obtained as follows. First, the removal of viruses due to grazing was estimated by subtracting the 24 h variation in virus abundance in the unfiltered system compared to the 24 h variation in virus abundance in the bacterivore-free treatment. Second, the effect of virus disappearance from the water column due to penetration into the hosts and consequent lysogenic infection was estimated by assuming that only one phage infected the host and that the percentage of bacteria with lysogenic infection was conservative with time. In both cases, differences in all values were expressed as percentages of viral production on an hourly basis.
Statistical analyses.
Analysis of variance (ANOVA) was used to investigate differences between stations and times and to test the effect of different treatments. Whenever factors were identified as significant a HSD-Tukey post hoc test was performed.
RESULTS
Bacterial parameters.
Total and metabolically active bacterial abundances, biomass, bacterial carbon production, and turnover rates are reported in Table 2. Bacterial abundance decreased significantly from the northern to the southern stations (1.88 ± 0.36 × 109 cells liter−1 versus 0.34 ± 0.03 × 109 cells liter−1, respectively; P < 0.01 [ANOVA]). At station SS08, bacterial abundance at the sediment-water interface was double that at the surface (P < 0.001 [ANOVA]). Active bacteria accounted for 42.2 to 99.9% of total bacterial abundance. Compared to the entire bacterial abundance, the metabolically active fraction displayed a different spatial pattern, with significantly higher values at station D051 (1.34 ± 0.02 ± 109 cells liter−1; P < 0.001 [ANOVA]).
TABLE 2.
Viral abundance, bacterial and NuCC abundance and percentage, virus-to-bacterium abundance ratio, bacterial biomass, and bacterial C production in the investigated areasa
| Station | Depth | Bacterioplankton (109 cells liter−1) | NuCC (109 cells liter−1) | NuCC abundance (%) | Viruses (109 VLP liter−1) | Virus/bacterium ratio | Bacterial biomass (μg C liter−1) | Bacterial C production (μg C liter−1 h−1) |
|---|---|---|---|---|---|---|---|---|
| A118 | Surface | 1.88 ± 0.36 | 0.79 ± 0.11 | 42.18 | 9.54 ± 3.30 | 5.08 ± 0.73 | 55.74 ± 10.86 | 0.31 ± 0.02 |
| D051 | Surface | 1.39 ± 0.07 | 1.34 ± 0.02 | 97.07 | 11.18 ± 2.24 | 8.07 ± 1.41 | 37.33 ± 1.96 | 0.14 ± 0.03 |
| SS08 | Surface | 0.34 ± 0.03 | 0.15 ± 0.02 | 42.95 | 3.16 ± 0.16 | 9.26 ± 0.00 | 8.87 ± 0.67 | 0.03 ± 0.01 |
| SS08 | Sediment-water interface | 0.76 ± 0.02 | 0.76 ± 0.03 | 99.99 | 3.60 ± 0.38 | 4.72 ± 0.04 | 25.69 ± 7.16 | 0.04 ± 0.00 |
Values are means ± the standard deviation where applicable.
Bacterial biomass decreased significantly moving southward (from eutrophic to oligotrophic), ranging from 55.74 ± 10.86 to 8.87 ± 0.67 μg C liter−1 at stations A118 and SS08 (surface), respectively (P < 0.001 [ANOVA]).
Bacterial carbon production displayed a similar pattern, being higher at station A118 (0.31 ± 0.02 μg C liter−1) and decreasing significantly southward (P < 0.001 [ANOVA]).
Viral abundance.
The highest VLP abundances were observed at station A118 and D051 [(9.54 ± 3.30) × 109 VLP liter−1 and (11.18 ± 2.24) × 109 VLP liter−1, respectively], whereas the lowest values were observed at station SS08 in both surface water and at the sediment-water interface (P < 0.01 [ANOVA] and P < 0.05 [HSD-Tukey], Table 2). The ratio of virus to total bacterial abundance increased from the northern station A118 (5.08) to the southern station SS08 (9.26).
Viral production, decay rates, burst size, and lysogenic fraction.
Viral production, decay rates, and turnover are reported in Table 3. Viral production varied significantly among stations (P < 0.001 [ANOVA]), with the highest values at the northern station A118 (4.87 × 109 virus liter−1 h−1, P < 0.001 [HSD-Tukey]). At all sampling stations, viral production nearly tripled in the first 6 h and remained constant until the end of the experiment (P < 0.05 [ANOVA]), whereas in the deep water layer (station SS08) viral production increased constantly to 12 h (P < 0.001 [ANOVA]). Viral turnover time ranged from 1.96 to 8.79 h (at stations A118 and D051, respectively).
