Abstract
Trametes villosa laccase was used for direct azo dye degradation, and the reaction products that accumulated after 72 h of incubation were analyzed. Liquid chromatography-mass spectrometry (LC-MS) analysis showed the formation of phenolic compounds during the dye oxidation process as well as a large amount of polymerized products that retain azo group integrity. The amino-phenol reactions were also investigated by 13C-nuclear magnetic resonance and LC-MS analysis, and the polymerization character of laccase was shown. This study highlights the fact that laccases polymerize the reaction products obtained during long-term batch decolorization processes with azo dyes. These polymerized products provide unacceptable color levels in effluents, limiting the application of laccases as bioremediation agents.
The chemical structure of dyes is comprised of a conjugated system of double bonds and aromatic rings. The major classes of dyes have antroquinoid, indigoid, and azo aromatic structures. All of these structures allow strong π-π* transitions in the UV-visible (UV-Vis) area, with high extinction coefficients that allow us to consider these structures dye chromophores. Of all of these structures, the azo aromatic one is the most widespread dye class in the industry. The main drawback of this class of dyes is that they are not easily degraded by aerobic bacteria, and with the action of anaerobic or microaerobic reductive bacteria, they can form toxic and/or mutagenic compounds such as aromatic amines (9, 10, 34). There is a great environmental concern about the fate of these dyes, with special emphasis on reactive dyeing of cellulosic fibers, where large amounts of unbound dye are discharged in the effluent (24).
Laccases have been extensively studied for their degradation of azo dyes (1, 5, 8, 17, 19, 21, 23, 33). These enzymes are multicopper phenol oxidases that decolorize azo dyes through a highly nonspecific free radical mechanism forming phenolic compounds, thereby avoiding the formation of toxic aromatic amines (8, 35). Over long periods of time, there can be a coupling between the reaction products, and even polymerization. It is known that laccases can catalyze the polymerization of various halogen-, alkyl-, and alkoxy-substituted anilines (15). In the literature, there is a large number of papers reporting on the decolorization of azo dyes; however, the fate of the products of azo dye laccase reactions and their possible polymerization have been ignored (7, 16, 25, 28). Therefore, the purpose of this work was the study of azo dye degradation products in the presence of laccase. Direct azo dye laccase degradation and amino-phenol polymerization were performed for several days. The formed soluble products were studied by liquid chromatography-mass spectrometry (LC-MS), and the polymerized insoluble products were studied by 13C-nuclear magnetic resonance (13C-NMR).
MATERIALS AND METHODS
Dyes, reagents, and enzymes.
The investigated dyes, 3-(4-dimethylamino-1-phenylazo) benzenesulfonic acid (dye I) and 3-(2-hydroxy-1-naphthylazo) benzenesulfonic acid (dye II), were synthesized as described before (36). All other reagents and dyes were purchased from Sigma-Aldrich, St. Louis, MO, and used without further purification.
Laccase (EC 1.10.3.2) from Trametes villosa (5.3 mg protein/ml, 600 U/ml) was kindly provided by Novo Nordisk, Denmark.
Dye decolorization with laccase.
Stirred azo dye solutions (10 mM; 50 ml) buffered with 0.1 M sodium acetate buffer, pH 5, were incubated with 20 μl of Trametes villosa laccase (5.3 mg protein/ml, 600 U/ml) at room temperature. Dye decolorization was measured in a UV-visible spectrophotometer (Unicam, Cambridge, England) at different times during the course of the experiment, and the percentage of effluent decolorization was calculated from these data.
Polymerization reactions with laccase.
Stirred equimolar solutions of 2,5-diamino benzenesulfonic acid (DBSA) and catechol (10 mM; total volume, 50 ml) buffered with 0.1 M sodium acetate buffer, pH 5, were incubated with 20 μl of laccase (5.3 mg protein/ml, 600 U/ml) at 25°C. The same experiments were performed with catechol and DBSA separately.
LC-MS and 13C-NMR analyses.
