Abstract
Porphyromonas gingivalis, a gram-negative obligate anaerobe, contains two homologs of an Escherichia coli resistance-nodulation-cell division-type multidrug exporter gene, acrB, in putative operons, together with homologs of membrane fusion protein gene acrA and outer membrane channel gene tolC. MIC determination and accumulation assays with mutants with disruptions of one or more genes showed that one cluster, named xepCAB, pumped out multiple agents including rifampin, puromycin, and ethidium bromide.
Bacteria must protect themselves from toxic compounds in the environment. Efflux pumps that excrete noxious substrates contribute to this process. Some transporters extrude a specific drug or a class of drugs. Others, the multidrug transporters, which pump out a variety of structurally unrelated compounds, are classified into several families (10). Among them, members of the resistance-nodulation-cell division (RND) family of transporters often have extremely wide substrate specificities, and the best-studied example, AcrB of Escherichia coli, is responsible for the intrinsic resistance of this organism to most antibiotics (10). AcrB, like many RND pumps, functions as a part of a multisubunit transport complex, together with TolC, an outer membrane channel belonging to the outer membrane factor (OMF) family (2), and AcrA, a periplasmic connector protein belonging to the membrane fusion protein (MFP) family (2). The genes coding for these subunits of the tripartite efflux complex often constitute an operon (for reviews, see references 10, 12, and 13).
Recently, an obligate anaerobe, Bacteroides fragilis, was shown to export fluoroquinolones by a transporter belonging to another family, the multidrug and toxic compound extrusion (MATE) family (9, 14). However, little else is known about xenobiotic transporters in anaerobic bacteria.
Search for putative RND-type efflux pump genes in P. gingivalis genome.
A puromycin-resistant, single-step mutant isolated from Porphyromonas gingivalis type strain ATCC 33277 was resistant to several drugs (data not shown), which suggested that a single mutation could have resulted in overexpression of a multidrug transporter. We noted that a DNA segment cloned for another purpose from Bacteroides uniformis, an obligate anaerobe, contained a homolog of an RND family pump gene, acrF of E. coli (19). Since most of the RND family members in gram-negative bacteria are either multidrug or toxic cation efflux pumps (16), we searched a database for genes homologous to acrB or acrF in the nucleotide sequence of the P. gingivalis W83 genome with the TBLASTN program (http://www.tigr.org [1]). An open reading frame (ORF) most similar to AcrB was PG0540, which was annotated as an AcrB/AcrD/AcrF family protein by The Institute for Genome Research (TIGR). Upstream of and adjacent to PG0540 were located ORFs PG0538, homologous to tolC (coding for an OMF), and PG0539, homologous to acrA (coding for an MFP).
The arrangement of these three genes suggests that their products assemble into a typical tripartite efflux complex, composed of an RND pump, a periplasmic MFP connector protein, and an outer membrane OMF channel (10). We propose that PG0538, PG0539, and PG0540 be called xepC, xepA, and xepB, respectively, where xep represents xenobiotic exporters of Porphyromonas.
Disruption of xepCAB genes.
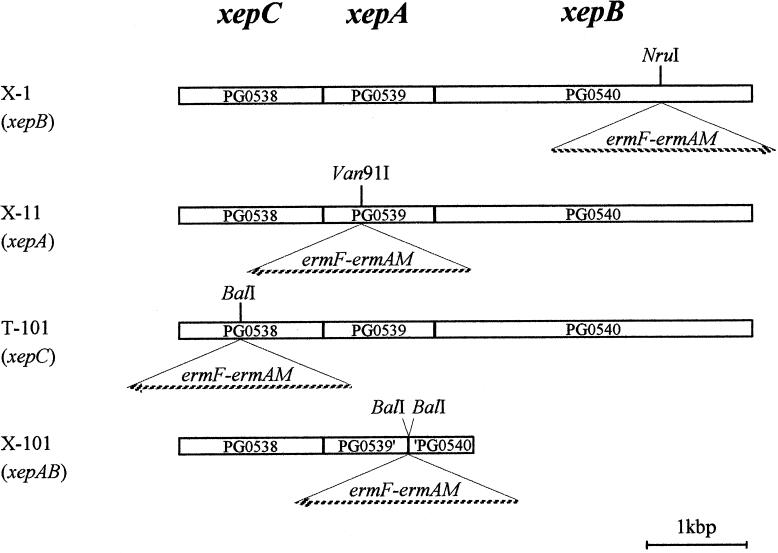
The xepCAB genes were disrupted in strain ATCC 33277 (Fig. 1). All DNA manipulations were performed as described in reference 17. For construction of the xepB mutant, the internal sequence of xepB of ATCC 33277 was amplified by PCR with a high-fidelity, blunt-end-producing Pyrobest DNA polymerase (Takara, Kyoto, Japan) with primers (Table 1) whose sequences were based on the W83 genome sequence. The amplicon was ligated at the HincII site into a pUC19 plasmid vector and transformed into the host E. coli JM109. An erythromycin cassette (erm cassette), in which both ermF (expressed in Porphyromonas and Bacteroides spp.) and ermAM (expressed in E. coli) were present, was purified from plasmid pVA2198 (4) by digestion with PvuII and was inserted at an NruI site in xepB. The region of xepB containing the erm cassette was cut out with PvuII. P. gingivalis ATCC 33277 was mutagenized by transformation with this linear DNA (4). The other disruption mutants and a deletion-insertion mutant, xepAB::erm, were constructed similarly; the details are shown in Table 1.
