Abstract
Eight anaerobic granular sludges were surveyed for Crenarchaeota using rRNA gene cloning. Microbial arrangement and substrate uptake patterns were elucidated by fluorescent in situ hybridization and beta imaging. Group 1.3 Crenarchaeota represented up to 50% of Archaea and 25% of the total microbiota in five sludges. Crenarchaeota were localized in close association with methanogenic Archaea.
Crenarchaeal phylogeny has been fundamentally changed by the isolation of novel small-subunit rRNA gene clones from mesophilic and psychrophilic habitats. Indeed, rRNA gene sequences representing nonthermophilic clades of Crenarchaeota have been reported from, for example, mesophilic soils in disparate locations (e.g., the United States [3], Japan [14], and Finland [12]) and granular biofilms from psychrophilic anaerobic bioreactors treating various wastewaters (7, 8, 9, 16).
Notwithstanding the many reports documenting their presence in submesophilic environmental samples, the absence of nonthermophilic isolates has hindered a biochemical and physiological characterization of these organisms. Thus, the ecological roles and physiological functions of these abundant and cosmopolitan Crenarchaeota remain a mystery. While the unusual properties of thermophilic Crenarchaeota have attracted the attention of both exobiologists wishing to study microbial evolution and biotechnology companies wishing to exploit the hyperthermotolerance of crenarchaeal cellular enzymes, the widespread occurrence of nonthermophilic Crenarchaeota, particularly in engineered environments such as anaerobic digesters, means that the biotechnological significance and potential of these organisms should now be explored.
This paper describes observations regarding the prevalence and spatial distribution of Crenarchaeota in anaerobic granules from wastewater treatment reactors. The microbial community structure was determined by using 16S rRNA clone library analysis. Fluorescent in situ hybridization (FISH) was applied in conjunction with radioactive tracer techniques and microbeta imaging to investigate substrate uptake patterns. This is the first application of this technology to anaerobic granular biofilms and represents an advancement in terms of both microbial ecology and efforts to describe the role of microorganisms involved in wastewater treatment.
Eight anaerobic granular sludges, S1 to S8, were obtained from various full (37°C)- and lab (15°C)-scale anaerobic biological wastewater treatment plants. S1 was from a full-scale upflow anaerobic sludge bed treatment plant treating citric acid production wastewater at Archer Daniels Midland, Ringaskiddy, County Cork, Ireland; S2 was from a lab-scale expanded granular sludge bed-anaerobic filter (EGSB-AF) reactor treating volatile fatty acid-based wastewater; S3 and S4 were from lab-scale upflow anaerobic sludge bed reactors used to treat acetate-based and propionate/butyrate/ethanol-based wastewaters, respectively; S5 and S6 were from full-scale internal circulation (IC) reactors at Archer Daniels Midland, County Cork, and Carbery Milk Products, Ballineen, County Cork, respectively; and S7 and S8 were from lab-scale EGSB-AF reactors treating high- and low-strength whey-based wastewater, respectively. All lab-scale reactors had been operated for extended trials of 250 to 500 days at 12 to 15°C (9).
Total genomic DNAs were extracted from all eight samples (S1 to S8) as described previously (7). Briefly, various methods were examined for the isolation of nucleic acids, the merits of which were assessed by determining the cell lysis efficiency, i.e., the best protocol for DNA collection from biomass was that which resulted in the smallest amount of unlysed cells while maintaining a high yield of good-quality DNA. Microorganisms in sludge samples before and after DNA extraction were observed according to the method of Bitton and coworkers (4) and as described in detail by Collins et al. (7). Sludge granules were initially disassociated by grinding or by sonication prior to microbial cell lysis using a chemical approach, as described by Zhou et al. (25), or a method using mechanical disruption by bead beating combined with chemical lysis. Different combinations of the above were tested, and it was found that gently crushing sludge granules with a pestle and mortar before passing the biomass, according to the manufacturer's instructions, through a soil DNA kit (MoBio Laboratories, Inc.) provided an optimal DNA yield with little shearing and provided the highest cell lysis efficiency (7). Although some Archaea possess a particularly thick and rigid outer layer named methanochondroitin (15), this DNA recovery procedure was sufficiently robust and efficient to retrieve representative DNA yields for community structure analyses.