LC-MS analyses were performed in negative-ion mode on an Agilent 1100 high-performance liquid chromatography system (degasser, binary pump, column compartment, and diode array detector [DAD]) coupled with a Q-trap LC-MS from Applied Biosystems, Canada. The flow coming from the high-performance liquid chromatography system was split 1:28 and introduced to an electrospray ionization turbo spray, which was operated at 350°C. A scan rate of 4,000 atomic mass units s−1 was used with negative ionization in the enhanced scan mode. All gases consisted of nitrogen produced from a gas generator from PEAK Science, Scotland. Further MS settings were as follows: ion spray voltage, −4,500 V; declustering potential, −50 V; entrance potential, −10 V; collision energy, −90 ÷ −10 V; curtain gas, 40 lb/in2; nebulizer gas, 45 lb/in2; and turbo gas, 80 lb/in2. Chromatographic separation was performed with the following chromatographic columns: Synergi Hydro (150 mm by 4.6 mm by 4 μm; Phenomenex), ProntoSIL AQ (60 mm by 4 mm by 3 μm; Bischoff, Germany), and Nucleosil HD (70 mm by 4 mm by 3 μm; Macherey-Nagel). The DAD performed a scan over the range of 200 to 800 nm with a sampling rate of 1.25 Hz at a slit width of 4 and a step width of 2 nm. A 753 suppressor module from Methrom, Switzerland, was used for cation suppression.
The 13C CP/MAS NMR spectra were recorded as described before (13).
RESULTS
Spectrophotometric analysis.
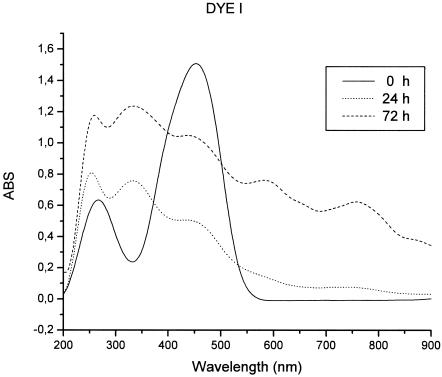
The biodegradation of the azo dyes was monitored by UV-Vis analysis. The UV-Vis spectrum showed dramatic changes in the enzymatically treated solutions. It was observed that this reaction is a multistep process, with a decrease in absorbance of the visible peak in the first stages of treatment and a general increase in absorbance in all UV-Vis spectra due to darkening of enzymatically treated solutions after longer treatment times, i.e., 72 h.
The decrease in the intensity of the visible peak (465 nm) of dye I indicates that the degradation of the molecule was not complete and that some undegraded dye was still present in the solution after 24 h of treatment with laccase. In the UV spectra, two new peaks emerged, at 250 and 320 nm (Fig. 1).
FIG. 1.
UV-Vis spectra of dye I (10 mM; 50 ml in 0.1 M sodium acetate buffer, pH 5) before and after laccase (20 μl; 5.3 mg protein/ml, 600 U/ml) decolorization at room temperature. ABS, absorbance.
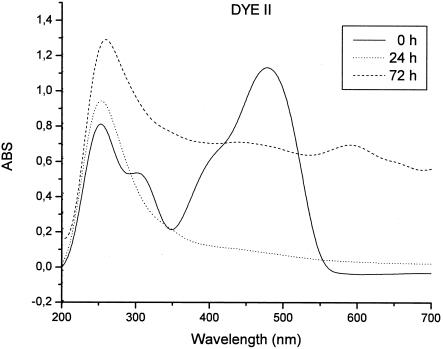
In the case of dye II, we observed a very rapid decrease in intensity of the peak in the visible absorption spectrum (465 nm), indicating almost complete decolorization with the disruption of the chromophoric group (azo bond disruption). The decrease in the absorbance of the two peaks in the UV region (242 and 307 nm) and the formation of a new peak at 250 nm suggest that there were changes in the aromatic group (Fig. 2).
FIG. 2.
UV-Vis spectra of dye II (10 mM; 50 ml in 0.1 M sodium acetate buffer, pH 5) before and after laccase (20 μl; 5.3 mg protein/ml, 600 U/ml) decolorization at room temperature. ABS, absorbance.
LC-MS/MS analysis of the degradation products of dye I.
LC-tandem MS (LC-MS/MS) is a versatile system which combines both selectivity and sensitivity, and it is generally considered the most reliable technique to quantify chemical compounds in complex matrices such as those studied here (12).
During the LC-MS/MS analysis of dye I [3-(4-dimethylamino-1-phenylazo) benzenesulfonic acid], 10 compounds were detected, 7 of which were identified. The mass spectra of the detected compounds were performed using enhanced ion scanning and enhanced product ion scanning with various level of collision energies (Table 1).
TABLE 1.
