Abstract
A field collected population of Plutella xylostella (SERD4) was selected in the laboratory with Bacillus thuringiensis endotoxins Cry1Ac (Cry1Ac-SEL) and Cry1Ab (Cry1Ab-SEL). Both subpopulations showed similar phenotypes: high resistance to the Cry1A toxins and little cross-resistance to Cry1Ca or Cry1D. A previous analysis of the Cry1Ac-SEL showed incompletely dominant resistance to Cry1Ac with more than one factor, at least one of which was sex influenced. In the present study reciprocal mass crosses between Cry1Ab-SEL and a laboratory susceptible population (ROTH) provided evidence that Cry1Ab resistance was also inherited as incompletely dominant trait with more than one factor, and at least one of the factors was sex influenced. Analysis of single pair mating indicated that Cry1Ab-SEL was still heterogeneous for Cry1Ab resistance genes, showing genes with different degrees of dominance. Binding studies showed a large reduction of specific binding of Cry1Ab and Cry1Ac to midgut membrane vesicles of the Cry1Ab-SEL subpopulation. Cry1Ab-SEL was found to be more susceptible to trypsin-activated Cry1Ab toxin than protoxin, although no defect in toxin activation was found. Present and previous results indicate a common basis of resistance to both Cry1Ab and Cry1Ac in selected subpopulations and suggest that a similar set of resistance genes are responsible for resistance to Cry1Ab and Cry1Ac and are selected whichever toxin was used. The possibility of an incompletely dominant trait of resistant to these toxins should be taken into account when considering refuge resistance management strategies.
Products formulated with Bacillus thuringiensis have been used as niche products for pest control for over 30 years (30). It had been presumed that resistance to B. thuringiensis toxins was unlikely because of its unique mode of action. However, the potential benefits of B. thuringiensis may rapidly be lost due to proliferation of highly resistant insect populations (5). The constitutive expression of B. thuringiensis toxins in transgenic plants will increase the selection pressure in insects (5). and it is considered a major threat to the long-term success of B. thuringiensis (36). Several B. thuringiensis-resistant populations have been selected in the laboratory, but the diamondback moth, Plutella xylostella (L.), and Trichoplusia ni have been reported to evolve resistance in the field following spray applications of B. thuringiensis-based insecticides (5, 14, 30).
Several mechanisms of insect resistance to B. thuringiensis toxins have been proposed (5). One involves changes in the binding of toxins to gut receptors. For example, decreases in toxin binding have been reported in resistant strains of P. xylostella (5, 28). Slower activation, faster degradation (7), differential proteolytic activation (16), and sequestration by proteases (21) have all been described as potential alternative mechanisms of resistance to B. thuringiensis toxins.
In a previous study, a population of P. xylostella from Malaysia (SERD4) with a history of exposure to B. thuringiensis subsp. kurstaki was collected and divided into several subpopulations. Each subpopulation was subjected to a different selecting B. thuringiensis toxin (29). A subpopulation selected with Cry1Ac (Cry1Ac-SEL) showed a very high level of resistance (142,000-fold compared to the susceptible population) to this toxin and cross-resistance to Cry1Ab (2,630-fold compared to the susceptible population). Genetic studies with Cry1Ac-SEL showed that the inheritance of resistance to Cry1Ac was incompletely dominant, sex influenced, and controlled by more than one locus (29). Similarly, the subpopulation selected with Cry1Ab (Cry1Ab-SEL) showed a high level of resistance (3,848-fold) to Cry1Ab and cross-resistance (at a much higher level) to Cry1Ac (64,210-fold). Likewise in a previous study (31) we had found that an alternative resistant P. xylostella population (SERD5) was more susceptible to trypsin-activated toxin than crystalline protoxin. The same study found that solubilized protoxin was considerably less toxic than crystalline protoxin.
We sought here to study the genetic and biochemical bases of resistance of Cry1Ab-SEL to Cry1Ab and to compare these to the nature of Cry1Ac resistance in Cry1Ac-SEL.
MATERIALS AND METHODS
Insect populations.
A field population of P. xylostella was collected from Serdang, Malaysia, in December 1998 and designated SERD4 (28). The Cry1Ab-selected (Cry1Ab-SEL) and Cry1Ac-selected (Cry1Ac-SEL) subpopulations of SERD4 showed resistance ratios of 3,848 and 2,630 to Cry1Ab and of 64,210 and 141,900 to Cry1Ac, respectively, compared to the susceptible population ROTH (29). Unfortunately, ROTH was then lost to disease, but it was replaced with an alternative susceptible population LAB-UK. Both susceptible populations were obtained from Rothamsted Research (Harpenden, Hertfordshire, United Kingdom), where they had been maintained in the laboratory for more than 250 generations. Insect larvae were reared and tested on 4- to 6-week-old pesticide-free greenhouse-grown Chinese cabbage (Brassica pekinensis cv. Tip Top) at 20°C and ca. 65% relative humidity under a 16-h photophase.
Inheritance of the resistance.
Degree of dominance for the 50% lethal concentration (LC50) (DLC) was calculated according to the method of Bourguet et al. (3). The resulting parameters range from 0 (completely recessive resistance) to 1 (completely dominant resistance). Maternal effects and sex linkage of the resistance to Cry1Ab were evaluated by testing F1 progeny of mass crosses using 40 insects of each sex and single-pair crosses. Mass crosses provided enough offspring for multiple concentration testing and calculation of LC50 values.
Interstrain complementation tests for allelism.
To determine whether the locus or loci responsible for resistance to Cry1Ab and Cry1Ac varied among populations, we performed complementation tests for allelism between Cry1Ab-SEL and Cry1Ac-SEL strains as described by Tabashnik et al. (34). Using bioassays, we tested the offspring of reciprocal crosses of selected strains. Since resistance to Cry1Ab and Cry1Ac is incompletely dominant at LC50 but depends upon the concentrations tested, we used a discriminatory dose of 8 μg ml−1, at which resistance was completely recessive. If two resistant strains are crossed, each with recessive alleles for resistance at separate loci, allelic complementation will restore susceptibility (the wild-type phenotype) in the progeny. However, if the recessive resistance alleles occur at the same locus in different populations, the progeny will be resistant because they will inherit resistance alleles at the same locus from both parents (34).
