Abstract
Antagonism has been described in vitro and in vivo for azole-polyene combinations against Aspergillus species. Using an established murine model of invasive pulmonary aspergillosis, we evaluated the efficacy of several amphotericin B (AMB) dosages given alone or following preexposure to itraconazole (ITC). Mice were immunosuppressed with cortisone acetate and cyclophosphamide. During immunosuppression, animals were administered either ITC solution (50 mg/kg of body weight) or saline by oral gavage twice daily for 3 days prior to infection. Infection was induced by intranasally inoculating mice with a standardized conidial suspension (1 × 108 CFU/ml) of Aspergillus fumigatus strain AF 293. AMB was then administered by daily intraperitoneal injections (0.25, 0.5, 1.0, and 3.0 mg/kg) starting 24 h after inoculation and continuing for a total of 72 h. Drug pharmacokinetics of AMB and ITC in plasma were determined by high-performance liquid chromatography. Four different endpoints were used to examine the efficacy of antifungal therapy: (i) viable counts from harvested lung tissue (in CFU per milliliter), (ii) the whole-lung chitin assay, (iii) mortality at 96 h, and (iv) histopathology of representative lung sections. At AMB doses of >0.5 mg/kg/day, fewer ITC-preexposed mice versus non-ITC-preexposed mice were alive at 96 h (0 to 20 versus 60%, respectively). At all time points, the fungal lung burden was consistently and significantly higher in animals preexposed to ITC, as measured by the CFU counts (P = 0.001) and the chitin assay (P = 0.03). Higher doses of AMB did not overcome this antagonism. ITC preexposure was associated with poorer mycological efficacy and survival in mice treated subsequently with AMB for invasive pulmonary aspergillosis.
Invasive pulmonary aspergillosis (IPA) has emerged as a common opportunistic fungal infection and is currently the leading infectious cause of death in patients with hematological malignancies (6, 17). In high-risk patient populations (those with acute leukemia or bone marrow transplant recipients), IPA is associated with a mortality rate of 60 to 80%, despite the administration of systemic antifungal therapy (2, 6). Given the rising frequency of Aspergillus infections and problems associated with its early diagnosis, antifungal prophylaxis has been increasingly recommended in high-risk leukemia patients and bone marrow transplant recipients as a means of reducing the risk for developing IPA (22, 25). Of the antifungals currently available, itraconazole (ITC) is often considered the most practical agent for IPA prophylaxis due to its broad spectrum, availability in both oral and intravenous formulations, and documented efficacy in reducing the frequency of Aspergillus infections among high-risk patients (3, 12, 15, 25). However, breakthrough mold infections still occur despite ITC prophylaxis, particularly in cancer patients with prolonged and profound neutropenia, steroid-refractory graft-versus-host disease, and suboptimal levels of ITC in plasma (11, 12, 22). When these breakthrough infections occur, clinical response to subsequent amphotericin B (AMB) or lipid formulation AMB therapy is especially poor (11).
Although numerous factors (i.e., underlying malignancy, prolonged neutropenia, and corticosteroid therapy, etc.) may account for the dismal activity of subsequent AMB therapy in patients who experience breakthrough IPA while on ITC prophylaxis, there is a possibility of pharmacological attenuation of AMB activity in Aspergillus spp. that have been previously exposed to azoles. A commonly cited theoretical concern is that azoles such as ITC, through their inhibition of sterol biosynthesis and depletion of ergosterol in the cell membrane, exhaust membrane binding targets and antagonize the fungicidal activity of AMB (28, 31). The attenuation of AMB activity by ITC has been documented in some, but not all, in vitro studies examining azole-polyene combinations for Aspergillus species (16, 19, 24). In vivo, concomitant combinations of AMB and ITC have displayed indifference to antagonistic interactions, with some studies reporting complete elimination of AMB activity in animals infected with invasive aspergillosis (10, 19, 23, 26-29, 31).
However, no animal study to date has specifically examined in a controlled fashion the effects of ITC preexposure on the subsequent mycological efficacy of AMB administered at various dosages in a pulmonary model of aspergillosis. To this end, we used a murine model of sinopulmonary aspergillosis to examine the impact of ITC preexposure on the subsequent mycological efficacy of various AMB dosages for acute IPA.
(This work was presented at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 16 to 19 December 2001.)
MATERIALS AND METHODS
Mice.
White female Swiss Webster Mice (Harlan Sprague-Dawley Inc., Indianapolis, Ind.) were used for all experiments and weighed 20 to 25 g at the time of infection. Animals were housed (n = 5 per cage) in presterilized filter-topped cages and provided with sterile food, water, and bedding in the biohazardous isolation suite at The University of Texas M. D. Anderson Cancer Center Animal Care Facilities. Animals were allowed access to food and water ad libitum. All procedures were performed in accordance with the highest standards for humane handling, care, and treatment of research animals as were approved by The University of Texas M. D. Anderson Cancer Center and University of Houston Institutional Animal Care and Use Committees.
Immunosuppression.