Mass spectra of products of dye 1 degradation
| Compound no. | Enhanced mass spectrum m/z (relative intensity [%]) | Value for enhanced product ion mass spectrum of quasi molecular ion
|
|
|---|---|---|---|
| Collision energy (V) | m/z (relative intensity [%]) | ||
| I | 157 (100), 93 (5) | −50 | 157 (8.1), 139 (9.5), 93 (27.5), 80 (100), 65 (11) |
| II | 173 (100), 172 (56), 171 (1.5), 109 (2), 80 (1) | −25 | 173 (100), 155 (5), 109 (15), 93 (5), 80 (22) |
| III | 185 (?), 93 (?)a | −30 | 185 (100), 121 (99), 93 (6), 80 (31) |
| IV | 1,382 (<0.5), 922 (0.6), 921 (2), 462 (9), 461 (16), 460 (68), 327 (14), 328 (20), 327 (100) | −35 | 921 (76), 786 (13), 761 (5), 592 (3), 474 (5), 446 (18), 431 (5), 415 (3), 380 (12), 340 (14), 327 (100) |
| V | 1,233 (<0.5), 618 (17), 617 (26), 616 (100), 329 (16), 328 (15), 327 (53) | −35 | 1,233 (18), 904 (11), 616 (100), 602 (18), 573 (12), 536 (33), 482 (26), 327 (63), 246 (25), 166 (30) |
| VI | 549 (6), 365 (3.5), 364 (16), 274 (78), 263 (3.5), 262 (13), 261 (27), 232 (3.5), 231 (8.5), 172 (7.5), 171 (19), 158 (2.5), 157 (3.5), 156 (100) | −35 | 549 (100), 521 (1.5), 364 (23), 336 (1.5), 260 (18), 232 (2.5), 171 (8.5), 156 (28.5) |
| VII | 448 (4), 447 (9.5), 446 (73), 261 (16), 260 (5), 172 (4), 171 (1.5), 157 (3), 156 (100) | −35 | 446 (13), 260 (11), 171 (29), 156 (100), 80 (10) |
Not stated because of weak chromatographic separation.
The identified compounds are products of the cleavage of the N-C bond in the dye molecule as well as polymeric products of coupling of these products with undegraded dye molecules. Compound I has been identified as benzenesulfonic acid, and compound II is hydroxy-benzenesulfonic acid. Compound III is tentatively identified as 3-diazenyl-benzenesulfonic acid, but this cannot be stated unambiguously. Compounds IV and V are dimeric coupling products of dye I and benzenesulfonic acid. Compound IV is a dimeric coupling product of dye I and one molecule of benzenesulfonic acid, while compound V is a dimeric coupling product of two molecules of benzenesulfonic acid and dye I (Fig. 3). Compound VII has been identified as the product of the coupling reaction of two 3-(4-methylamino-phenylazo)-benzenesulfonic acid molecules with one nitrogen molecule. In the sample, a coupling product between a contaminant in the sample and benzenesulfonic acid (compound VI) was identified.
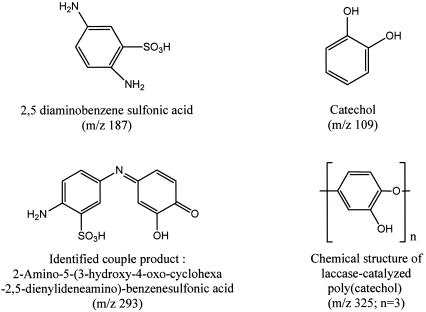
FIG. 3.
Identified catechol polymer and coupling product between DBSA and catechol.
The other three products, which appear at m/z 327, 366, and 480, could not be identified. It should be noted that these products were not stable and disappeared when the sample was frozen.
LC-MS analysis of the degradation products of dye II.
During the analysis of dye II [3-(2-hydroxy-1-naphthylazo) benzenesulfonic acid], seven compounds were identified.
The mass spectra of identified compounds were recorded in the same way as those for dye I, using enhanced ion scanning and enhanced product ion scanning with various values of collision energies. The mass spectra of compounds I, II, and III are listed in Table 1, and the mass spectra of compounds VIII, IX, X, and XI are listed in Table 2.
TABLE 2.