TABLE 3.
Viral production, decay rates, viral turnover and burst size (VLP released per lysed bacterial cell) in the investigated systemsa
| Station | Depth | Viral production (108 VLP liter−1 h−1) | Viral decay (108 VLP liter−1 h−1) | Turnover (h−1) | Burst size |
|---|---|---|---|---|---|
| A118 | Surface | 48.66 ± 3.37 | 19.67 ± 5.84 | 1.96 | 109 |
| D051 | Surface | 12.72 ± 3.33 | 5.27 ± 1.52 | 8.79 | 105 |
| SS08 | Surface | 11.54 ± 0.27 | 0.15 ± 0.05 | 2.74 | 41 |
| SS08 | Sediment-water interface | 16.29 ± 2.40 | 1.74 ± 0.56 | 2.21 | 15 |
Values are means ± the standard deviations where applicable.
Viral decay rates were highest at station A118 (1.97 × 109 virus liter−1 h−1) and decreased along the transect (P < 0.001 [ANOVA]).
The largest burst size was observed at station A118 (109 VLP cell−1), while the smallest burst sizes (15 and 41 VLP cell−1) were reported at station SS08, at the sediment-water interface, and in surface water, respectively.
The percentage of lysogenic bacteria was significantly higher at station SS08 (at both depths) and lower at the northern stations D051 and A118 (P < 0.01 [HSD-Tukey], Fig. 2).
FIG. 2.
Active and lysogenic bacterial fractions in the four sampling stations along a transect in the northern basin of the Adriatic Sea.
Bacterivore-free treatment.
The removal of nanoflagellates and other bacterivores resulted in significant and dramatic increases in bacterial abundance at stations A118 and D051 (624 and 185%, respectively [P < 0.001, ANOVA] for both stations, Fig. 3A), whereas no significant changes were observed in the unfiltered microcosms. After bacterivore removal, bacterial carbon production increased significantly at stations A118 and D051 (P < 0.01 [ANOVA] for both), whereas only a slight increase was observed at station SS08 in both surface waters and at the sediment-water interface (Fig. 3B). The “bacterivore-free” treatment resulted in a significant increase (ca. 580%) of viruses at station A118 (P < 0.001 [ANOVA], Fig. 3C), while demonstrating a decrease in viral abundance (P < 0.001 [ANOVA]) at station D051. No significant changes were observed at station SS08 at both sampling depths.
FIG. 3.
Temporal variations over 24 h of viral abundance (A), bacterial abundance (B), and bacterial carbon production (C) in samples where bacterivores were removed (bacterivore-free treatments).
DISCUSSION
Bacterial and viral abundances in the north basin of the Adriatic Sea followed the trophic gradient, displaying highest values in the northern station and a significant decrease southward. Viral abundance and production are influenced by nutrient enrichment (3, 9, 29, 34). The metabolic status of the host is crucial for viral development as high host growth rates can support higher viral turnover rates (3, 11). The eutrophic conditions of the northern Adriatic induced a higher growth rate of bacterioplankton. This was evident by the analysis of the (BCP/cell), which indicates that the metabolic status of virus-hosts was significantly higher at station A118 (3.86 × 10−10 μg C cell−1) than at all other stations (P < 0.01 [HSD-Tukey test]). The significant relationship between bacterial C production and virus abundance (Spearman correlation r2 = 0.62; n = 12; P < 0.05) further confirmed the key role of bacterial activity in controlling viral dynamics.
On the other hand, grazing by nanoflagellates has been generally considered an important causative agent of bacterial mortality, controlling bacterial carbon production, thus affecting, directly and/or indirectly, viral production (7, 14, 34). Our results indicate that the removal of nanoflagellates and of all other potential bacterivores caused a substantial increase in bacterial abundance and production and viral abundance within 24 h. However, such an effect was significant at the eutrophic station close to the Po River but not at the more oligotrophic stations. These results are in contrast with previous findings reporting a low control of grazing on bacterial abundance in eutrophic waters (31). Moreover, in contrast to reports by Weinbauer et al. (34) and Šimek et al. (26, 27) involving the use of medium time length experiments (24 to 96 h), we observed a significant and relatively rapid increase in viral abundance when bacterivores were removed. Our findings suggest that in the northern basin of the Adriatic Sea, where conditions are not nutrient limited, grazing can exert a relevant top-down control on bacteria and, consequently, on viruses.