All immunosuppressive agents were prepared the day of use. Cyclophosphamide (Sigma Chemical Co., St. Louis, Mo.) was dissolved in sterile saline (15 mg/ml) and administered by intraperitoneal injections (200 to 250 μl) on days −4 and −1 prior to inoculation. This regimen renders mice neutropenic (an absolute neutrophil count of <100/ml) within 4 days of the first cyclophosphamide injection, and neutropenia lasts for 4 days after the last booster injection (day −1) (14). Cortisone acetate (Sigma) was suspended in sterile saline (60 mg/ml) containing 0.1% Tween 80 and administered by subcutaneous injections (200 μl) on days −4 and −1 prior to infection. Based on our previous experience with this model and the relatively short experimental period, it was not necessary to supplement food or drinking water with antibiotic therapy.
Antifungals.
AMB deoxycholate (Pharma-Tek, Huntington, N.Y.) was reconstituted according to the manufacturer's instructions in sterile water and diluted 1:10 in sterile 5% dextrose water for dosing in animals. ITC (Sporanox) oral solution (10 mg/ml) (Janssen Pharmaceuticals, Titusville, N.J.) was administered to animals without dilution.
Test organism.
Aspergillus fumigatus 293 (AF 293; kindly provided by David Denning, University of Manchester, Manchester, United Kingdom) was used to infect animals. The isolate was recovered from lung tissue taken at autopsy from a patient with fatal IPA (Fungal Research Trust [www.aspergillus.man.ac.uk]).
Inoculum.
Cultures were grown on potato dextrose agar slants (Remel, Lenexa, Kans.) at 37°C for 5 days. The inoculum was prepared by washing the surface growth with 0.1% Tween 80 in sterile physiological saline (10 ml) and filtering the suspension through two layers of sterile gauze to remove hyphal fragments. This procedure results in an inoculum of ∼2 × 107 conidia per ml. The spore suspension was then transferred to a sterile 15-ml screw-top tube and centrifuged at 10,000 × g to pellet the conidia. The supernatant (8 ml) was then carefully removed with a sterile pipette, and the conidia were resuspended in the remaining 2 ml to produce the final inoculum of 1 × 108 conidia/ml. Conidium numbers were confirmed with a hemocytometer and counting software (Image Pro Plus; Media Cybernetics, Silver Spring, Md.). Conidium viability was greater than 99%, as determined by quantitative plating of serial dilutions taken from the original inoculum.
Susceptibility testing.
Susceptibility testing was performed by broth microdilution methods as proposed by the NCCLS (standard M38-P) (20). Additionally, Etest strips were used to evaluate the effect of ITC preexposure (0.02 mg/liter) on the MICs of AMB for the test isolate by using previously described methods (16). In selected animals, isolates recovered from the lung were transferred from agar plates to potato dextrose agar slants, grown for 72 h at 37°C, and retested by broth microdilution and Etest without exposure to ITC.
Infection and treatment.
Mice were infected by the sinopulmonary route by using a modification of the method proposed by Dixon et al. (8). Modifications employed in our study included the use of higher cyclophosphamide and cortisone acetate doses as well as a higher inoculum to ensure that we could consistently induce IPA in mice receiving ITC. Immunosuppressed mice were rendered unconscious by using an anesthesia chamber attached to a nebulizer delivering isoflurane (5%) and oxygen (5 liters per min). Anesthesia times for mice typically ranged from 180 to 240 s. During inoculation, mice were held perpendicular to the countertop with the mouth held shut and a single droplet (30 μl) was delivered to the nares with a micropipette. The droplet was then inhaled involuntarily by the unconscious mouse. Noses were then wiped with 70% ethanol, and mice were returned to filter-topped cages to recover for 24 h before treatment with AMB. Animals were observed for 96 h (day +4) after infection and weighed daily to monitor for drug toxicity. Any animal that appeared moribund before day +4 was euthanized by CO2 asphyxiation, and death was recorded as occurring 8 h later.
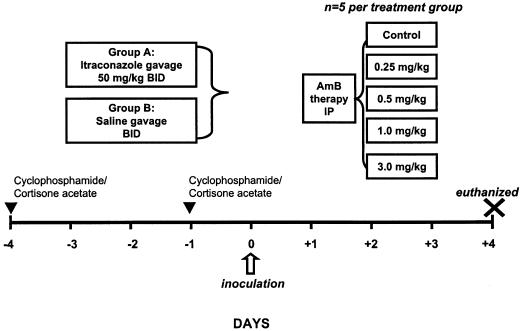
Antifungals were administered to animals by using the dosing scheme represented in Fig. 1. Doses were selected on the basis of previous studies performed in a murine model of bloodstream candidiasis (32). ITC (50 mg/kg of body weight) or saline was administered by feeding needle to animals every 12 h (q12h) for 3 days (days −3 through −1) prior to infection. Mice (5 per treatment group) were then administered saline (control) or AMB (0.25, 0.5, 1, and 3, mg/kg q24h) by intraperitoneal injection for 3 days. At 96 h after inoculation (day +4), all surviving mice were euthanized by CO2 asphyxiation and the lungs were immediately removed for quantitative lung cultures.
FIG. 1.
Schematic of antifungal dosing in the animal model of IPA. BID, twice a day; IP, intraperitoneal.