Mass spectra of products of dye II degradation
| Compound no. | Enhanced mass spectrum m/z (relative intensity [%]) | Value for enhanced product ion mass spectrum of quasi molecular ion
|
|
|---|---|---|---|
| Collision energy (V) | m/z (relative intensity [%]) | ||
| VIII | 484 (26), 483 (100), 457 (4.5), 456 (24), 455 (80), 429 (<1), 428 (2.5), 427 (7), 158 (7), 157 (8), 156 (73) | −30 | 483 (26), 455 (28), 427 (7), 347 (0.5), 312 (0.5), 172 (6.5), 156 (100), 80 (11.5) |
| IX | 641 (1), 640 (3), 639 (12.5), 612 (3), 611 (5.5), 456 (7), 455 (21), 454 (100), 399 (1), 398 (5), 397 (19), 362 (3), 361 (8), 158 (6), 157 (6.5), 156 (84), 145 (22) | −50 | 639 (2), 611 (38), 583 (7.5), 531 (12), 503 (11.5), 454 (12.5), 441 (7), 425 (5), 397 (4), 326 (14.5), 282 (7.5), 172 (4), 156 (100), 80 (9.5) |
| X | 487 (9), 486 (26.5), 485 (100), 458 (0.5), 457 (8) | −50 | 485 (72), 457 (70), 441 (6), 405 (13), 385 (7), 373 (70), 361 (14), 349 (100), 301 (9), 273 (7), 245 (6), 156 (16), 80 (6) |
| XI | 331 (1.5), 330 (18.5), 329 (100), 302 (4), 301 (27) | −35 | 329 (100), 301 (51.5), 285 (11), 273 (68.5), 249 (64.5), 221 (70) |
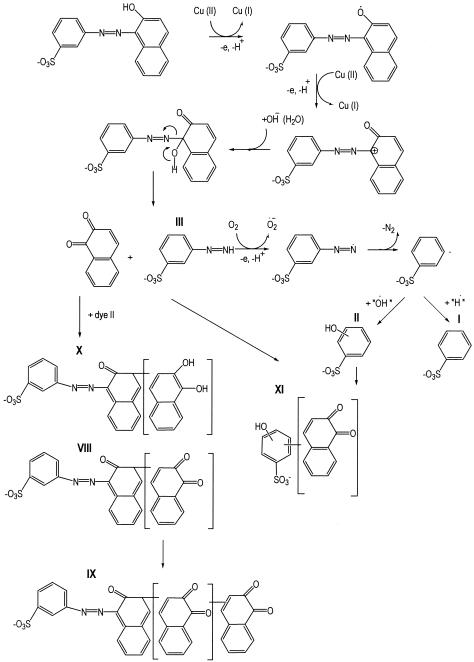
The products of the degradation of dye II, namely, hydroxy-benzenesulfonic acid (II) and benzenesulfonic acid (I), have been found. Unfortunately, the identification of 3-diazenyl-benzenesulfonic acid (III) could not be stated unambiguously.
The products obtained from the coupling processes of dye II with products obtained from azo dye oxidation by laccase were identified. Compound VIII is a coupling product of dye II with one 1,2-naphthoquinone molecule, while compound IX is obtained from the coupling of dye II with two molecules of 1,2-naphthoquinone. In addition, another two compounds were identified, denominated compound X and compound XI, which are the reaction products of coupling of the dye with 1,2-naphthodiol and of hydroxy-benzenesulfonic acid with 1,2-naphthoquinone, respectively.
Polymerization experiments.
The reaction between a phenol (catechol) and an aromatic amine (DBSA) was performed in the presence of Trametes villosa laccase. The insoluble polymer and the effluent were investigated by means of 13C-NMR and LC-MS analysis.
LC-MS analysis of the products showed the presence in the solution of coupling (m/z 293) and oligopolymeric (m/z 325, 369, 672, 525, 408, 259, 391, and 647) products. The structure of the oligopolymer has not yet been fully elucidated and will be the subject of future studies. The coupling product found in small amounts was identified as 2-amino-5-(3-hydroxy-4-oxo-cyclohexa-2,5-dienylideneamino)-benzenesulfonic acid (m/z 293), and the oligopolymeric product was identified as poly(catechol) (m/z 325) (Fig. 3).
The 13C-NMR spectrum of the precipitated catechol polymer at 7 kHz showed two large peaks in the aromatic range, at 144.7 and 122.5 ppm (Table 3). These peaks correspond to the carbons linked with the -OH groups and to the carbons at meta and para positions, respectively, confirming a structure that is related to the catechol polymer structure (2). The DBSA polymer spectrum at 7 kHz showed a series of peaks in the aromatic range, which was different from the noticeable unreacted structure of the diamine, confirming the oligomerization of DBSA under the influence of laccase action (111.7, 113.5, 123.5, 125.8, 128, 129, 131.8, and 134.6 ppm). In the spectrum of the polymer obtained from the reaction between DBSA and catechol in the presence of laccase, intense peaks at 144 and 116.2 ppm were observed.
TABLE 3.