High viral abundances have been often coupled with high values of burst size. In the present study, the burst size clearly decreased from the more eutrophic to the more oligotrophic station. Burst sizes, therefore, increased according with the productivity of the system. As previously suggested, this is likely related to the higher nutrient availability, which promotes a faster host growth and a larger cell size (32, 33). Burst size, indeed, varied according with bacterial carbon production, suggesting that this factor can influence viral dynamics.
Several studies have suggested that availability of nutrients may have an important influence also on viral strategies. Lysogenic infection is considered the most favorable way of bacterial infection in waters characterized by low bacterial and primary production (10, 32, 39). Our findings support previous studies indicating that oligotrophic conditions drive viral life strategies toward lysogenic rather than lytic infection.
An accurate analysis of viral dynamics requires the independent estimations of both viral production and decay rates. Here we provide, for the first time, a budget for viral abundance and net production along a trophic gradient. Our results indicate that in all stations investigated, viral production considerably exceeds viral decay, causing an apparent positive net balance of viral abundance. This effect was more evident at the northern eutrophic station, where the viral net balance was highly positive (2.9 × 109 VLP liter−1 h−1); viral production in particular was two to four times higher than in all other stations. Viral decay only partly balanced viral production in eutrophic waters (ca. 40 to 41% at stations A118 and D051), and this fraction dropped significantly to 1.3 and 10.7% at the more oligotrophic station SS08 (in the surface water and in the sediment-water interface, respectively). These results indicated that environmental conditions of eutrophic systems promoted significantly higher viral decay rates compared to oligotrophic systems.
The use of different approaches for estimating viral production and decay (i.e., dilution versus filtration) could also be partially responsible for the observed results. The dilution method that we applied for estimating viral production is based on the use of prefiltered (virus-free) seawater, collected synoptically with the samples on which the measurements were performed. As such, it is unlikely that this approach might have stimulated lytic cycles in lysogenic infections, with consequent overestimates in the measured viral production rates. In addition, this method has the advantage of being relatively simple and is widely utilized in literature (9, 15, 38, 40), so that our results can be compared to most of the available information. On the other hand, the filtration-based method for estimating viral decay (18) is based on a completely different approach, since the removal of bacteria, suspended particles, and organic molecules or aggregates could result in a lower rate of virus removal from the system. As such our viral decay rates could be partially underestimated. The extent of the potential bias caused by this latter method is difficult to quantify. However, if the removal of all particles and suspended loads is the main cause of a biased estimate of the viral decay, this factor should be much more evident in eutrophic waters than in the oligotrophic systems, which are characterized by a particle concentration approximately six times lower than in the northern eutrophic station (see transmittance values in Table 1). Conversely, our results clearly indicate that oligotrophic waters, where this bias should be minimal, displayed a much larger fraction of viral production not balanced by viral decay. Therefore, although these results must be further substantiated by other comparative approaches and additional analyses based also on different methodologies, we conclude that differences between rates of viral production and decay cannot be entirely a result of artifacts in the measurements.
The identification of factors responsible for differences in removal and/or decay rates between different environments is difficult. These factors can be summarized as (i) colloidal and heat-labile substances; (ii) direct grazing on virus or indirect grazing, through ingestion of infected bacteria; (iii) lysogenic bacterial infection; (iv) adsorption onto settling particles; and (v) UV solar radiation. Several authors suggested that, in estuarine and riverine waters, a substantial fraction of viruses is attached to colloidal particles with a size of <0.3 μm (16, 22). Heat-labile substances (extracellular enzymes) and adsorption of viruses onto particles have been considered the main causative agents responsible of virus decay in marine coastal waters (18, 37). However, grazing can represent another important cause of virus removal, either by selective ingestion of viruses or by protozoan predation of infected bacteria (2, 7). In addition, a non-negligible fraction of viruses can be temporarily undetectable in the water because they are involved in lysogenic infections.
The predominance of one factor over the others can produce different ecological consequences. Enzymatic degradation of viral particles can result in their removal from the water column; adsorption onto particles may imply sinking out of viruses toward the benthic domain, while grazing results in an increased C transfer toward higher trophic levels (9).