Toxin preparation for binding experiments.
Cry1Ab and Cry1Ca protoxins were expressed as inclusion bodies in Escherichia coli (kindly supplied by Ruud A. de Maagd, Plant Research International B.V., Wageningen, The Netherlands). E. coli was grown at 37°C in TB medium (17 mM KH2PO4, 72 mM K2HPO4, 10.8 g of tryptone/liter, 21.6 g of yeast extract/liter, 0.36% glycerol) supplemented with 25 μg of ampicillin/ml. Each 1 g of cell culture expressing the different toxins was resuspended in 3 ml of lysis buffer (50 mM Tris-HCl [pH 8.0], 5 mM EDTA, 100 mM NaCl), and then 800 μg of lysozyme was added per g of pellet. After incubation at room temperature for 20 min, 1 mg of deoxycholic acid/ml was added, followed by incubation at 37°C for 30 min. DNA was digested by 50 μg of DNase I/ml, and the incubation was continued for another 30 min. After a centrifugation at 40,000 × g for 20 min, pellets containing the protoxin inclusion bodies were washed several times with wash buffer (20 mM Tris-HCl [pH 7.5], 1% Triton X-100, 1 M NaCl). Protoxin in the inclusion bodies was solubilized by incubation for 2 h at 37°C with solubilization buffer (50 mM sodium carbonate [pH 10.0], 100 mM NaCl, 10 mM dithiothreitol). Soluble protoxin was separated from insoluble fraction by centrifugation at 40,000 × g for 20 min. Protoxin was activated adding trypsin at a ratio of 1:10 (trypsin-protoxin [wt/wt]), followed by incubation for 2 h at 37°C. Any insoluble material was removed by centrifugation at 15,800 × g for 15 min at room temperature. Cry1Ac toxin was purified from a recombinant B. thuringiensis strain EG11070 (Ecogen, Inc.). Bacteria were grown in CCY medium (33) supplemented with 3 μg of chloramphenicol/ml for 48 h at 30°C. Crystals and spores were collected by centrifugation at 9,700 × g for 12 min. The pellet was washed three times with ice-cold 1 M NaCl-10 mM EDTA. The pellet was solubilized with solubilization buffer (50 mM carbonate buffer [pH 10.5], 10 mM dithiothreitol). The protoxin solubilized was separated from spores by centrifugation at 9,700 × g for 10 min. Protoxin was activated with trypsin as indicated above.
Trypsin-activated toxins Cry1Ab, Cry1Ac, and Cry1Ca for binding experiments were dialyzed against Tris-NaCl buffer (20 mM Tris-HCl, 150 mM NaCl [pH 9.0]) overnight at 4°C. The dialyzed solution was purified by MonoQ HR 5/5 anion-exchange column (fast-protein liquid chromatography system; Amersham, Uppsala, Sweden) as described previously (32). For Cry1Ca, an additional purification step was performed, following the procedure described by Zhao et al. (40).
Binding experiments.
Analyses with brush border membrane vesicles (BBMV) from 1 g (approximately, 250 larvae) of whole Cry1Ab-SEL and LAB-UK last-instar larvae were conducted as described by Sayyed et al. (28). Cry1Ab was 125I labeled by a chloramine-T method (38). Cry1Ac and Cry1Ca were 125I labeled by the Iodo-Bead (Pierce, Rockford, Ill.) method (15). Specific activities of the labeled proteins were 125I-labeled Cry1Ab (125I-Cry1Ab; 0.48 mCi/mg), 125I-Cry1Ac (0.08 mCi/mg), and 125I-Cry1Ca (0.02 mCi/mg) as determined by an enzyme-linked immunosorbent sandwich assay (39). Binding experiments were performed as described previously (28). Cry1Ca binding experiments were performed as a control to check qualitatively the function of the BBMVs in both populations. All binding experiments were duplicated. Control experiments with ROTH population using increasing amounts of labeled toxins determined the optimal concentrations used in the experiments (1, 6, and 24 nM for 125I-Cry1Ab, 125I-Cry1Ac, and 125I-Cry1Ca, respectively). The radioactivity in the pellet was measured in a model 1282 Compugamma CS gamma counter (LKB Pharmacia). The results from binding experiments with the LAB-UK population were previously presented in Sayyed et al. (27) and were performed in parallel with the Cry1Ab-SEL ones. Nonspecific binding was determined with an excess of unlabeled toxin, obtaining the following values at 0.3 mg of BBMV/ml from the total binding: 12% for Cry1Ab, 1.5% for Cry1Ac, and 2.2% for Cry1Ca.
Bioassays.
Cry1Ab for bioassays was expressed as inclusion bodies in E. coli. The inclusions were purified by sonication and successive washes with 0.5 M NaCl and water. Activated toxin was produced by incubation of crystals in a 50 mM sodium carbonate-bicarbonate buffer (pH 9.8) with 1 mg of trypsin ml−1 at 37°C overnight (32). All bioassays were conducted with third-instar larvae of P. xylostella on leaf disks as described by Sayyed et al. (32). Each leaf disk (4.8-cm diameter) was immersed in a test solution for 10 s and allowed to dry at ambient temperature for 1 to 1.5 h (29). Control leaf disks were immersed in distilled water with Triton X-100. The leaf disks were placed in individual petri dishes (5-cm diameter) containing moistened filter paper. Five larvae were placed in each dish, and each treatment was repeated eight times. Mortality was determined after 5days. Estimates of LC50 values and their 95% fiducial limits (FL) were obtained by maximum-likelihood logit regression analysis in a generalized linear modeling using the statistical package GLIM 3.77 (Numerical Algorithms Group, 1985), from which differences between sets were extracted by analysis of deviance. Differences between the LC50 values of two sets were considered significant (P < 0.01) if their 95% FL did not overlap (29).
Protoxin digestions.