Chemical shifts in CP-MAS 13C NMR spectra of samples treated with laccase
| Description | Catechol δ | DBSA δ | Catechol + DBSA δ |
|---|---|---|---|
| C or CH ring | 144.0 | 111.7 | 144.7 |
| 122.5 | 113.5 | 116.2 | |
| 123.5 | |||
| 125.8 | |||
| 128 | |||
| 129 | |||
| 131.8 | |||
| 134.6 |
DISCUSSION
A spectrophotometric analysis of dye I in the UV region showed peaks that could be attributed to the conversion of the degraded dye into unknown reaction products. Furthermore, the increased absorption in the visible spectra could be good evidence that a polymerization reaction occurred. The breaking down of the dye into smaller fragments, including the breakage of the azo bond, can lead to a decrease in the absorbance of the visible spectra and in a colorless solution. However, according to the literature (22), from this reaction a product could result that simply loses color due to a shift in the UV spectrum rather than a direct degradation of the molecule into smaller fragments. This slow degradation model based on a demethylation process could explain the persistence of a yellowish color in the solution, which remains even in the presence of very low concentrations of dye I. Bianco Prevot et al. suggested that after the demethylation reaction, a nonenzymatic oxidation disrupts the azo bond (4). Based on this model (Bianco Prevot) and on the results we obtained by means of LC-MS, the absence of N,N-dimethylaniline and 4-hydroxy-N,N-dimethylaniline from the reaction products can be explained.
The dye II peak near 250 nm is normally associated with the presence of phenolic and naphthoquinone compounds (20). Based on these findings once again, the laccase model oxidation pathway of the hydroxy-naphthylazo dyes is supported, where the laccase action allows direct and rapid dye degradation (8).
Furthermore, the two dyes showed a general increase in the absorption bands in the visible spectra after 48 h, indicating the formation of coupling products which retain azo group integrity in their molecules. Both dyes retained some color after maximal decolorization (∼70% for dye I and ∼90% for dye II) at 24 h. The products of laccase degradation participated in linking reactions with unreacted and reacted dye. Thus, the formation of polymerized products stopped the degradation process due to laccase action, leading to incomplete decolorization of the dye solutions.
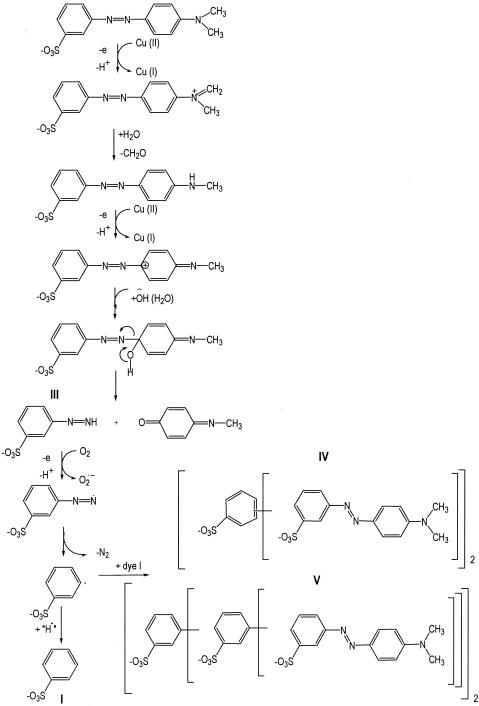
This hypothesis was supported by LC-MS analysis of the solutions after enzymatic treatment for a long period of time. The literature on the chemical oxidation of azo dyes and the products found during LC-MS/MS analysis could be helpful for understanding the degradation pathways of dye I (8, 14). Even though dye I is not a phenol azo dye that would be a typical substrate for laccase, extensive degradation was observed. Earlier reports on the chemical oxidation of methyl azo dyes suggested that a one-electron extraction from the amino substituent occurs in the initial step of dye degradation. The resulting radical cation undergoes oxidation, which leads to the formation of an iminium ion, and then the secondary amine can be formed through solvolysis processes. A loss of both N-methyl groups has also been reported (11). In this case, the mechanism could follow a similar pathway, performing a one-electron oxidation of the tertiary amine and subtraction of a hydrogen radical, with subsequent demethylation followed by the oxidation of the secondary amine by laccase. Nucleophilic attack by water on the phenolic ring carbon bearing the azo linkage causes N-C bond cleavage and produces 3-diazenyl-benzenesulfonic acid and 4-methylimino-cyclohexa-2,5-dienone. The 3-diazenyl-benezenesulfonic acid loses a nitrogen molecule to produce a benzenesulfonic acid radical, which could further undergo hydrogen radical addition or polymerization with a dye I molecule. However, this mechanism is not fully elucidated.