Estimating the relative importance of these different factors causing viral decay or removal from the water column is, consequently, a key task in ecological investigations. In the present study, viral decay measured in 0.2-μm-pore-size-prefiltered seawater was exclusively dependent upon the effect of dissolved substances (including colloidal and heat-labile material).
Our results indicate that grazing by bacterivores and dissolved-colloidal substances were responsible for the removal of ca. 62% of viral production in eutrophic conditions (station A118), while in oligotrophic waters (station SS08) these factors together were responsible for the removal of ca. 1.6% of viral production. Virus involved in lysogenic infection accounted for 4% at the eutrophic station and for ca. 15% at the oligotrophic stations. If the steady-state condition is assumed, the remaining 34% of virus particles produced in eutrophic conditions and ca. 83% in oligotrophic waters are subjected to a different fate. We did not directly measure the removal of viruses by adsorption onto settling particles. However, measurements of turbidity can be indicative of the total suspended solids loads. At our eutrophic station, turbidity was sixfold higher than in the more oligotrophic stations. If we assume that suspended solids loads are proportional to the downward fluxes of particles and consequent removal of viruses adsorbed onto the particle surface, the eutrophic northern Adriatic station could experience a rate of virus removal from the water column approximately six times higher than in the oligotrophic areas.
Overall, our results suggest that, in different trophic conditions, different causative agents are primarily responsible for the removal of viruses from the water (Fig. 4). Summing up all of the components, it is evident that the fate of viral production in oligotrophic and eutrophic systems follows different pathways. (i) In eutrophic waters, viral adsorption onto settling particles accounts for 34% of the viral production. (ii) In oligotrophic waters this fraction is six times lower. Therefore, our results indicate that in oligotrophic systems the fraction of viral production not subjected to apparent decay could be very high (ca. 78%). In oligotrophic waters the impact of UV on virus survival is likely to be higher than in more eutrophic systems because of the penetration of UV into the water column. However, we did not directly measure the impact of UV on viruses in these samples, so we cannot draw any definitive conclusions in this area.
FIG. 4.
Simplified scheme of the fate of viral production under different trophic conditions. Eutrophic (top panel) and oligotrophic waters (bottom panel) are depicted. Arrow dimensions are roughly proportional to the relative importance of the process described.
Our experiments also indicate that eutrophic waters can sustain a higher viral production rate through higher rates of bacterial production and host cell metabolic activities. Under these conditions, grazing can also exert measurable and important control over both bacteria and viruses. The present study suggests that viral production and decay rates may not necessarily be balanced in the short term, resulting in a net increase of viruses in the system. In the future, in order to get reliable production estimates, we recommend independent measurements of viral decay and production rates. Only accurate and complete analyses of the balance between viral production and decay rates will allow testing of the steady-state assumption and permit further understanding of the role of viruses under different environmental conditions.
Acknowledgments
We thank the crew of the R/V Alliance and the NATO SACLANT Undersea Research Centre for assistance and two anonymous reviewers for useful suggestions. We also thank A. Joyner at UNC Chapel Hill for graphic designs.
This study was supported by NITIDA (COFIN-PRIN) and by MEDVEG (EU project, contract no. Q5RS-2001-02456).