Quantitative lung cultures.
Lungs were removed after euthanization of the animals, weighed, and homogenized in 2 ml of sterile saline with sterile tissue grinders. Numbers of CFU were determined by performing serial dilutions of the homogenized tissue in sterile saline and plating 50 μl of lung homogenate on 110-mm-diameter potato dextrose agar plates (Remel). Plates were incubated at 37°C, and numbers of CFU were counted at 24 and 48 h. To further assess the mycelial burden of Aspergillus in the lungs of mice, we pooled lung homogenate samples (5 animals per treatment group per sample) and performed the whole-lung chitin assay as described by Lehmann and White (18). Briefly, homogenized tissue is treated with a hot concentrated alkaline solution to form the insoluble product chitosan. An aldehyde product of chitosan is then made upon reaction with NaNO2, and the aldehyde groups are assayed colorimetrically as a glucosamine equivalent versus a glucosamine standard. Each chitin assay included a negative control and a positive spiked control, which consisted of lung homogenate plus 500 μg of purified chitin (Sigma).
Histopathology.
Whole-lung tissue was submitted 72 h after infection from non-ITC-preexposed and ITC-preexposed animals to compare the disease severity by histopathology. Additionally, the whole lungs from three infected mice who did not receive antifungals (control), three infected mice treated with AMB alone, and three infected mice preexposed to ITC and then treated with AMB (1 mg/kg) were evaluated by an experienced veterinary pathologist who was blinded to the treatment groups. Lungs were harvested as described before from mice euthanized at 72 h, fixed with 10% (vol/vol) formaldehyde, and then routinely processed and embedded in paraffin wax. Matched sections were stained with hematoxylin and eosin and Grocott's methamine silver nitrate. Each tissue section was subjectively scored (on a scale from zero to five) by the pathologist during the examination of the slides on the basis of (i) bronchial involvement, (ii) extent of hemorrhage, necrosis, and congestion, and (iii) visible burden of hyphae. A score of zero represented no disease; a score of one represented mild lung congestion, no hemorrhage, and rare hyphae; a score of two represented mild hemorrhage, lung congestion, occasional hemorrhage, and focal hyphae; a score of three represented hemorrhage with necrosis, congestion, and focal hyphae; a score of four represented marked hemorrhage with necrosis, congestion, and multifocal hyphae; and a score of five represented extensive hemorrhage and necrosis, congestion, and multifocal hyphae.
Antifungal pharmacokinetics.
Drug concentrations in animals were verified by a single-dose pharmacokinetic study of AMB administered by three intraperitoneal doses (0.5, 1.0, and 3.0 mg/kg) in infected animals. For each dose, groups of three mice at five separate time points after dosing (0.5, 4, 8, 12, and 24 h) were anesthetized with isoflurane and sampled and/or euthanized by cardiac puncture with a heparanized syringe. Blood was transferred to microcentrifuge tubes and centrifuged at 10,000 × g for 10 min. Plasma was subsequently removed and stored at −70°C until analysis.
Trough levels of ITC in plasma were measured in groups (three immunosuppressed mice each) at three separate time points: days 0, +1, and +2 (12, 36, and 60 h after the last oral dose). At each time point, plasma was collected in a fashion similar to that used in the AMB pharmacokinetic studies.
Drug levels in plasma were verified with high-performance liquid chromatograph assays described by Gubbins et al. (for ITC) and Ng et al. (for AMB) (13, 21). A six-point standard curve method including a blank sample was used. The lower limits of quantitation were the lowest standard concentrations for each compound, 0.125 and 0.025 mg/liter for AMB and ITC, respectively. Accuracies were ≥90% for each compound, and the intra- and interday variations were less than 10% for the range of concentrations tested. The pharmacokinetic parameters of interest were the maximum and minimum concentrations in plasma, the area under the plasma concentration-time curve from 0 to 24 h (AUC0-24), the elimination rate constant, and the half-life. The values for these parameters were calculated by using a noncompartmental method for extravascular administration (WinNonLin, version 3.0; Pharsight, Mountain View, Calif.).
Data analysis.
Mean CFU counts per treatment group and the pooled chitin assay served as the primary endpoints for mycological efficacy. Survival and mean histopathology scores were secondary endpoints of drug efficacy. Analysis of variance was used to assess differences in the primary endpoints, with Tukey's test used for all post hoc comparisons. For secondary endpoints, log rank and Wilcoxon tests were used. For all comparisons, a P value of ≤0.05 was deemed significant.
RESULTS
Susceptibility testing.
Antifungal susceptibility measured by broth microdilution testing (n = 5 determinations) revealed 48-h median MICs of AMB and ITC of 0.5 and 0.25 mg/liter, respectively. When AMB MICs were determined after preexposure to ITC, the median (n = 3) AMB MIC increased from 0.5 to 8 mg/liter. MICS of AMB and ITC for isolates recovered from infected lungs were not elevated, with MICs falling within 1 dilution of the original MIC determination (0.5 and 0.25 mg/liter, respectively).
Infection and quantitative lung cultures.