In the case of dye II, the presence of the coupling products of 1,2-naphthoquinone confirms the most accepted model of azo dye degradation by laccase (8, 27).
According to this model, laccase oxidizes the phenolic group of the phenolic azo dye, with the participation of one electron generating a phenoxy radical which is sequentially followed by oxidation to a carbonium ion. A nucleophilic attack by water on the phenolic ring carbon bearing the azo linkage to produce 3-diazenyl-benzenesulfonic acid (III) and 1,2-naphthoquinone then takes place. Quinones can form stable structures by addition reactions with the radicals in the reaction environment (31, 32). The reaction pathways published earlier (8) allow us to explain the formation of the coupling products of 1,2-naphthoquinone and its derivative, 1,2-naphthodiol, obtained during laccase degradation of dye II. In the present case, it is possible that the radicals which are formed by the one-electron oxidation of dye II by laccase will react with 1,2-naphthoquinone rather than be oxidized. The formed radicals can undergo oxidation to yield compound VIII, reduction to yield compound X, or further polymerization and again oxidation to form compound IX. Compound XII can be produced by the process of one-electron oxidation of hydroxy-benzenesulfonic acid, which can undergo a coupling reaction with 1,2-naphthoquinone, followed by further oxidation. The proposed reaction pathways of dye degradation by laccases are shown in Fig. 4 and 5. The positions of 1,2-naphthoquinone and 1,2-naphthodiol in the identified oligomeric molecules cannot be stated at present. Bollag observed dimerization and polymerization of phenoxy radicals during a Rhizoctonia practicola laccase treatment of organic compounds (6). According to that investigation, cross-coupling between the reactive species results in the formation of C-C and C-O bonds between phenolic molecules and of C-N and N-N bonds between aromatic amines. By phenolic cross-coupling, an electron is removed from the hydroxyl group, generating an alkoxy radical. Alkoxy free radicals forms dimers in the ortho and para positions with the hydroxyl groups. Phenolic radicals can be further oxidized to yield oligomeric products. Under certain conditions, the C-C-formed dimers can take part in coupling reactions to form extended quinines.
FIG. 4.
Proposed mechanism of laccase degradation of dye I.
FIG. 5.
Proposed mechanism of laccase degradation of dye II.
In order to confirm the efficiency of laccase polymerization, a preliminary study of the ability of the Trametes villosa laccase to catalyze polymerization and coupling reactions was performed. Usually, the laccase is able to catalyze polymerization reactions with various substituted anilines, performing an oxidative oligomerization established by a nonenzymatic coupling reaction between substituted anilines (15). The catechol is a known substrate for laccase that polymerizes, forming a poorly soluble product (2). In the present study, the presence of a phenolic compound in the system, in this case catechol, offers the advantage of enhancing the degree of polymerization. It was suggested earlier that the presence of catechol in the reaction medium disfavors aromatic amine self-coupling and enhances coupling between catechol and the amines (3, 30). We assumed that the biggest part of the coupling products was included in the identified polymeric matrix of catechol and precipitates from the solution as copolymers. In our studies, we observed that the amounts of polymers obtained from the laccase-catalyzed processes of DBSA or catechol alone in solution or of both DBSA and catechol in the reaction medium were significantly different, confirming earlier published work (29).
13C-NMR analysis confirmed the differences in the structures of the polymers obtained when both reactants were in solution and of the polymers obtained when catechol and DBSA were reacted separately. The differences in peak positions between catechol, DBSA, and DBSA/catechol polymers allowed an interpretation of the inhibition effect of the catechol on aromatic amine self-coupling with the formation of a copolymer between the oxidized anilines and catechol from the simultaneous nonenzymatic coupling and enzymatic polymerization reactions (18, 26).
In the present work, we have shown that laccase modifies azo dye structures by destroying their chromophoric structure. This is observed visually in azo dye solutions as a discoloration. However, over long periods of oxidation, the products obtained during the degradation reaction can undergo further reaction so that they can couple between themselves or with the unreacted dye, producing a large amount of coupled products leading to a darkening of the solution. The laccase-catalyzed reaction of the phenolic and aminophenolic compounds is a coupling reaction which occurs in the same manner as that specified above. The presence of the laccase in the solution leads to the oxidation of all compounds present in the system due to the fact that this enzyme has a high oxidative potential. For batch decolorization processes of azo dyes in the presence of laccase, polymerization reactions must be considered because in several cases acceptable dye degradation could not be attained, which limits the application of laccases as bioremediation agents.
It is our view that for any bioremediation processes (if possible) using laccases, one would have to consider the use of the immobilized enzyme in order to avoid coupling reactions of the reaction products with the undegraded dye.