REFERENCES
- 1.Artegiani, A., D. Bregant, E. Paschini, N. Pinardi, F. Raicich, and A. Russo. 1997. The Adriatic Sea general circulation. J. Phys. Oceanogr. 27:1497-1514. [Google Scholar]
- 2.Binder, B. 1999. Reconsidering the relationship between virally induced bacterial mortality and frequency of infected cells. Aquat. Microb. Ecol. 18:207-215. [Google Scholar]
- 3.Corinaldesi, C., E. Crevatin, P. Del Negro, M. Marini, A. Russo, S. Fonda-Umani, and R. Danovaro. 2003. Large-scale spatial distribution of virioplankton in the Adriatic Sea: testing the trophic state control hypothesis. Appl. Environ. Microbiol. 69:2664-2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fischer, U. R., W. Weisz, C. Wieltschnig, A. K. T. Kirschner, and B. Velimirov. 2004. Benthic and pelagic viral decay experiments: a model-based analysis and its applicability. Appl. Environ. Microbiol. 70:6706-6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuhrman, J. A. 1999. Marine viruses and their biogeochemical and ecological effects. Nature 399:541-548. [DOI] [PubMed] [Google Scholar]
- 6.Fry, J. C. 1990. Direct methods and biomass estimation. Methods Microbiol. 22:41-85. [Google Scholar]
- 7.González, J. M., and C. A. Suttle. 1993. Grazing by marine nanoflagellates on viruses and virus-sized particles: ingestion and digestion. Mar. Ecol. Prog. Ser. 94:1-10. [Google Scholar]
- 8.Guixa-Boixereu, N., K. Lysnes, and C. Pedrós-Alió. 1999. Viral lysis and bacterivory during a phytoplankton bloom in a coastal water microcosm. Appl. Environ. Microbiol. 65:1949-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hewson, I., J. M. O'Neil, J. A. Fuhrman, and W. C. Dennison. 2001. Virus-like particles distribution and abundance in sediments and overlying waters along eutrophication gradients in two subtropical estuaries. Limnol. Oceanogr. 46:1734-1746. [Google Scholar]
- 10.Jiang, S. C., and J. H. Paul. 1996. Occurrence of lysogenic bacteria in marine communities as determined by prophage induction. Mar Ecol. Prog. Ser. 142:27-38. [Google Scholar]
- 11.Lenski, R. E. 1988. Dynamics of interactions between bacteria and virulent bacteriophage. Adv. Microb. Ecol. 10:1-44. [Google Scholar]
- 12.Luna, G. M., and R. Danovaro. 2003. Active, dormant and dead bacterial fractions in aquatic sediments. Rec. Res. Dev. Microbiol. 7:135-145. [Google Scholar]
- 13.Malone, T. C., A. Malej, L. W. Harding, Jr., and N. Smodlaka (ed.). 1999. Coastal and estuarine studies. Ecosystems at the land-sea margin. Drainage basin to coastal sea, vol. 55. American Geophysical Union Press, Washington, D.C.
- 14.McManus, G. B., and J. A. Fuhrman. 1988. Control of marine bacterioplankton populations: measurement and significance of grazing. Hydrobiologia 159:51-62. [Google Scholar]
- 15.Mei, M. L., and R. Danovaro. 2004. Viral production and life strategies in aquatic sediments. Limnol. Oceanogr. 49:459-470. [Google Scholar]
- 16.Metcalf, T. G., V. C. Rao, and J. L. Melnick. 1984. Solid associated viruses in a polluted estuary. Monogr. Virol. 15:97-110. [Google Scholar]
- 17.Middelboe, M., and P. G. Lyck. 2002. Regeneration of dissolved organic matter by viral lysis in marine microbial communities. Aquat. Microb. Ecol. 27:187-194. [Google Scholar]
- 18.Noble, R. T., and J. A. Fuhrman. 1997. Viral decay and its causes in coastal waters. Appl. Environ. Microbiol. 63:77-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noble, R. T., and J. A. Fuhrman. 1998. Use of SYBR Green I for rapid epifluorescence counts of marine viruses and bacteria. Aquat. Microb. Ecol. 14:113-118. [Google Scholar]
- 20.Noble, R. T., and J. A. Fuhrman. 2000. Rapid virus production and removal as measured with fluorescently labeled viruses as tracers. Appl. Environ. Microbiol. 66:3790-3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ortmann, A., J. Lawrence, and C. Suttle. 2002. Lysogeny and lytic viral production during a bloom of the cyanobacterium Synechococcus spp. Microb. Ecol. 43:225-231. [DOI] [PubMed] [Google Scholar]
- 22.Payment, P., E. Morin, and M. Trudel. 1988. Coliphages and enteric viruses in the particulate phase of river water. Can. J. Microbiol. 34:907-910. [DOI] [PubMed] [Google Scholar]
- 23.Peduzzi, P., and F. Schimier. 2004. Bacteria and viruses in the water column of tropical freshwater reservoirs. Environ. Microbiol. 6:707-715. [DOI] [PubMed] [Google Scholar]