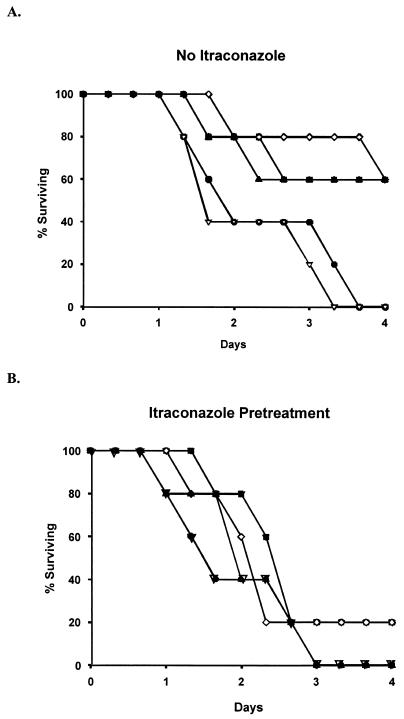
A dose-response relationship for AMB therapy versus survival was observed among non-ITC-pretreated mice (Fig. 2A). At AMB dosages of <0.5 mg/kg/day, the 96-h survival rate was 0 to 20%. At dosages of ≥0.5 mg/kg/day, animal survival improved to 60%. Among animals that were preexposed to ITC, however, AMB doses of ≥0.5 mg/kg/day decreased 96-h survival rates to 20% (Fig. 2B). This survival difference between the non-ITC-preexposed and ITC-preexposed groups was not statistically significant because of the small numbers of animals used in each treatment arm, as survival was not a primary endpoint (P = 0.31). No discernible differences in animal weights were observed between mice that received ITC plus AMB versus those who received AMB alone.
FIG. 2.
Survival curves at various AMB doses for animals pretreated (A) and not pretreated (B) with ITC. •, control; ▿, 0.25 mg of AMB/kg q24h; ▪, 0.5 mg of AMB/kg q24h; ◊, 1.0 mg of AMB/kg q24h; ▴, 3.0 mg of AMB/kg q24h.
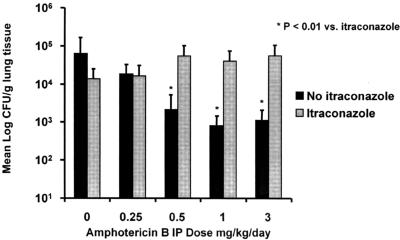
CFU sampling of homogenized lung tissue demonstrated attenuation of AMB dose-response activity in ITC-preexposed animals (Fig. 3). AMB doses that prolonged survival (≥0.5 mg/kg/day) were associated with a 1 to 1.5 log10 reduction in fungal lung burden as measured by CFU counts recovered from lung homogenate. This reduction was not seen in animals preexposed to ITC. Lung CFU counts were significantly higher in ITC-preexposed versus non-ITC-preexposed animals at effective AMB doses (≥0.5 mg/kg/day) (P < 0.01).
FIG. 3.
CFU counts from homogenized lung tissue. IP, intraperitoneal.
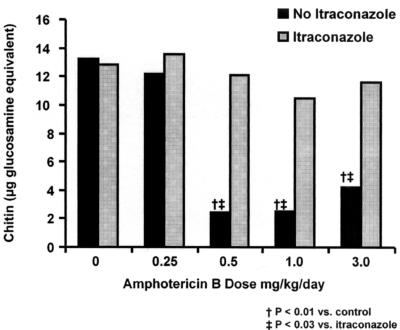
Attenuation of AMB mycological activity could also be appreciated with the whole-lung chitin assay (Fig. 4). Significantly lower chitin concentrations (measured as glucosamine equivalents) were seen among pooled sample groups that received AMB dosages of ≥0.5 mg/kg/day without ITC preexposure (P < 0.01). However, this reduction was not seen with ITC-preexposed animals. Pooled whole-lung chitin concentrations were significantly higher among ITC-preexposed animals at AMB doses of >0.5 mg/kg (P < 0.03).
FIG. 4.
Pooled whole-lung chitin assay results.
Histopathology.
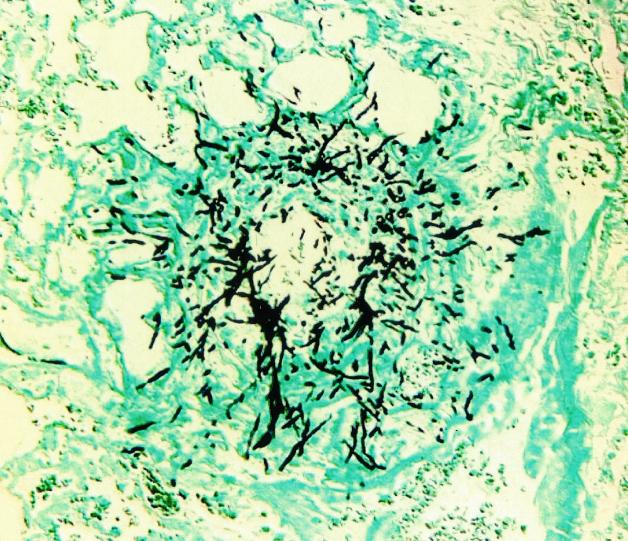
A representative histopathology is presented in Fig. 5. After 72 h of infection, multiple areas of unencapsulated hemorrhage and necrosis, typically surrounding airways, were seen throughout the lung tissue. In each area of necrosis, multiple branching segmented hyphae were observed in the classic sunburst pattern described for human IPA (5). Mean histopathology scores for the treatment groups were as follows: control (untreated), 4.3; AMB (1 mg/kg q24h), 3.3; and ITC (50 mg/kg q12h) then AMB (1 mg/kg q24h), 3.8. The difference in mean histopathology scores between ITC-preexposed and non-ITC-preexposed animals was not significant (P > 0.05).