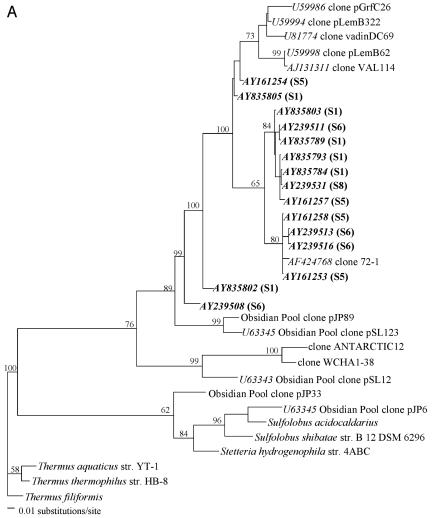
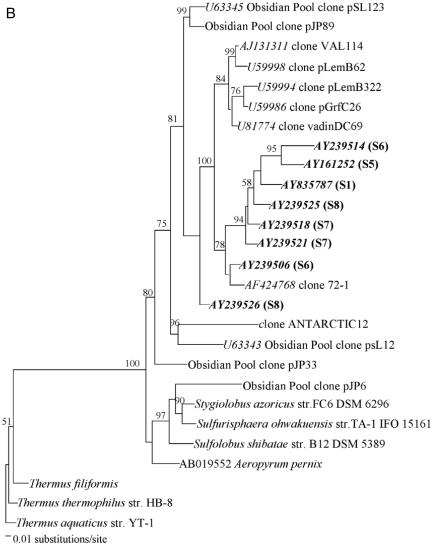
Archaeal 16S rRNA genes were amplified with the forward primer 21F (5′-TTCCGGTTGATCCYGCCGGA-3′ [21]) and the reverse primer 958R (5′-YCCGGCGTTGAMTCCAATT-3′ [10]); sequences were obtained from 16S rRNA gene clone libraries, and phylogenetic reconstruction was carried out as described in detail previously (7). No Crenarchaeota-like clones were detected in S2, S3, or S4, while high levels of uncultured crenarchaeotes were found in S1, S5, S6, S7, and S8 (69%, 55%, 59%, 14%, and 78% of all archaeal clones, respectively). While Crenarchaeota from sediment and soils group into several phylogenetic subclusters (11), there appears to be a well-defined phylogenetic coherence among all crenarchaeal clones recovered from this panel of anaerobic sludges (Fig. 1). Our clones fall into group 1.3 of the Crenarchaeota (11, 13), or group 1.3b proposed by Ochsenreiter et al. (17).
FIG. 1.
Phylogeny of crenarchaeal small-subunit rRNA genes based on the Kimura two-parameter algorithm. Partial 16S rRNA gene clonal sequences not supporting comparative alignment were placed in separate phylograms. Bootstrap replicates (from a total of 100 replicate samplings) that supported the branching order are shown at relevant nodes. Scale, 1 nucleotide substitution per 100 sequence positions. GenBank accession numbers of crenarchaeal clones from this study are presented in bold, with the source biomass shown in parentheses.
Anaerobic granules were fixed, and cross sections were prepared as described previously (20). FISH was performed as described by Schramm et al. (19), using the following hierarchical set of Cy3-labeled 16S rRNA-targeted oligonucleotide probes (Biomers.net, Germany): (i) Arch915 (22), specific for Archaea; (ii) Eub338 (1), specific for Bacteria; (iii) Cren499 (5), specific for most of the Crenarchaeota; and (iv) Mx825 (18), specific for Methanosaeta spp. In the case of simultaneous hybridizations using two of the above oligonucleotides together, one probe was labeled with fluorescein instead of Cy3. Hybridization stringencies in the hybridization buffer used were achieved by adding formamide to final concentrations (vol/vol) of 35%, 30%, 0%, and 20% for Arc915, Eub338, Cren499, and Mx825, respectively. The Non338 probe (24), complementary to the Eub338 sequence, was used as a negative control. Pure cultures of Methanosaeta concilii and Sulfolobus solfataricus P2 were used to positively control the use of Mx825/Arc915 and Cren499, respectively. Based on a comparative analysis of recently available aligned 16S rRNA gene sequences by BLAST at the NCBI website (http://www.ncbi.nlm.nih.gov/) as well as the assistance of the Probe Match program of the RDP-II (6), the specificity of the probes was examined theoretically with target and nontarget microorganisms.