Gut extracts from P. xylostella were obtained by dissecting guts from approximately 50 fourth-instar larvae from a given population and resuspending them in 50 mM sodium carbonate-bicarbonate buffer (pH 9.8; 10 μl per gut). After vigorous mixing and centrifugation for 10 min in a microfuge, the soluble fraction was retained. For digestion studies, Cry1Ab protoxin was suspended at 0.5 mg/ml in 50 mM sodium carbonate-bicarbonate buffer (pH 9.8), gut extract (2.5% [vol/vol]) was added, and the sample was incubated at 37°C. Samples were removed at 1 and 24 h and centrifuged for 10 min in a microfuge. The soluble products from this incubation were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
RESULTS
Inheritance of the resistance.
Mass reciprocal crosses were performed between the Cry1Ab-SEL colony and ROTH, and the susceptibility to Cry1Ab of F1 offspring was analyzed (Fig. 1). The LC50 and slope values (Table 1) of F1 progeny of Cry1Ab-SEL males × ROTH females were significantly (P < 0.01 and P < 0.05, respectively) different from F1 progeny of Cry1Ab-SEL females × ROTH males, indicating that parental sex influenced the expression of resistance in offspring.
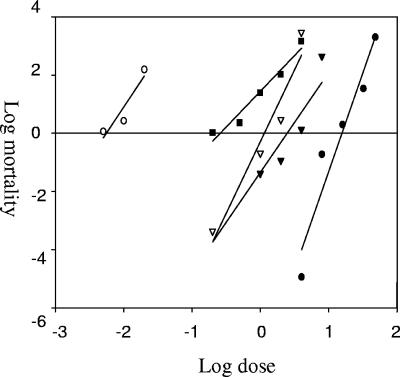
FIG. 1.
Response to Cry1Ab of P. xylostella larvae from susceptible (ROTH population) (○), resistant (Cry1Ab-SEL population) (•), F1 progeny (resistant male × susceptible female) (▾), F1 progeny (resistant female × susceptible male) (▿), and backcross progeny (susceptible × F1 (pooled) (▪).
TABLE 1.
Toxicity of Cry1Ab protoxin to resistant (Cry1Ab-SEL) and susceptible (ROTH) populations, F1, and back-cross progenies
| Population | Nh | LC50 (μg/ml) | 95% FL | Slope ± SE | RRa |
|---|---|---|---|---|---|
| Cry1Ab-SEL | 182 | 14.73 | 12.60-17.64 | 6.52 ± 0.69 | 2,455 |
| ROTH | 182 | 0.006 | 0.002-0.009 | 2.67 ± 0.95 | |
| Reciprocal crosses | |||||
| F1b | 184 | 2.94 | 2.26-3.90 | 3.65 ± 0.63 | 490 |
| F1c | 154 | 1.38 | 1.25-1.72 | 5.4 ± 0.95 | 230 |
| F1 (pooled) | 338 | 2.02 | 1.66-2.45 | 3.62 ± 0.46 | 337 |
| Backcrosses | |||||
| BCd | 214 | 0.12 | 0.019-0.22 | 2.36 ± 0.69 | 20 |
| BCe | 210 | 0.40 | 0.20-0.61 | 1.82 ± 0.40 | 67 |
| BCf | 182 | 0.18 | 0.04-0.31 | 2.09 ± 0.62 | 30 |
| BCg | 191 | 0.43 | 0.26-0.60 | 2.52 ± 0.47 | 72 |
| BC (pooled) | 660 | 0.24 | 0.17-0.31 | 2.21 ± 0.24 | 40 |
RR, resistance ratio (the LC50 for the strain divided by the LC50 for ROTH population).
Progeny of mass crosses between 40 males of Cry1Ab-SEL and 40 females of ROTH.
Progeny of mass crosses between 40 females of Cry1Ab-SEL and 40 males of ROTH.
Progeny of backcrosses between 40 females of Cry1Ab-SEL female F1 progeny and 40 males of ROTH.
Progeny of backcrosses between 40 males of Cry1Ab-SEL female F1 progeny and 40 females of ROTH.
Progeny of backcrosses between 40 females of Cry1Ab-SEL male F1 progeny and 40 males of ROTH.
Progeny of backcrosses between 40 males of Cry1Ab-SEL male F1 progeny and 40 females of ROTH.
N = number of larvae tested, including controls.
The LC50s of F1 progenies from mass crosses yielded dominance values (DLC) of 0.79 and 0.70, respectively, suggesting resistance was incompletely dominant at LC50.
The estimated slope of the log mortality line for pooled backcross progeny was 2.21, which can be considered similar to the estimated slope for ROTH, half of the slope of F1 pooled progeny and one-third of the slope of the resistant population (Cry1Ab-SEL) (Table 1). This pattern of slopes indicates an increase of genetic variance in the backcross progeny compared to Cry1Ab-SEL and F1 progeny. This increased genetic variance suggests that the number of loci with major effects on resistance to Cry1Ab was high. The direct test of a monogenic model showed significant deviation (P < 0.05) between observed and expected mortality at all concentrations tested (Table 2).
TABLE 2.
Direct test of monogenic inheritance for resistance to Cry1Ab protoxin by comparing expected and observed mortality of the backcross F1 and ROTH populations of P. xylostella
| Concn (μg ml−1) | No. of larvae tested | Observed mortality (%) | Expected mortalitya (%) | χ2 (df = 1)b | P |
|---|---|---|---|---|---|
| 0.2 | 130 | 50 | 2 | 114.87 | <0.001 |
| 0.5 | 134 | 58 | 21 | 125.69 | <0.001 |
| 1 | 135 | 80 | 26 | 210.29 | <0.001 |
| 2 | 133 | 89 | 31 | 207.16 | <0.001 |
| 4 | 128 | 96 | 42 | 153.76 | <0.001 |
Expected number of larvae dead at given dose = 0.5 (number of F1 larvae that die + number of selected larvae that die).
df = degrees of freedom.
The homozygosity of insects for resistance genes in Cry1Ab-SEL population was tested by the analysis of F1 progeny from single-pair crosses (Fig. 2). Progeny were tested with two doses of Cry1Ab that provide 100% of mortality for ROTH population and 0% mortality for the selected population.