FIG. 5.
Representative histopathologic section (Grocott's methamine silver nitrate stain) of lung from an animal model 72 h after dosing. Magnification, ×200.
Antifungal pharmacokinetics.
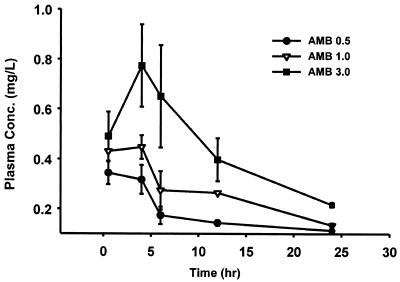
The time course of AMB in serum of infected neutropenic mice following intraperitoneal doses of 0.5, 1.0, and 3.0 mg/kg is shown in Fig. 6. A dose-dependent increase in peak levels in serum was seen within 6 h and ranged from 0.34 ± 0.04 mg/liter (AMB at 0.5 mg/kg) to 0.78 ± 0.17 mg/liter (AMB at 3.0 mg/kg). The elimination half-life ranged from 16 to 22 h, which was similar to previous data in murine models of systemic candidiasis (1). The AUC0-24 ranged from 5.4 to 12.2 mg · h/liter with the lowest and highest doses, respectively.
FIG. 6.
Single-dose serum pharmacokinetics of intraperitoneal AMB doses in infected neutropenic animals.
ITC concentrations in plasma measured in the animals are presented in Table 1. Relatively high concentrations of ITC in plasma and its active hydroxylated metabolite were found 12 h after the last oral dose (day 0) with mean concentrations of 10.9 and 6.9 mg/liter, respectively. The high concentrations achieved in this model may be attributable to the fact that we used a formulation of oral ITC solution marketed for human use rather than a formulation of pulverized ITC capsules administered in a corn oil vehicle, as was reported in a previous study (32). Nevertheless, ITC concentrations were nearly undetectable 24 h later (time of the first AMB dose) and undetectable 60 h after the last dose of ITC.
TABLE 1.
Trough ITC levels in plasma in neutropenic mice
| Compound | Mean concn ± SD (mg/liter) in plasma on day:
|
||
|---|---|---|---|
| 0 | +1 | +2 | |
| ITC | 10.9 ± 3.8 | 0.07 ± 0.03 | Undetectable |
| OH-ITC | 6.9 ± 2.1 | Undetectable | Undetectable |
DISCUSSION
Our study extends observations from previous animal models of systemic aspergillosis that have documented antagonism of AMB activity against Aspergillus infection when used concomitantly with ITC. In this study, we specifically examined the sequential activity of AMB following ITC therapy. We purposely sought to simulate breakthrough IPA during ITC prophylaxis, since this is an increasingly common clinical scenario for the azole-polyene combinations in patients at high risk for IPA (3, 7, 11). The design of our study differs from those of previous animal studies of antifungal combinations in several respects. First, we utilized a sinopulmonary model of infection that more closely simulates the pathophysiology and histopathology of invasive aspergillosis that is seen in humans. In contrast, the majority of previous studies have used an inoculation method of intravenously injecting Aspergillus conidia, which results in disease that localizes predominantly to the kidneys and spreads secondarily to the lungs (17). Because many antifungal agents concentrate in the kidneys of animals (similar to humans), it is conceivable that antifungal activity in these models may be overestimated or not directly translatable to other more clinically relevant sites of human infection such as the lung. Second, we evaluated the effects of ITC pretreatment for multiple increasing daily doses of AMB and found that increasing doses in this model did not overcome the apparent attenuation of AMB activity. Third, we used multiple endpoints to assess this interaction of antifungals, including mycological and biochemical surrogate markers of disease burden, animal survival, and histopathology.
The sequence and timing of antifungal therapies when used in combination are important factors in determining the overall efficacy of the combination. Sugar and Liu examined the interactions of ITC and AMB in a murine model of invasive candidiasis (32). Similar to our study, pretreatment with ITC markedly decreased the ability of effective AMB dosages (1 mg/kg/day) to prolong survival in their model. This antagonism was also seen if AMB and ITC were administered simultaneously but was not present if AMB was administered (sequentially) before ITC. In our study, we only examined a single scenario, the sequential use of AMB after ITC, as we felt this would be the most common clinical scenario for sequential antifungal therapy in high-risk patients who develop breakthrough IPA.