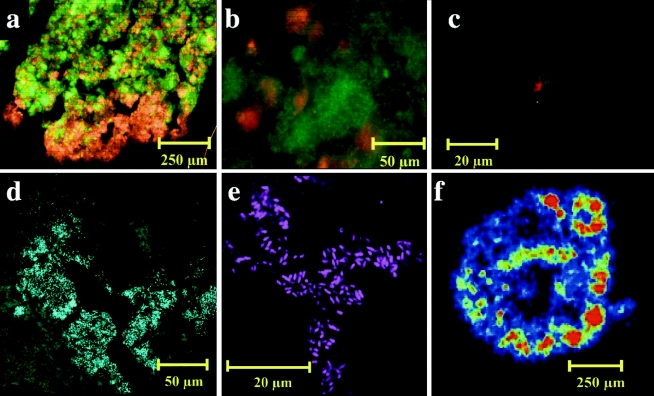
FISH experiments revealed an apparent overestimation of Crenarchaeota-like species by clone library analysis in some sludges, thus highlighting the value of a polyphasic approach to ecological inventories. Nevertheless, the relative abundance of Crenarchaeota was determined as a fraction of the total area of granule sections, and cells hybridizing to Cren499 comprised up to 50% of all Archaea and 15 to 25% of the total sludge microbiota in S1, S5, S6, S7, and S8. Cells of the Crenarchaeota were rods of 1.5 μm in length and 0.7 μm in width which occurred in dense clusters within a layered, granular biofilm architecture (Fig. 2).
FIG. 2.
In situ hybridizations using typical S1 granule sections. (a) Archaea (red) and Bacteria (green); (b) simultaneous Crenarchaeota (red) and Methanosaeta (green) probing; (c) Crenarchaeota cluster (red) surrounded by filamentous Methanosaeta cells (green); (d) Crenarchaeota clusters located at the granule edge and around granule channels and cavities; (e) rod-shaped Crenarchaeota cells at granule periphery; (f) microbeta image illustrating localized uptake of acetate at the surface of an S1 granule at 15°C after 8 h.
Substrate uptake patterns in S1 were investigated using a radioactive tracer technique (2) and beta imaging (23). Aliquots of 50 μl of sludge (2.8 mg suspended solids) were added to 1.5-ml microcentrifuge tubes for radiotracer incubations. Sterilized biomass samples were used as negative controls. An organic substrate, [1(2)-14C]acetic acid sodium salt (specific radioactivity, 59 mCi mmol−1), was tested. The unlabeled substrate was pure-grade acetic acid from Sigma. Tests were carried out in triplicate, and the concentration of acetate in each tube was 100 mM. Incubations were carried out at 15°C and 37°C for 8 h; a time-series experiment was achieved by stopping some incubation mixtures after 2 h and 4 h.
After incubation, the organic substrate was replaced with chilled 4% paraformaldehyde in 1× phosphate-buffered saline (PBS) and incubated at 4°C for 6 h. Samples were washed in PBS and stored in PBS-ethanol (1:1) at −20°C before being prepared for sectioning as described previously. Sectioned samples were scanned for radioactivity using a microbeta imager. In situ hybridizations were carried out as described before.
Beta imaging suggested the localized accumulation of radioactivity in the outer layers of biofilm sections from acetate incubations. This coincided with an abundance of filamentous Methanosaeta cells in juxtaposition with Cren499-positive cell clusters around the outer layer of these S1 granules (Fig. 2). Such an arrangement is unusual with respect to the other granular sludges studied here and to previously described granule structures in which archaeal cores are enveloped by a bacterial surface (20). Crenarchaeota were also detected along channels extending from the surface towards the granule core of S1 (Fig. 2). Given the position (for example, the intimate relationship between crenarchaeal clusters and Methanosaeta cells) and abundance of these uncultured Crenarchaeota in active areas of the S1 granular biofilm, we postulate that these organisms are active members of the granular consortium and may interact metabolically with acetate-utilizing methanogens. Further research is required to investigate a putative symbiosis between these Archaea.