FIG. 1.
Insertion positions of erythromycin cassette in the xep genes. Broken arrows indicate the directions of orientation of the erythromycin cassettes that were inserted. The Institute for Genome Research locus names are indicated in the boxes of the xep genes. PG0539′ and ′PG0540 indicate the C-terminal truncated xepA and the N-terminal truncated xepB, respectively. In X-101, the region between a BalI site in xepA and that in xepB was deleted and an erythromycin cassette was inserted there.
TABLE 1.
Construction of xepCAB mutants
| Mutant | Mutation | Primersa for amplification | Amplicon digested with: | pUC19 cut with: | Site for erm insertion | Digestion before P. gingivalis transformation |
|---|---|---|---|---|---|---|
| X-1 | xepB::erm | PgenvD1F | —-b | HincII | NruI | PvuII |
| PgenvD1R | ||||||
| X-11 | xepA::erm | PgenvCF | BclI | BamHI | Van91I | NaeI |
| PgenvCR | HincII | |||||
| T-101 | xepC::erm | PgTLCF | — | HincII | BalI | PvuII |
| PgTLCR | ||||||
| X-101 | xepAB::erm | PgacrFF1 | BamHI | BamHI | BalIc | NdeI |
| PgenvCR | HincII |
The nucleotide sequences of the primers used for amplification of the internal regions of xep genes are as follows: PgenvD1F, 5′-AGGCAGAATATCCGGTGGGCA-3′; PgenvD1R, 5′-GCACAATACCGTTCTTCACCAC-3′; PgenvCF, 5′-CCGGCTGTAACCACTGTCTCG-3′; PgenvCR, 5′-ATGAAAAGATCGAATACACTGGGC-3′; PgTLCF, 5′-CGTACTCTCCGAAGCCGATGTG-3′; PgTLCR, 5′-TAATTGAGTCGAGCCTGCAAAAGA-3′; PgacrFF1, 5′-CTACGCTGTCTCCTCATCCC-3′.
—, not digested. The ends of the amplicon were blunt.
This removed a 2.7-kbp internal fragment, which was then replaced with the erm cassette.
The insertion of an erm cassette into the target genes was confirmed by PCR. The amplicons were subjected to size analysis and restriction endonuclease mapping (data not shown). Mutant X-1 (Table 1) had no detectable growth defect, and its morphology was indistinguishable from that of the parent strain, even when it was grown at 43°C.
Mutants of xepCAB were hypersusceptible to various agents.
The MICs of drugs for the parent and mutant strains were measured. The cells were precultured in Anaerobic Bacteria Culture Medium (ABCM; Eiken, Tokyo, Japan) broth supplemented with 5 μg of hemin per ml and 10 μg of menadione per ml (sABCM broth) anaerobically at 37°C. One-half milliliter of the preculture was inoculated into 2 ml of fresh sABCM broth and incubated anaerobically at 37°C for 4 to 6 h. Five microliters of each culture was spotted onto sBHK agar containing twofold dilutions of drugs, and the plates were incubated anaerobically at 37°C for 7 days. The MICs (Table 2) of various agents were decreased for mutants X-1, X-11, X-101, and T-101. The extent of the decrease was particularly large for ethidium bromide, puromycin, rifampin, and sodium dodecyl sulfate (SDS). For fluoroquinolones (except sparfloxacin), berberine, acriflavine, tetracycline, and minocycline, the difference in MICs was only twofold, but this difference was reproducibly seen in measurements obtained by retesting more than five times.