Pharmacokinetic data derived from our model may partially explain why increasing doses of AMB do not overcome the apparent antagonism of AMB activity. At the highest dosage tested, peak AMB concentrations in plasma averaged 0.8 mg/liter with an AUC0-24 of 12.2 mg · h/liter (average 24-h concentration in plasma of approximately 0.5 mg/liter). This range of concentrations in plasma falls well below the median AMB MICs (8 to 16 mg/liter) reported for clinical Aspergillus isolates that were tested after preexposure to ITC (16). For the isolate used in this study (AF 293), the Etest method measured a 16- to 32-fold increase in the MIC of AMB (final MIC of 8 mg/liter) when the isolate was grown on subinhibitory concentrations (0.02 mg/liter) of ITC. This phenomenon is somewhat more difficult to appreciate by NCCLS methods or standard checkerboard dilution studies in RPMI medium because of problems associated with MIC clustering of AMB (20). On the basis of previous animal pharmacokinetic studies with AMB, we estimate that intraperitoneal AMB doses of >20 mg/kg q24h would be required to achieve drug levels in plasma in the range of these AMB MICs for ITC-preexposed strains to overcome this attenuation (1). Given the narrow therapeutic index of AMB, it is unlikely we could administer these doses without significant toxicity to the animals. It may be possible, however, to achieve higher AMB concentrations in plasma by administering the liposomal formulation of AMB, possibly overcoming attenuation of mycological activity that occurred after ITC exposure.
Repeat susceptibility testing was performed for isolates recovered from infected lung tissue that was harvested from animals preexposed to ITC. However, the AMB susceptibility of Aspergillus isolates recovered from these animals did not change by more than one dilution compared to baseline AMB MICs recorded prior to infection. Previous in vitro studies performed in our laboratories have suggested that the in vitro antagonism of AMB by ITC is reversible when fungal material displaying an antagonistic interaction is transferred to an ITC-free liquid culture and then retested on ITC-free agar (16). Studies examining the reversibility of antifungal antagonism, however, are complicated by technical challenges associated with transferring mature drug-exposed hyphae on agar to liquid medium and then back to drug-free agar. Because it would be difficult to isolate drug-exposed hyphae from homogenized lung tissue, isolates grown from infected lungs of ITC-preexposed animals were allowed to germinate and then conidia were harvested and prepared as a standardized suspension for subsequent susceptibility testing (time period of 6 days without ITC exposure). It is possible that this in vitro testing method would not accurately reflect the in vivo susceptibility of ITC-preexposed hyphae to AMB.
Another interesting finding is that the attenuation of AMB activity occurred despite low or nearly undetectable levels of ITC in plasma measured in the animals on the first day of AMB dosing. Concentrations of ITC in tissue, however, are often 2- to 10-fold higher than concurrent levels in plasma (22). Although we cannot extrapolate what the concentrations of ITC were in the lungs of animals at the time of the first AMB dose, it is possible that this attenuation phenomenon may persist for some period after the removal of ITC. Persistent antagonism of AMB activity has been described in a previous animal study of disseminated aspergillosis, even when ITC therapy was stopped before starting AMB therapy (26). Future studies are needed to determine the washout period or reversibility of ITC-mediated antagonism of AMB activity in vivo.
Like any animal study of invasive mycoses, our study has several limitations that need to be considered before data can be extrapolated to the clinical setting. We induced a hyperacute model of IPA in mice that produced disease that is histopathologically similar to that seen in humans with rapidly progressing IPA (17). However, the high tissue fungal burden in our model and the poor efficacy of sequential AMB following ITC may not necessarily reflect the activity of AMB seen in other, potentially more common, subacute forms of invasive aspergillosis. The acute nature of IPA in this model also prevented us from correlating mycological endpoints used in this study with histopathological findings. Finally, we cannot completely exclude the possibility that preexposure to ITC enhances the toxicological effects of AMB, although animal weight did not significantly differ among the treatment groups. Irrespective of toxicity concerns, mycological endpoints used in this model clearly demonstrated attenuation of AMB activity in animals that were previously exposed to ITC.
In terms of mycological endpoints, it is generally acknowledged that CFU quantitation does not accurately reflect the number of viable cells or disease burden in animal lungs (17). For this reason, we also employed the chitin assay as a means of indirectly quantitating the mycelial burden in infected lung tissue. Like previous investigators, we found that this assay is a good index of fungal burden in IPA and correlates well with animal survival (9, 18, 30). However, this assay was labor intensive to perform and required pooling of samples from animals in each treatment group. Sample pooling hindered our ability to assess the potential variability of antifungal attenuation within each dosage arm. It is of interest that we actually saw a correlation between CFU quantitation and the chitin assay, which may be due to the acute nature of our model. Recent work by Bowman et al. examining real-time PCR as a method for quantitation of Aspergillus burden in tissue found a correlation between PCR and CFU counts in early infection, when mycelial mass is made up largely of small germlings (4). As the infection progressed over time, however, the fungal burden increased by extension of the mycelial network, which was not reflected by an increase in CFU counts. Therefore, we conclude that at the time animals were euthanized in our study, the filamentous network in tissue was not too extensive and CFU counts continued to serve as a relatively accurate index of fungal burden. This conclusion is supported by histopathology at 72 h, which did not demonstrate an extensive filamentous network of hyphae in the lung (Fig. 5).