- 24.Riemann, L., and M. Middelboe. 2002. Viral lysis of marine bacterioplankton: implications for organic matter cycling and bacterial clonal composition. Ophelia 56:57-68. [Google Scholar]
- 25.Sekulić, B., and A. Vertačnik. 1997. Comparison of anthropological and “natural” input of substances through waters into Adriatic, Baltic, and Black Sea. Water Res. 31:3178-3182. [Google Scholar]
- 26.Šimek, K., J. Pernthaler, M. G. Weinbauer, K. Hornák, J. R. Dolan, J. Nedoma, M. Masín, and R. Amann. 2001. Changes in bacterial community composition and dynamics and viral mortality rates associated with enhanced flagellate grazing in a mesoeutrophic reservoir. Appl. Environ. Microbiol. 67:2723-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Šimek, K., K. Hornák, M. Masín, U. Christaki, J. Nedoma, M. G. Weinbauer, and J. R. Dolan. 2003. Comparing the effects of resource enrichment and grazing on a bacterioplankton community of a meso-eutrophic reservoir. Aquat. Microb. Ecol. 31:123-125. [Google Scholar]
- 28.Smith, D. C., and F. Azam. 1992. A simple, economical method for measuring bacterial protein synthesis rates in sea using 3H-leucine. Mar. Microb. Food Webs 6:107-114. [Google Scholar]
- 29.Stopar, D., A. Černe, M. Žigman, M. Poljšak-Prijatelj, and V. Turk. 2004. Viral abundance and a high proportion of lysogens suggest that viruses are important members of the microbial community in the Gulf of Trieste. Microb. Ecol. 47:1-8. [DOI] [PubMed] [Google Scholar]
- 30.Van Hannen, E. H., G. Zwart, M. P. Van Agtervald, H. J. Gons, J. Ebert, and H. J. Laanbrook. 1999. Changes in bacterial and eukaryotic community structure after mass lysis of filamentous cyanobacteria associated with viruses. Appl. Environ. Microbiol. 65:795-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weinbauer, M. G., and P. Peduzzi. 1995. Significance of viruses versus heterotrophic nanoflagellates for controlling bacterial abundance in the northern Adriatic Sea. J. Plankton Res. 17:1851-1856. [Google Scholar]
- 32.Weinbauer, M. G., and C. A. Suttle. 1999. Lysogeny and prophage induction in coastal and offshore bacterial communities. Aquat. Microb. Ecol. 18: 217-225. [Google Scholar]
- 33.Weinbauer, M. G., D. Fuks, and P. Peduzzi. 1993. Distribution of viruses and dissolved DNA along a coastal trophic gradient in the northern Adriatic Sea. Appl. Environ. Microbiol. 59:4074-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weinbauer, M. G., U. Christaki, J. Nedoma, and K. Šimek. 2003. Comparing the effects of resource enrichment and grazing on viral production in a meso-eutrophic reservoir. Aquat. Microb. Ecol. 31:137-144. [Google Scholar]
- 35.Wen, K., A. C. Ortmann, and C. A. Suttle. 2004. Accurate estimation of viral abundance by epifluorescence microscopy. Appl. Environ. Microbiol. 70:3862-3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilhelm, S. W., and C. A. Suttle. 1999. Viruses and nutrient cycles in the sea. BioScience 49:781-788. [Google Scholar]
- 37.Wilhelm, S. W., M. G. Weinbauer, C. A. Suttle, and W. H. Jeffrey. 1998. The role of sunlight in the removal and repair of viruses in the sea. Limnol. Oceanogr. 43:586-592. [Google Scholar]
- 38.Wilhelm, S. W., S. M. Bridgen, and C. A. Suttle. 2002. A dilution technique for the direct measurement of viral production: a comparison in stratified and tidally mixed coastal waters. Microb. Ecol. 43:168-173. [DOI] [PubMed] [Google Scholar]
- 39.Williamson, S. J., L. A. Houchin, L. McDaniel, and J. H. Paul. 2002. Seasonal variation in lysogeny as depicted by prophage induction in Tampa Bay, Florida. Appl. Environ. Microbiol. 68:4307-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Winter, C., A. Smit, T. Szoeke-Dénes, G. J. Herndl, and M. G. Weinbauer. 2005. Modelling viral impact on bacterioplankton in the North Sea using artificial neural networks. Environ. Microbiol. doi: 10.1111/j.1462-2920.2005.00768.x [DOI] [PubMed]
- 41.Wommack, K. E., and R. L. Colwell. 2000. Virioplankton: viruses in aquatic ecosystems. Microb. Mol. Biol. Rev. 64:69-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zweifel, U. L., and A. Hagström. 1995. Total counts of marine bacteria include a large fraction of non-nucleoid-containing bacteria (ghosts). Appl. Environ. Microbiol. 61:2180-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]