The following conclusions may now be drawn: (i) group 1.3 Crenarchaeota are widespread and highly abundant in anaerobic wastewater treatment biofilms and can be cultivated as members of methanogenic consortia in laboratory-scale bioreactors, (ii) FISH illustrated a definite crenarchaeal population structure comprising dense cell clusters associated with acetoclastic methanogens within the granular biofilm architecture, (iii) Crenarchaeota clusters associated with methanogenic Archaea were colocalized with acetate uptake in the biofilm structure, and (iv) further beta imaging work and microautoradiography-FISH with a broader range of substrates are necessary to elucidate the full in situ functionalism and ecophysiology of Crenarchaeota in anaerobic granules. In summary, this is an important first step toward understanding the role of the previously mysterious Crenarchaeota in biotechnologically important systems.
Nucleotide sequence accession numbers.
Gene sequences from this study were deposited in the GenBank database under accession numbers AY161236 to AY161261, AY239506 to AY239582, and AY835782 to AY835826.
Acknowledgments
The receipt of financial support from the Higher Education Authority (HEA) of Ireland Programme for Research in Third Level Institutes (PRTLI)-Cycle II, through the Environmental Change Institute (ECI) and the National Centre for Biomedical Engineering Sciences (NCBES) at NUI, Galway, Ireland, and an Enterprise Ireland research scholarship to G.C. are acknowledged.
S3, S4, S7, and S8 biomass was kindly donated by Seán Connaughton and Sharon McHugh, NUI, Galway, Ireland.
REFERENCES
- 1.Amann, R. I., B. J. Binder, R. J. Olson, S. W. Chisholm, R. Devereux, and D. A. Stahl. 1990. Combination of 16S rRNA-targeted oligonucleotide probes with flow cytometry for analyzing mixed microbial populations. Appl. Environ. Microbiol. 56:1919-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreasen, K., and P. H. Nielsen. 1997. Application of microautoradiography to the study of substrate uptake by filamentous microorganisms in activated sludge. Appl. Environ. Microbiol. 63:3662-3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bintrim, S. B., T. J. Donohue, J. Handelsman, G. P. Roberts, and R. M. Goodman. 1997. Molecular phylogeny of Archaea from soil. Proc. Natl. Acad. Sci. USA 94:277-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bitton, G., B. Koopman, K. H. Jung, G. Voiland, and M. Kotob. 1993. Modification of the standard epifluorescence microscopic method for total bacterial counts in environmental-samples. Water Res. 27:1109-1112. [Google Scholar]
- 5.Burggraf, S., T. Mayer, R. I. Amann, S. Schadhauser, C. R. Woese, and K. O. Stetter. 1994. Identifying members of the domain Archaea with rRNA-targeted oligonucleotide probes. Appl. Environ. Microbiol. 60:3112-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cole, R., B. Chai, T. L. Marsh, R. J. Farris, Q. Wang, S. A. Kulam, S. Chandra, D. M. McGarrell, T. M. Schmidt, G. M. Garrity, and J. M. Tiedje. 2003. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 1:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins, G., A. Woods, S. McHugh, M. W. Carton, and V. O'Flaherty. 2003. Microbial community structure and methanogenic activity during start-up of psychrophilic anaerobic digesters treating synthetic industrial wastewaters. FEMS Microbiol. Ecol. 46:159-170. [DOI] [PubMed] [Google Scholar]
- 8.Collins, G., C. Foy, S. McHugh, and V. O'Flaherty. 2005. Anaerobic treatment of 2,4,6-trichlorophenol in an expanded granular sludge bed-anaerobic filter (EGSB-AF) bioreactor at 15°C. FEMS Microbiol. Ecol. 53:167-178. [DOI] [PubMed] [Google Scholar]
- 9.Collins, G., S. McHugh, A. Kearney, and V. O'Flaherty. June. 2004. Psychrophilic anaerobic digestion of a range of wastewaters: process technology, microbial activity and population dynamics. Water Intelligence Online. [Online.] http://www.waterintelligenceonline.com.