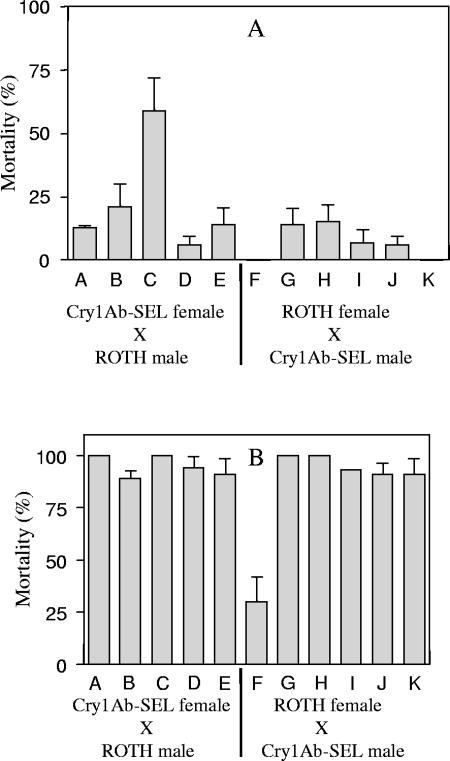
FIG. 2.
Mortality (%) in the F1 from reciprocal single-pair hybrid families between Cry1Ab-SEL and ROTH populations of P. xylostella. Each letter represents a single-pair cross. The mortality is obtained at two different doses of Cry1Ab: 0.2 μg/ml (Α) and 4 μg/ml (Β). The expected mortalities of the F1 progeny were 50 and 48% at 0.2 and 4 μg/ml, respectively. Some families in Fig. 2B do not show error bars because of 100% mortality.
In general, results of the family analysis are similar with the mass crosses at 0.2 μg ml−1 (Fig. 1); however, differential mortality observed at 4 μg ml−1 in mass crosses (Fig. 1) was not observed in most of the single-pair families. The mortality observed in a single pair (Fig. 2) was heterogeneous at 0.2 μg ml−1 dose, whereas family C showed a significantly higher mortality (59%) (t = 8.57, df = 70, P < 0.05) compared to the other families (mean 14%). At a 4-μg ml−1 dose, mortality was more homogeneous among the families except for the family F that showed a significantly lower mortality (30%) (t = 4.83, df = 70, P < 0.05) compared to the mean (89%) obtained with the rest of families.
Interstrain complementation tests for allelism.
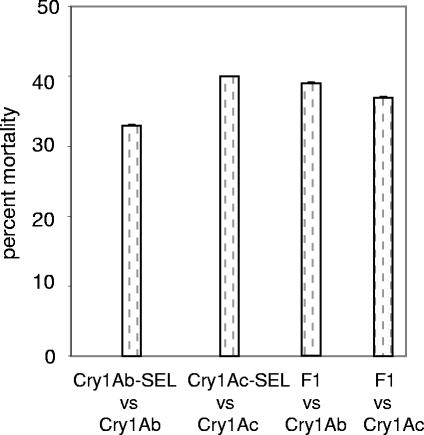
F1 progeny from crosses between Cry1Ab-SEL and Cry1Ac-SEL were similar to parents in resistance to Cry1Ab and Cry1Ac (range in mortality, 33 to 40%; n = 20,100 larvae per toxin; Fig. 3). These results suggest that Cry1Ab-SEL and Cry1Ac-SEL share a genetic locus that controls resistance to both Cry1Ab and Cry1Ac.
FIG. 3.
Response to B. thuringiensis protoxins Cry1Ab and Cry1Ac in Cry1Ab-SEL, Cry1Ac-SEL SERD4, and F1 (Cry1Ab-SEL × Cry1Ac-SEL), respectively. Error bars represent standard errors, and two populations had very low standard errors.
Susceptibility of Cry1Ab-SEL to activated Cry1Ab toxin.
In a previous study we showed ROTH population to be equally susceptible to the activated and protoxin forms of Cry1Ac (31). In this present study there was no significant difference in susceptibility to protoxin or toxin of ROTH and LAB-UK for Cry1Ab (Table 3). In contrast, activated Cry1Ab toxin was significantly more toxic toward the resistant population than the protoxin.
TABLE 3.
Toxicity of Cry1Ab protoxin and activated toxin on susceptible and resistant populations of P. xylostella
| Population | Toxin | LC50 (95% FL) (nM) | Toxicity ratioa |
|---|---|---|---|
| LAB-UK | Cry1Ab protoxin | 0.23 (0.07-0.54) | |
| Cry1Ab toxin | 0.092 (0.015-0.24) | ||
| ROTH | Cry1Ab protoxin | 0.07 (0.015-0.2) | |
| Cry1Ab toxin | 0.015 (0.0015-0.054) | ||
| Cry1Ab-SEL | Cry1Ab protoxin | 444 (214-1117) | 12 |
| Cry1Ab toxin | 37 (12-73) |
LC50 of protoxin/LC50 of activated toxin.
Cleavage of Cry1Ab and Cry1Ac by gut extracts from the resistant populations.
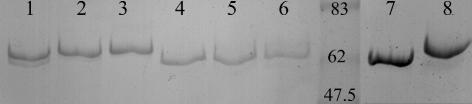
To test the possibility that the larvae were defective in toxin activation Cry1Ab protoxin was treated with gut extracts from Cry1Ab-SEL, Cry1Ac-SEL, or LAB-UK. The soluble products from these incubations were analyzed by SDS-PAGE (Fig. 4). The relative sizes of the toxin fragments obtained are compared in Fig. 4 with trypsin-activated Cry1Ac (lane 7) and the results of a trypsin digest of a mutant Cry1Ac toxin (2) that is not cleaved at the N terminus by trypsin (lane 8). The lower band in lane 7 should correspond to fully N and C terminally activated toxin and the larger band in lane 8 should correspond to N terminally intact, but C terminally activated toxin. The results show that in all cases proteolytic processing of the protoxin for 1 h produced a partial digestion with the relative size of the main band, a finding consistent with an N terminally intact but a C terminally activated toxin. A faint band representing fully processed toxin can be seen particularly with the Cry1Ab protoxin treated with gut extract from Lab-UK. Prolonged incubation over 24 h resulted in complete activation of the toxin by all gut extracts. There was no obvious difference in the final Cry1Ab products whichever gut extract was used, suggesting that the gut extracts from the resistant larvae were clearly capable of activating the toxin in solution.