Overall, our results point to the possibility of a clinically significant attenuation of AMB activity when used sequentially after ITC therapy. Whether or not this attenuation can be demonstrated in vivo after exposure to other broad-spectrum azoles (voriconazole and posaconazole) or agents with minimal Aspergillus activity (fluconazole) should be examined in future animal studies. Given the high baseline mortality of IPA in patients with hematological malignancies, this attenuation may be difficult to clinically appreciate in the current era of poor diagnostics and disease monitoring tools for Aspergillus infections. With the introduction of new treatment options for IPA, it will be important to differentiate the sequence and timing of antifungal therapy for IPA, as well as which therapies retain activity against Aspergillus species after breakthrough infections develop with a previously administered class of antifungals. Specifically, we agree with Shaffner and Frick that future clinical studies examining the use of azole antifungals for either prophylaxis or preemptive therapy during persistent febrile neutropenia should include analysis of subsequent AMB therapy response rates among patients who develop breakthrough IPA (27).
Acknowledgments
We thank Paul Gubbins (University of Arkansas) for help with the itraconazole assay and L. Clifton Stevens (University of Texas M. D. Anderson Cancer Center) for assistance with the histopathology studies.
This work was supported by The University of Texas M. D. Anderson Cancer Center Physician Referral Service grant awarded to D.K. The Animal Care Unit at M. D. Anderson Cancer Center is supported by NIH-NCI Cancer Center CORE Support grant no. 16672.
REFERENCES
- 1.Andes, D., T. Stamsted, and R. Conklin. 2001. Pharmacodynamics of amphotericin B in a neutropenic-mouse disseminated-candidiasis model. Antimicrob. Agents Chemother. 45:922-926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bodey, G., B. Bueltmann, W. Duguid, D. Gibbs, H. Hanak, M. Hotchi, G. Mall, P. Martino, F. Meunier, S. Milliken, et al. 1992. Fungal infections in cancer patients: an international autopsy survey. Eur. J. Clin. Microbiol. Infect. Dis. 11:99-109. [DOI] [PubMed] [Google Scholar]
- 3.Boogaerts, M., J. Maertens, A. van Hoof, R. de Bock, G. Fillet, M. Peetermans, D. Selleslag, B. Vandercam, K. Vandewoude, P. Zachee, and K. De Beule. 2001. Itraconazole versus amphotericin B plus nystatin in the prophylaxis of fungal infections in neutropenic cancer patients. J. Antimicrob. Chemother. 48:97-103. [DOI] [PubMed] [Google Scholar]
- 4.Bowman, J. C., G. K. Abruzzo, J. W. Anderson, A. M. Flattery, C. J. Gill, V. B. Pikounis, D. M. Schmatz, P. A. Liberator, and C. M. Douglas. 2001. Quantitative PCR assay to measure Aspergillus fumigatus burden in a murine model of disseminated aspergillosis: demonstration of efficacy of caspofungin acetate. Antimicrob. Agents Chemother. 45:3474-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandler, F. W., W. Kaplan, and L. Ajello. 1980. Color atlas and text of the histopathology of mycotic diseases. Year Book Medical Publishers, Chicago, Ill.
- 6.Denning, D. W. 1996. Therapeutic outcome in invasive aspergillosis. Clin. Infect. Dis. 23:608-615. [DOI] [PubMed] [Google Scholar]
- 7.Denning, D. W., A. Marinus, J. Cohen, D. Spence, R. Herbrecht, L. Pagano, C. Kibbler, V. Kcrmery, F. Offner, C. Cordonnier, U. Jehn, M. Ellis, L. Collette, R. Sylvester, et al. 1998. An EORTC multicentre prospective survey of invasive aspergillosis in haematological patients: diagnosis and therapeutic outcome. J. Infect. 37:173-180. [DOI] [PubMed] [Google Scholar]
- 8.Dixon, D. M., A. Polak, and T. J. Walsh. 1989. Fungus dose-dependent primary pulmonary aspergillosis in immunosuppressed mice. Infect. Immun. 57:1452-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenstein, D. J., P. W. Biddinger, and J. C. Rhodes. 1990. Experimental murine invasive pulmonary aspergillosis. Am. J. Clin. Pathol. 93:510-515. [DOI] [PubMed] [Google Scholar]
- 10.George, D., D. Kordick, P. Miniter, T. F. Patterson, and V. T. Andriole. 1993. Combination therapy in experimental invasive aspergillosis. J. Infect. Dis. 168:692-698. [DOI] [PubMed] [Google Scholar]
- 11.Glasmacher, A., C. Hahn, C. Leutner, E. Molitor, E. Wardelmann, C. Losem, T. Sauerbruch, G. Marklein, and I. G. Schmidt-Wolf. 1999. Breakthrough invasive fungal infections in neutropenic patients after prophylaxis with itraconazole. Mycoses 42:443-451. [DOI] [PubMed] [Google Scholar]
- 12.Glasmacher, A., E. Molitor, C. Hahn, K. Bomba, S. Ewig, C. Leutner, E. Wardelmann, I. G. Schmidt-Wolf, J. Mezger, G. Marklein, and T. Sauerbruch. 1998. Antifungal prophylaxis with itraconazole in neutropenic patients with acute leukaemia. Leukemia 12:1338-1343. [DOI] [PubMed] [Google Scholar]
- 13.Gubbins, P. O., B. J. Gurley, and J. Bowman. 1998. Rapid and sensitive high performance liquid chromatographic method for the determination of itraconazole and its hydroxy-metabolite in human serum. J. Pharm. Biomed. Anal. 16:1005-1012. [DOI] [PubMed] [Google Scholar]
- 14.Gudmundsson, S., and H. Erlenddottir. 1999. Murine thigh infection model, p. 137-144. In O. Zak and M. A. Sande (ed.), Handbook of animal models of infection. Academic Press, London, England.