Acknowledgments
We thank the Portuguese Foundation of Science and Technology (FCT) for providing grants to Andrea Zille (SFRH/BD/4720/2001) and the German MUNLV (Ministerium für Umwelt, Naturschutz, Landwirtschaft und Verbraucherschutz) of North Rhine Westfalia (NRW) for providing a grant to Barbara Gornacka.
REFERENCES
- 1.Adosinda, M., M. Martins, N. Lima, A. J. D. Silvestre, and M. J. Queiroz. 2003. Comparative studies of fungal degradation of single or mixed bioaccessible reactive azo dyes. Chemosphere 52:967-973. [DOI] [PubMed] [Google Scholar]
- 2.Aktas, N., and A. Tanyolac. 2003. Kinetics of laccase-catalyzed oxidative polymerization of catechol. J. Mol. Cat. B 22:61-69. [Google Scholar]
- 3.Anderson, J. S. 2000. The chemistry of hair colorants. JSDC 116:193-196. [Google Scholar]
- 4.Bianco Prevot, A., A. Basso, C. Baiocchi, M. Pazzi, G. Marcí, V. Augugliaro, L. Palmisano, and E. Pramauro. 2004. Analytical control of photocatalytic treatment degradation of sulfonated azo dye. Anal. Bioanal. Chem. 378:214-220. [DOI] [PubMed] [Google Scholar]
- 5.Blanquez, P., N. Casas, X. Font, X. Gabarrell, M. Sarra, G. Caminal, and T. Vicent. 2004. Mechanism of textile metal dye biotransformation by Trametes versicolor. Water Res. 38:2166-2172. [DOI] [PubMed] [Google Scholar]
- 6.Bollag, J. M. 1992. Enzymes catalyzing oxidative coupling reactions of pollutants. Met. Ions Biol. Syst. 28:205-217. [Google Scholar]
- 7.Chagas, P. E., and R. L. Durrant. 2001. Decolorization of azo dyes by Phanerochaete chrysosporium and Pleurotus sajorcaju. Enzyme Microb. Technol. 29:473-477. [Google Scholar]
- 8.Chivukula, M., and V. Renganathan. 1995. Phenolic azo dye oxidation by laccase from Pyricularia oryzae. Appl. Environ. Microbiol. 61:4347-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung, K. T., and C. E. Cerniglia. 1992. Mutagenicity of azo dyes: structure activity relationship. Mutat. Res. 277:201-220. [DOI] [PubMed] [Google Scholar]
- 10.Chung, K. T., and S. E. Stevens, Jr. 1993. Decolorization of azo dyes by environmental microorganisms and helminthes. Environ. Toxicol. Chem. 12:2121-2132. [Google Scholar]
- 11.Darwent, J. R., and A. Lepre. 1986. Photo-oxidation of methyl orange sensitised by zinc oxide. J. Chem. Soc. Faraday Trans. 282:1457-1468. [Google Scholar]
- 12.De Hoffman, E., J. Charette, and V. Stroobant. 2001. Mass spectrometry, principles and applications. Wiley & Sons, New York, N.Y.
- 13.Del Arco, M., S. Gutiérrez, C. Martín, V. Rives, and J. Rocha. 2004. Synthesis and characterization of layered double hydroxides (LDH) intercalated with non-steroidal anti-inflammatory drugs (NSAID). J. Solid State Chem. 177:3954-3962. [Google Scholar]
- 14.Galindo, C., P. Jacques, and A. Kalt. 2000. Photodegradation of the aminobenzene acid orange 52 by three advanced oxidation processes: UV/H2O2, UV/TiO2 and VIS/TiO2 comparative mechanistic and kinetic investigation. J. Photochem. Photobiol. A 130:35-47. [Google Scholar]
- 15.Hoff, T., S. Y. Liu, and J. M. Bollag. 1985. Transformation of halogen-, alkyl-, and alkoxy-substituted anilines by a laccase of Trametes versicolor. Appl. Environ. Microbiol. 49:1040-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jarosz-Wilkolazka, A., J. Kochmanska, E. Malarczyk, W. Wardas, and A. Leonowicz. 2002. Fungi and their ability to decolorize azo and anthraquinonic dyes. Enzyme Microb. Technol. 30:566-572. [Google Scholar]
- 17.Kirby, N., R. Marchant, and G. McMullan. 2000. Decolourisation of synthetic textile dyes by Phlebia tremellosa. FEMS Microbiol. Lett. 188:93-96. [DOI] [PubMed] [Google Scholar]