FIG. 4.
SDS-PAGE analysis of the proteolytic processing of Cry1Ab protoxin by gut extracts from resistant and susceptible insects. Lanes 1 to 3, 1-h incubation; lanes 4 to 6, 24-h incubation; lanes 1 and 4, gut extract from LAB-UK; lanes 2 and 5, gut extract from Cry1Ab-SEL; lanes 3 and 6, gut extract from Cry1Ac-SEL; lane 7, trypsin-activated Cry1Ac control; lane 8, trypsin-activated mutant Cry1Ac control. The molecular weight marker is included in the gel between lanes 6 and 7.
Binding assays.
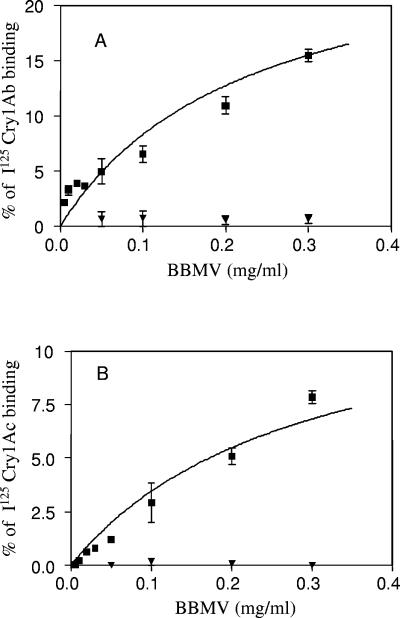
Binding of trypsin-activated Cry1Ab and Cry1Ac toxin to BBMV was evaluated in the resistant Cry1Ab-SEL and susceptible LAB-UK colonies by incubating iodinated toxins with different concentrations of BBMV (Fig. 5). Experiments with labeled Cry1Ab (Fig. 5A) and Cry1Ac (Fig. 5C) showed a large difference in the specific binding to vesicles from susceptible and resistant strains. At the highest concentration of BBMV used (0.3 mg ml−1) specific binding of 15% and 8% was found for Cry1Ab and Cry1Ac, respectively, in the susceptible strain. On the other hand, with the Cry1Ab-SEL population, only 0.64% of specific binding was obtained for Cry1Ab and no binding of Cry1Ac. Control experiments of binding with BBMV using 125I-labeled Cry1Ca toxin showed similar results in both populations (1 to 3% specific binding using 0.1 to 0.3 mg ml−1 of BBMV), confirming the binding ability of the BBMV from resistant insects.
FIG. 5.
Specific binding of 125I-labeled pure activated toxins Cry1Ab (A) and Cry1Ac (B) as a function of P. xylostella BBMV concentration for susceptible strain LAB-UK (▪) and resistant population Cry1Ab-SEL (▾). Nonspecific binding values were subtracted from each binding point for obtaining the specific binding. Each point represents the mean of two experiments for Cry1Ab and Cry1Ac. Standard errors are represented by error bars.
DISCUSSION
Unlike the majority of cases of resistance to B. thuringiensis (5), in reciprocal mass crosses between Cry1Ab-SEL and ROTH populations the LC50 and slope values of F1 progeny of Cry1Ab-SEL males × ROTH females were significantly different from F1 progeny of Cry1Ab-SEL females × ROTH males, suggesting an influence of parent sex that carried the resistance. These results were similar to the ones found in another subpopulation selected from the SERD4 collected sample, Cry1Ac-SEL (29). It has previously been reported that resistance to Cry1Ab in F1 progeny of P. xylostella from the Philippines was influenced by the sex of the resistant parent, but sex linkage was discarded since no significant difference was found in the number of male and female survivors (19). All other studies on P. xylostella, (13, 32, 34, 37), P. interpunctella (20), and H. virescens (11) have indicated autosomal inheritance for resistance to B. thuringiensis and its Cry1 toxins.
Another significant feature of the resistance common to the selected subpopulations Cry1Ab-SEL and Cry1Ac-SEL was the multigenic basis of inheritance to Cry1Ab in the Cry1Ab-SEL subpopulation and Cry1Ac in the Cry1Ac-SEL subpopulation (29), even though we cannot discard other genetic alternatives based on a single gene. Indeed, the heterogeneity observed in the analysis of single-pair families, suggests that each insect was resistant due to different combinations of resistance alleles. In addition, parental sex influence was not a general property of all of the alleles, since F1 from single-pair crosses did not render the expected results. In Lepidoptera, females are heterogametic (ZW); the F1 results could be explained by single resistance gene linkage to the Z chromosome. However, this hypothesis was discarded as the results obtained in backcrosses, in which LC50 values differed from the expected (Table 2); for example, BCf should have provided the same LC50 as F1c and, similarly, LC50 of BCd should be similar to ROTH (Table 1). There is no simple genetic explanation for these results, but the LC50 values suggest that the progeny were more resistant when the male parent contributed the resistance alleles. The results of single pair-crosses suggest that the Cry1Ab-SEL population was not homogeneous and that each insect carried a different amount of resistance alleles. In addition, the expected different mortality between reciprocal crosses at 4 μg ml−1 dose (Fig. 1) was not observed in single-pair F1 families (Fig. 2), suggesting that sex influence was present only on some resistant loci.
The results of the backcross experiments (Table 2) with deviance between observed and expected dose-responses suggest that resistance to Cry1Ab in Cry1Ab-SEL was due to more than one locus. One general assumption of studies of the inheritance of resistance is that the parent populations are homozygous. If the susceptible parental population did contain individuals that possessed a resistant genotype, it is likely that some of the F1 progeny would be resistant and assessment of the dose response would have been confounded by the mixed genotype. However, this is unlikely since the ROTH population has been in laboratory culture for over 250 generations. The numbers of classes used in the test are limited (one degree of freedom), and in this case the potential of committing a type II error accepting Ho (lack of differences between observed and expected outcome) is high. One way to minimize this would be to test different crosses and toxin concentrations.