- 10.DeLong, E. F. 1992. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA 89:5685-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeLong, E. F. 1998. Everything in moderation: Archaea as “non-extremophiles.” Curr. Opin. Genet. Dev. 8:649-654. [DOI] [PubMed] [Google Scholar]
- 12.Jurgens, G., and A. Saano. 1999. Diversity of soil Archaea in boreal forest before and after clear-cutting and prescribed burning. FEMS Microbiol. Ecol. 29:205-213. [Google Scholar]
- 13.Jurgens, G., F. Glockner, R. I. Amann, A. Saano, L. Montonen, M. Likolammi, and U. Munster. 2000. Identification of novel Archaea in bacterioplankton of a boreal forest lake by phylogenetic analysis and fluorescent in situ hybridization. FEMS Microbiol. Ecol. 34:45-56. [DOI] [PubMed] [Google Scholar]
- 14.Kudo, Y., T. Nakajima, T. Miyaki, and H. Oyaizu. 1997. Methanogen flora of paddy soils in Japan. FEMS Microbiol. Ecol. 22:39-48. [Google Scholar]
- 15.Lange, M., and B. K. Ahring. 2001. A comprehensive study into the molecular methodology and molecular biology of methanogenic Archaea. FEMS Microbiol. Rev. 25:553-555. [DOI] [PubMed] [Google Scholar]
- 16.McHugh, S., M. W. Carton, G. Collins, and V. O'Flaherty. 2004. Reactor performance and microbial community dynamics during anaerobic biological treatment of wastewaters at 16-37°C. FEMS Microbiol. Ecol. 48:369-378. [DOI] [PubMed] [Google Scholar]
- 17.Ochsenreiter, T., D. Selezi, A. Quaiser, L. Bonch-Osmolovskaya, and C. Schleper. 2003. Diversity and abundance of Crenarchaeota in terrestrial habitats studied by 16S RNA surveys and real time PCR. Environ. Microbiol. 5:787. [DOI] [PubMed] [Google Scholar]
- 18.Raskin, L., J. M. Stromley, B. E. Rittmann, and D. A. Stahl. 1994. Group-specific 16S rRNA hybridization probes to describe natural communities of methanogens. Appl. Environ. Microbiol. 60:1232-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schramm, A., D. de Beer, M. Wagner, and R. I. Amann. 1998. Identification and activities in situ of Nitrosospira and Nitrospira spp. as dominant populations in a nitrifying fluidized bed reactor. Appl. Environ. Microbiol. 64:3480-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekiguchi, Y., Y. Kamagata, K. Nakamura, A. Ohashi, and H. Harada. 1999. Fluorescence in situ hybridization using 16S rRNA-targeted oligonucleotides reveals localization of methanogens and selected uncultured Bacteria in mesophilic and thermophilic sludge granules. Appl. Environ. Microbiol. 65:1280-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stackebrandt, E., and M. Goodfellow. 1991. Nucleic acid techniques in bacterial systematics. Wiley, Chichester, England.
- 22.Stahl, D. A., and R. I. Amann. 1991. Development and application of nucleic acid probes, p. 205-248. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons Ltd., Chichester, England.
- 23.Treude, T., K. Nauhaus, A. Gieseke, K. Knittel, A. Boetius, B. B. Joergensen, and W. Michaelis. 2003. Methanotrophic microbial mats forming reefs in the anoxic Black Sea. Geophys. Res. Abstr. 5:01732. [Google Scholar]
- 24.Wallner, G., R. I. Amann, and W. Beisker. 1993. Optimizing fluorescent in situ hybridisation with rRNA-targeted oligonucleotide probes for flow cytometric identification of microorganisms. Cytometry 14:136-143. [DOI] [PubMed] [Google Scholar]
- 25.Zhou, J., M. A. Bruns, and J. M. Teidje. 1996. DNA recovery from soils of diverse composition. Appl. Environ. Microbiol. 62:316-332. [DOI] [PMC free article] [PubMed] [Google Scholar]