- 18.Klibanov, A. M., T. M. Tu, and K. P. Scott. 1983. Peroxidase-catalyzed removal of phenols from coal-conversion wastewaters. Science 221:259-261. [DOI] [PubMed] [Google Scholar]
- 19.Maximo, C., and M. Costa-Ferreira. 2004. Decolourisation of reactive textile dyes by Irpex lacteus and lignin modifying enzymes. Proc. Biochem. 39:1475-1479. [Google Scholar]
- 20.Mielgo, I., M. T. Moreira, G. Feijoo, and J. M. Lema. 2001. A packed-bed fungal bioreactor for continuous decolourisation of azo-dyes (Orange II). J. Biotechnol. 89:99-106. [DOI] [PubMed] [Google Scholar]
- 21.Novotny, C., K. Svobodova, A. Kasinath, and P. Erbanova. 2004. Biodegradation of synthetic dyes by Irpex lacteus under various growth conditions. Int. Biodeterior. Biodegrad. 54:215-223. [Google Scholar]
- 22.Oakes, J., and P. Gratton. 1998. Kinetic investigation of the oxidation of methyl orange and substituted arylazonaphthol dyes by peracids in aqueous solution. J. Chem. Soc. Perkin Trans. 2:2563-2568. [Google Scholar]
- 23.Peralta-Zamora, P., C. M. Pereira, E. R. L. Tiburtius, S. G. Moraes, M. A. Rosa, R. C. Minussi, and N. Duran. 2003. Decolorization of reactive dyes by immobilized laccase. Appl. Catal. B 42:131-144. [Google Scholar]
- 24.Pierce, J. 1994. Colour in textile effluents—the origins of the problem. J. Soc. Dyers Colour. 110:131-134. [Google Scholar]
- 25.Robinson, T., B. Chandran, and P. Nigam. 2001. Studies on the production of enzyme by white-rot fungi for the decolourisation of textile dyes. Enzyme Microb. Technol. 29:575-579. [Google Scholar]
- 26.Simmons, K. E., R. D. Minard, and J. M. Bollag. 1989. Oxidative co-oligomerization of guaiacol and 4-chloroaniline. Environ. Sci. Technol. 23:115-121. [DOI] [PubMed] [Google Scholar]
- 27.Soares, G. M. B., M. T. Pessoa Amorim, R. Hrdina, and M. Costa-Ferreira. 2002. Studies on the biotransformation of novel disazo dyes by laccase. Process Biochem. 37:581-587. [Google Scholar]
- 28.Tauber, M. M., G. M. Guebitz, and A. Rehorek. 2005. Degradation of azo dyes by laccase and ultrasound treatment. Appl. Environ. Microbiol. 71:2600-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thiele, S., E. Fernandes, and J. M. Bollag. 2002. Enzymatic transformation and binding of labelled 2,4,6-trinitrotoluene to humic substances during an anaerobic/aerobic incubation. J. Environ. Qual. 31:437-444. [PubMed] [Google Scholar]
- 30.Thorn, K. A., P. J. Pettigrew, W. S. Goldenberg, and E. J. Weber. 1996. Covalent binding of aniline to humic substances. II. Nitrogen N15-NMR studies of nucleophilic addition reactions. Environ. Sci. Technol. 30:2764-2775. [Google Scholar]
- 31.Thurston, C. F. 1994. The structure and function of fungal laccase. Microbiology 140:19-26. [Google Scholar]
- 32.Ulbricht, J. 1992. Grundlagen der Synthese von Polymeren, p. 76-78. Hüthig and Wepf Verlag, Heidelberg, Germany.
- 33.Wesenberg, D., I. Kyriakides, and S. N. Agathos. 2003. White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol. Adv. 22:161-187. [DOI] [PubMed] [Google Scholar]
- 34.Wong, P. K., and P. Y. Yuen. 1996. Decolorization and biodegradation of methyl red by Klebsiella pneumoniae RS-13. Water Res. 30:1736-1744. [Google Scholar]
- 35.Wong, Y., and J. Yu. 1999. Laccase catalyzed decolorization of synthetic dyes. Water Res. 33:3512-3520. [Google Scholar]
- 36.Zille, A., P. Ramalho, T. Tzanov, R. Millward, V. Aires, M. H. Cardoso, M. T. Ramalho, G. M. Gubitz, and A. Cavaco-Paulo. 2004. Predicting dye biodegradation from redox potentials. Biotechnol. Prog. 20:1588-1592. [DOI] [PubMed] [Google Scholar]