- 15.Harousseau, J. L., A. W. Dekker, A. Stamatoullas-Bastard, A. Fassas, W. Linkesch, J. Gouveia, R. De Bock, M. Rovira, W. F. Seifert, H. Joosen, M. Peeters, and K. De Beule. 2000. Itraconazole oral solution for primary prophylaxis of fungal infections in patients with hematological malignancy and profound neutropenia: a randomized, double-blind, double-placebo, multicenter trial comparing itraconazole and amphotericin B. Antimicrob. Agents Chemother. 44:1887-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kontoyiannis, D. P., R. E. Lewis, N. Sagar, G. May, R. A. Prince, and K. V. Rolston. 2000. Itraconazole-amphotericin B antagonism in Aspergillus fumigatus: an E-test-based strategy. Antimicrob. Agents Chemother. 44:2915-2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Latge, J. P. 1999. Aspergillus fumigatus and aspergillosis. Clin. Microbiol. Rev. 12:310-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehmann, P. F., and L. O. White. 1975. Chitin assay used to demonstrate renal localization and cortisone-enhanced growth of Aspergillus fumigatus mycelium in mice. Infect. Immun. 12:987-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewis, R. E., and D. P. Kontoyiannis. 2001. Rationale for combination antifungal therapy. Pharmacotherapy 21:149S-164S. [DOI] [PubMed]
- 20.National Committee for Clinical Laboratory Standards. 1999. Reference method for broth dilution antifungal susceptibility testing of conidium forming filamentous fungi. Proposed standard M38-P. NCCLS, Wayne, Pa.
- 21.Ng, T. K., R. C. Chan, F. A. Adeyemi-Doro, S. W. Cheung, and A. F. Cheng. 1996. Rapid high performance liquid chromatographic assay for antifungal agents in human sera. J. Antimicrob. Chemother. 37:465-472. [DOI] [PubMed] [Google Scholar]
- 22.Poirier, J. M., and G. Cheymol. 1998. Optimisation of itraconazole therapy using target drug concentrations. Clin. Pharmacokinet. 35:461-473. [DOI] [PubMed] [Google Scholar]
- 23.Polak, A. 1987. Combination therapy of experimental candidiasis, cryptococcosis, aspergillosis and wangiellosis in mice. Chemotherapy 33:381-395. [DOI] [PubMed] [Google Scholar]
- 24.Polak, A. 1999. The past, present and future of antimycotic combination therapy. Mycoses 42:355-370. [DOI] [PubMed] [Google Scholar]
- 25.Prentice, A. G., and P. Donnelly. 2001. Oral antifungals as prophylaxis in haematological malignancy. Blood Rev. 15:1-8. [DOI] [PubMed] [Google Scholar]
- 26.Schaffner, A., and A. Bohler. 1993. Amphotericin B refractory aspergillosis after itraconazole: evidence for significant antagonism. Mycoses 36:421-424. [DOI] [PubMed] [Google Scholar]
- 27.Schaffner, A., and P. G. Frick. 1985. The effect of ketoconazole on amphotericin B in a model of disseminated aspergillosis. J. Infect. Dis. 151:902-910. [DOI] [PubMed] [Google Scholar]
- 28.Scheven, M., C. Scheven, K. Hahn, and A. Senf. 1995. Post-antibiotic effect and post-expositional polyene antagonism of azole antifungal agents in Candida albicans: dependence on substance lipophilia. Mycoses 38:435-442. [DOI] [PubMed] [Google Scholar]
- 29.Schmitt, H. J., E. M. Bernard, F. F. Edwards, and D. Armstrong. 1991. Combination therapy in a model of pulmonary aspergillosis. Mycoses 34:281-285. [DOI] [PubMed] [Google Scholar]
- 30.Spreadbury, C. L., T. Krausz, S. Pervez, and J. Cohen. 1989. Invasive aspergillosis: clinical and pathological features of a new animal model. J. Med. Vet. Mycol. 27:5-15. [DOI] [PubMed] [Google Scholar]
- 31.Sugar, A. M. 1995. Use of amphotericin B with azole antifungal drugs: what are we doing? Antimicrob. Agents Chemother. 39:1907-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugar, A. M., and X. P. Liu. 1998. Interactions of itraconazole with amphotericin B in the treatment of murine invasive candidiasis. J. Infect. Dis. 177:1660-1663. [DOI] [PubMed] [Google Scholar]