Tabashnik et al. (35) defined a “mode 1” form of resistance in P. xylostella in which there is loss of binding of a Cry1A toxin, no cross-resistance to Cry1Ca, and a recessive inheritance of the phenotype. Although Cry1Ab-SEL and Cry1Ac-SEL meet the first two criteria (Cry1Ab binding is also reduced in Cry1Ac-Sel [unpublished data]), their resistance to Cry1Ab and Cry1Ac, respectively, cannot be classified as mode 1 since it is inherited as an incompletely dominant trait. Since the lack of toxin binding to midgut receptors is generally considered a major mechanism of resistance, the involvement of other loci suggested by the backcross analysis may indicate the presence of additional resistance mechanisms in Cry1Ab-SEL and Cry1Ac-SEL subpopulations. Increased toxicity of the activated form of a toxin is not a universal observation since other studies with both susceptible and B. thuringiensis-resistant P. xylostella populations have seen no difference in activity between protoxin and activated toxin (22, 31). The differential activity seen here would therefore suggest the presence of an alternative resistance mechanism specifically targeted toward protoxin. Protease-mediated resistance mechanisms have been suggested for a number of insect species, including the Plodia interpunctella 198r population, where a reported defect in protoxin activation (25) correlated with a resistance allele closely linked to a protease null mutation (26). A resistant population of Ostrinia nubilalis has also been found to have significantly lower trypsin-like proteinase activity than a nonselected, susceptible subpopulation (15). Protoxin processing studies with this population also found a slower rate of protoxin activation although, as with the present study, the end products from resistant and susceptible populations were seemingly identical. Other studies have suggested that slower activation, faster degradation (7), differential proteolytic activation (16), and sequestration by proteases (21) can all affect the relative activity of toxins. We have previously reported (31) that for an alternative P. xylostella population (SERD5) solubilized protoxin was less toxic than crystalline protoxin and that in vitro activated toxin was significantly more active than either soluble or crystalline protoxin. That result suggested that it was not a consequence of the toxin being soluble that increased its toxicity. Interestingly, the Cry1Ab-SEL subpopulation of SERD4 was reselected with trypsin-activated toxin, making the observation that the resistant subpopulation showed a greater susceptibility to toxin than protoxin rather unexpected.
Attempts to correlate the greater relative activity of activated toxin with an inability to activate protoxin were not successful. Although differences were observed in the first 24 h in the rate of activation in vitro, it is difficult to correlate this with a difference in toxicity in vivo since we do not yet have a complete understanding of the fate of toxin within the gut. We are therefore not in a position to predict whether slight changes in the rate of activation would affect toxicity. Likewise, it is possible that subtle differences in the site of processing might exist that could affect the activity of the toxin. An alternative explanation is that the increased activity of activated toxin is not a direct consequence of a defect in activation. Various suggestions can be put forward as to why the activated toxin should be more effective than the protoxin. A mechanism by which protoxin is specifically sequestered is one such possibility. Milne et al. (21) and Gunning et al. (12) have both described examples where toxins are sequestered by either proteases or esterases decreasing the susceptibility of the larvae to these toxins. Sequestration of a protoxin through interaction with its C terminus for example, could explain our results.
Insect resistance to chemical insecticides can be monogenic or polygenic, with the latter most common when the population is continually exposed to the insecticide (27). The resistance phenotype may result from a single locus with major effect, as seen for the overexpression of a detoxifying enzyme, or it may be due to the additive effect of multiple loci (6). It is not unreasonable to suppose that a similar situation will exist with B. thuringiensis-resistant insects. The mode 1 resistance mechanism proposed by Tabashnik et al. (35) could be due to a single mutation affecting toxin binding to its receptor. In a B. thuringiensis-resistant population of H. virescens, a transposon insertion into the cadherin-like receptor is believed to be responsible for the resistance phenotype (9). Similarly, deletions in cadherin genes in Pectinophora gossypiella are believed to be responsible for the resistance phenotype (24). There are reported instances where there is no defect in toxin binding to explain resistance, suggesting an alternative resistance mechanism might be involved in these P. xylostella populations (17, 40). Aside from the protease-mediated mechanisms described above, few alternative B. thuringiensis resistance mechanisms have been proposed, although Forcada et al. (7, 8) suggested that a resistant population of H. virescens had acquired a more efficient cellular repair mechanism. A proteomic comparison between B. thuringiensis-resistant and susceptible P. interpunctella populations revealed a number of significant differences in expressed proteins that may help to elucidate the resistant phenotype (4). Of particular note were an increase in glutathione utilization and a general increase in oxidative metabolism within the cells of the resistant population. Increased activities of glutathione S-transferase and microsomal monooxygenase, as well as that of carboxylesterase, have also been reported in field populations of P. xylostella that were highly resistant to the insecticides fenvalerate and flufenoxuron (23). However, these field populations remained susceptible to B. thuringiensis products, suggesting that the stress responses may not directly protect against B. thuringiensis. Nonetheless, it remains possible that some aspect of the P. xylostella innate immune system can act to inactivate the B. thuringiensis toxin and that this response can be upregulated in the resistant population.
The findings presented here suggest that resistance to Cry1Ab in Cry1Ab-SEL was similar to Cry1Ac in Cry1Ac-SEL. This was perhaps not surprising given that both Cry toxins have been previously demonstrated to bind to the same receptor (5). Also, both insect lines were derived from the same gene pool. When both strains were crossed, the resulting progeny were similar to parents (Fig. 3) More surprising was the complexity of this common response with evidence for the involvement of multiple resistance genes and mechanisms.
This and our previous study (29) illustrate that resistance to Cry1Ab and Cry1Ac is incompletely dominant but the environment can alter dominance (by increasing toxin concentration). The most widely adopted resistance management strategy—high-dose refugia—relies on the principle that dominance can be altered by environment (3). Reduced dominance of resistance decreases the heritability of resistance, thereby delaying evolution of resistance. Various studies have confirmed that the high concentration of B. thuringiensis toxins in transgenic plants is effective for delaying resistance (40). However, the hypothesis that high doses are best for delaying pest resistance remains to be confirmed.
Acknowledgments
We thank S. Herrero for supplying purified Cry1Ca and R. A. de Maagd for supplying the Cry1Ab-expressing E. coli strain.
The work in Spain was supported by a Generalitat Valenciana grant (GV04B-165) and a research contract for B.E. from the “Ramón y Cajal” program. M.S.I.-P was supported by the Ministerio de Educación, Cultura, y Deportes with a fellowship (AP 2001-0972). The work in the United Kingdom was conducted under Plant Health License PHL 189/3973 (10/2001) amended (02/2002).
REFERENCES
- 1.Ballester, V., F. Granero, B. E. Tabashnik, T. Malvar, and J. Ferre. 1999. Integrative model for binding of Bacillus thuringiensis toxins in susceptible and resistant larvae of the diamondback moth (Plutella xylostella). Appl. Environ. Microbiol. 65:1413-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bravo, A., J. Sanchez, T. Kouskoura, and N. Crickmore. 2002. N-terminal activation is an essential early step in the mechanism of action of the Bacillus thuringiensis Cry1Ac insecticidal toxin. J. Biol. Chem. 277:23985-23987. [DOI] [PubMed] [Google Scholar]
- 3.Bourguet, D., A. Genissel, and M. Raymond. 2000. Insecticide resistance and dominance levels. J. Econ. Entomol. 93:1588-1595. [DOI] [PubMed] [Google Scholar]
- 4.Candas, M., O. Loseva, B. Oppert, P. Kosaraju, L. A. Bulla. 2003. Insect resistance to Bacillus thuringiensis: alterations in the Indian meal moth larval gut proteome. Mol. Cell Proteomics 2:19-28. [DOI] [PubMed] [Google Scholar]
- 5.Ferré, J., and J. Van Rie. 2002. Biochemistry and genetics of insect resistance to Bacillus thuringiensis. Annu. Rev. Entomol. 47:501-533. [DOI] [PubMed] [Google Scholar]
- 6.ffrench-Constant, R. H., P. J. Daborn, and G. Le Goff. 2004. The genetics and genomics of insecticide resistance. Trends Genet. 20:163-170. [DOI] [PubMed] [Google Scholar]
- 7.Forcada, C., E. Alcácer, M. D. Garcerá, A. Tato, and R. Martinez. 1996. Differences in the midgut proteolytic activity of two Heliothis virescens strains, one susceptible and one resistant to Bacillus thuringiensis toxins. Arch. Insect Biochem. Physiol. 31:257-272. [DOI] [PubMed] [Google Scholar]
- 8.Forcada, C., E. Alcacer, M. D. Garcera, A. Tato, and R. Martinez. 1999. Resistance to Bacillus thuringiensis Cry1Ac toxin in three strains of Heliothis virescens: proteolytic and SEM study of the larval midgut. Arch. Insect Biochem. Physiol. 42:51-63. [DOI] [PubMed] [Google Scholar]
- 9.Gahan, L. J., F. Gould, and D. G. Heckel. 2001. Identification of a gene associated with bit resistance in Heliothis virescens. Science 293:857-860. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Carreno, F. L., L. E. Dimes, and N. F. Haard. 1993. Substrate-gel electrophoresis for composition and molecular weight of proteinases or proteinaceous proteinase inhibitors. Anal. Biochem. 214:65-69. [DOI] [PubMed] [Google Scholar]
- 11.Gould, F., A. Anderson, A. Reynolds, L. Bumgarner, and W. Moar. 1995. Selection and genetic analysis of a Heliothis virescens (Lepidoptera: Noctuidae) strain with high levels of resistance to Bacillus thuringiensis toxins. J. Econ. Entomol. 88:1545-1559. [Google Scholar]
- 12.Gunning, R. V., H. T. Dang, F. C. Kemp, I. Nicholson, and G. D. Moores. 2005. New Helicoverpa armigera resistance mechanism threat to Bacillus thuringiensis Cry1Ac toxin, in transgenic crops. Appl. Environ. Microbiol. 71:2558-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herrero, S., B. Oppert, and J. Ferré. 2001. Different mechanisms of resistance to Bacillus thuringiensis toxins in the Indianmeal moth. Appl. Environ. Microbiol. 67:1085-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janmaat, A. F., and J. Myers. 2003. Rapid evolution and the cost of resistance to Bacillus thuringiensis in greenhouse populations of cabbage loopers, Trichoplusia ni. Philos. Trans. R. Soc. London B 270:2263-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li, H. R., B. Oppert, R. A. Higgins, F. N. Huang, K. Y. Zhu, L. L. Buschman. 2004. Comparative analysis of proteinase activities of Bacillus thuringiensis resistant and susceptible Ostrinia nubilalis (Lepidoptera: Crambidae). Insect Biochem. Mol. Biol. 34:753-762. [DOI] [PubMed] [Google Scholar]
- 16.Lightwood, D. L., D. J. Ellar, and P. Jarrett. 2000. Role of proteolysis in determining potency of Bacillus thuringiensis Cry1Ac δ-endotoxin. Appl. Environ. Microbiol. 66:5174-5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu, Y. B., B. E. Tabashnik, L. Masson, B. Escriche, and J. Ferré. 2000. Binding and toxicity of Bacillus thuringiensis protein Cry1Ca to susceptible and resistant diamondback moth (Lepidoptera: Plutellidae). J. Econ. Entomol. 93:1-6. [DOI] [PubMed] [Google Scholar]
- 18.MacIntosh, S. C., T. B. Stone, R. T. Jokerst, and R. L. Fuchs. 1991. Binding of Bacillus thuringiensis proteins to a laboratory-selected line of Heliothis virescens. Proc. Natl. Acad. Sci. USA 88:8930-8933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martínez-Ramírez, A. C., B. Escriche, M. D. Real, F. J. Silva, and J. Ferré. 1995. Inheritance of resistance to a Bacillus thuringiensis toxin in a field population of diamondback moth (Plutella xylostella). Pestic. Sci. 43:115-120. [Google Scholar]
- 20.McGaughey, W. H., and R. W. Beeman. 1988. Resistance to Bacillus thuringiensis in colonies of Indianmeal moth and almond moth (Lepidoptera, Pyralidae). J. Econ. Entomol. 81:28-33. [Google Scholar]
- 21.Milne, R. E., A. S. D. Pang, and H. Kaplan. 1995. A protein complex from Choristoneura fumiferana gut-juice involved in the precipitation of δ-endotoxin from Bacillus thuringiensis subsp. sotto. Insect Biochem. Mol. Biol. 25:1101-1114. [DOI] [PubMed] [Google Scholar]
- 22.Mohan, M., and G. T. Gujar. 2003. Characterization and comparison of midgut proteases of Bacillus thuringiensis susceptible and resistant diamondback moth (Plutellidae: Lepidoptera). J. Invert. Pathol. 82:1-11. [DOI] [PubMed] [Google Scholar]
- 23.Mohan, M., and G. T. Gujar. 2003. Local variation in susceptibility of the diamondback moth, Plutella xylostella (Linnaeus) to insecticides and role of detoxification enzymes. Crop Prot. 22:495-504. [Google Scholar]
- 24.Morin, S., R. W. Biggs, M. S. Sisterson, L. Shriver, C. Ellers-Kirk, D. Higginson, D. Holley, L. J. Gahan, D. G. Heckel, Y. Carriére, T. J. Dennehy, J. K. Brown, and B. E. Tabashnik. 2003. Three cadherin alleles associated with resistance to Bacillus thuringiensis in pink bollworm. Proc. Natl. Acad. Sci. USA 100:5004-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oppert, B., K. J. Kramer, D. Johnson, S. J. Upton, W. H. McGaughey. 1996. Luminal proteinases from Plodia interpunctella and the hydrolysis of Bacillus thuringiensis Cry1Ac protoxin. Insect Biochem. Mol. Biol. 26:571-583. [DOI] [PubMed] [Google Scholar]
- 26.Oppert, B., K. J. Kramer, R. W. Beeman, D. Johnson, and W. H. McGaughey. 1997. Proteinase-mediated insect resistance to Bacillus thuringiensis toxins J. Biol. Chem. 272:23473-23476. [DOI] [PubMed] [Google Scholar]
- 27.Roush, R. T. 1998. Two-toxin strategies for management of insecticidal transgenic crops: can pyramiding succeed where pesticide mixtures have not? Philos. Trans. R. Soc. London B 353:1777-1786. [Google Scholar]
- 28.Sayyed, A. H., B. Raymond, M. S. Ibiza-Palacios, B. Escriche, and D. J. Wright. 2004. Genetic and biochemical characterization of field evolved resistance to Bacillus thuringiensis toxin Cry1Ac in diamondback moth, Plutella xylostella. Appl. Environ. Microbiol. 70:7010-7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sayyed, A. H., and D. J. Wright. 2001. Cross-resistance and inheritance of resistance to Bacillus thuringiensis toxin Cry1Ac in diamondback moth (Plutella xylostella L.) from lowland Malaysia. Pest Manag. Sci. 57:413-421. [DOI] [PubMed] [Google Scholar]
- 30.Sayyed, A. H., and D. J. Wright. 2002. Genetic diversity of B. thuringiensis. resistance: implications for resistance management. Pak. J. Biol. Sci. 5: 1330-1344. [Google Scholar]
- 31.Sayyed, A. H., R. Gatsi, T. Kouskoura, D. J. Wright, and N. Crickmore. 2001. Susceptibility of a field-derived, Bacillus thuringiensis-resistant strain of diamondback moth to in vitro-activated Cry1Ac toxin. Appl. Environ. Microbiol. 67:4372-4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sayyed, A. H., R. Haward, S. Herrero, J. Ferré, and D. J. Wright. 2000. Genetic and biochemical approach for characterization of resistance to Bacillus thuringiensis toxin Cry1Ac in a field population of the diamondback moth, Plutella xylostella. Appl. Environ. Microbiol. 66:1509-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stewart, G. S. A. B., K. Johnstone, E. Hagelberg, and D. J. Ellar. 1981. Commitment of bacterial spores to germinate. Biochem. J. 198:101-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tabashnik, B. E., Y. B. Liu, T. Malvar, D. G. Heckel, L. Masson, V. Ballester, F. Granero, J. L. Ménsua, and J. Ferré. 1997. Global variation in the genetic and biochemical basis of diamondback moth resistance to Bacillus thuringiensis. Proc. Natl. Acad. Sci. USA 94:12780-12785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabashnik, B. E., Y. B. Liu, T. Malvar, D. G. Heckel, L. Masson, and J. Ferré. 1998. Insect resistance to Bacillus thuringiensis: uniform or diverse? Philos. Trans. R. Soc. London Ser. B 353:1751-1756. [Google Scholar]
- 36.Tabashnik, B. E., T. J. Dennehy M.A. Sims, K. Larkin, G. P. Head, W. J. Moar, and Y. Carriere. 2002. Control of resistant pink bollworm (Pectinophora gossypiella) by transgenic cotton that produces Bacillus thuringiensis toxin Cry2Ab. Appl. Environ. Microbiol. 68:3790-3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang, J. D., S. Gilboa, R. T. Roush, and A. M. Shelton. 1997. Inheritance, stability, and lack of fitness costs of field-selected resistance to Bacillus thuringiensis in diamondback moth (Lepdoptera: Plutellidae) from Florida. J. Econ. Entomol. 90:732-741. [Google Scholar]
- 38.Van Rie, J., S. Jansens, H. Höfte, D. Degheele, and H. Van Mellaert. 1989. Specificity of Bacillus thuringiensis delta-endotoxins. Importance of specific receptors on the brush border membrane of the midgut of target insects. Eur. J. Biochem. 186:239-247. [DOI] [PubMed] [Google Scholar]
- 39.Van Rie, J., W. H. McGaughey, D. E. Johnson, B. D. Barnett, and H. Van Mellaert. 1990. Mechanism of insect resistance to the microbial insecticide Bacillus thuringiensis. Science 247:72-74. [DOI] [PubMed] [Google Scholar]
- 40.Zhao, J. Z., H. L. Collins, J. D. Tang, J. Cao, E. D. Earle, R. T. Roush, S. Herrero, B. Escriche, J. Ferré, A. M. Shelton. 2000. Development and characterization of diamondback moth resistance to transgenic broccoli expressing high levels of Cry1Ca. Appl. Environ. Microbiol. 66:3784-3789. [DOI] [PMC free article] [PubMed] [Google Scholar]