TABLE 2.
MICs of various agents for P. gingivalis strains
| Strain | MIC (μg/ml)a
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ethidium bromide | Puro- mycin | Rifam- pin | Nor- floxacin | Oflox- acin | Cipro- floxacin | Spar- floxacin | Tetra- cycline | Mino- cycline | Ber- berine | Acri- flavine | SDS | |
| ATCC 33277 (parent)b | 5 | 12.5 | 0.012 | 4 | 0.5 | 1 | 0.4 | 0.12 | 0.05 | 32 | 2.5 | 500 |
| X-1 (xepB) | 0.6 | 3.1 | 0.003 | 2 | 0.25 | 0.5 | 0.4 | 0.06 | 0.025 | 16 | 1.25 | 125 |
| X-11 (xepA)c | 0.6 | 3.1 | 0.003 | 2 | 0.25 | 0.5 | 0.4 | 0.06 | 0.025 | 16 | 1.25 | 125 |
| T-101 (xepC) | 0.6 | 3.1 | 0.003 | 2 | 0.5 | 1 | 0.2 | 0.12 | 0.05 | 16 | 1.25 | 125 |
No changes seen in the MICs of the following agents for the xep mutants were seen (MICs are given in parentheses): nalidixic acid (150 μg/ml), ampicillin (0.4 μg/ml), crystal violet (5 μg/ml), chloramphenicol (10 μg/ml), cetylpyridinium chloride (10 μg/ml), kanamycin (>200 μg/ml), and streptomycin (>200 μg/ml).
Two other wild-type strains tested (strains W83 and 381) showed very similar susceptibility patterns.
The MIC pattern for mutant X-101 (xepAB::erm) was identical to those for both X-1 and X-11.
Decreased drug efflux from mutant cells.
The accumulation of acriflavine, a fluorescent dye, was measured in growing, presumably fully energized cells, as described previously (8). ATCC 33277 and X-1 cells were grown anaerobically to the mid-log phase. Acriflavine was added to 0.1 μg/ml. The cells were incubated in an AnaeroBox (Hirasawa Co. Ltd., Tokyo, Japan) chamber. At time zero (Fig. 2), each culture was divided into two halves; carbonyl cyanide m-chlorophenylhydrazone (CCCP), a proton conductor, was added to one half at 200 μM, and the same volume of acetone, the solvent for CCCP, was added to the other half. Samples of 6 ml were taken at various time points, and 5 ml was filtered through a glass-fiber filter (GF75; Advantec, Tokyo, Japan). The filters were washed with 5 ml of ice-cold 0.1 M LiCl and then air dried. The optical density at 600 nm (OD600) was determined by using the remainders of the samples. Intracellular acriflavine was eluted with 1.6 ml of dimethyl sulfoxide. After removal of debris by centrifugation, the fluorescence of the eluate was measured with a Shimadzu (Kyoto, Japan) RF-540 spectrofluorophotometer at excitation and emission wavelengths of 474 and 500 nm, respectively. Fluorescence was normalized to that for the cellular protein by using the relation that at 0.7 OD600 the cell suspension contained 0.3 mg of protein per ml (H. Nikaido, personal communication).
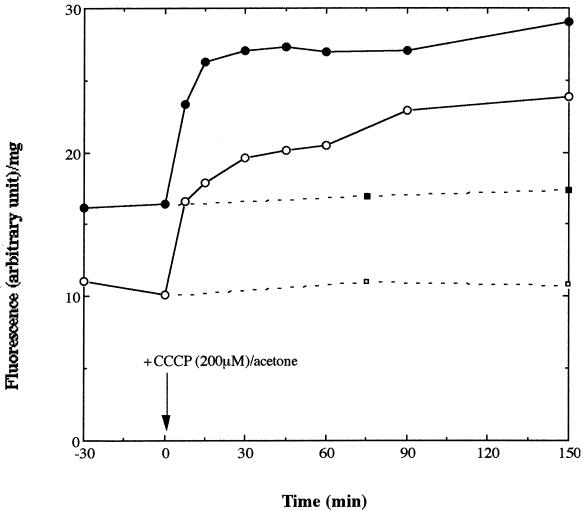
FIG. 2.
Accumulation of acriflavine in P. gingivalis cells. At time zero (indicated by an arrow), CCCP was added to one half of the bacterial cultures (open circle, ATCC 33277; closed circle, X-1) to a final concentration of 200 μM. The same volume of acetone was added to the other half (open square, ATCC 33277; closed square, X-1), which served as controls. Ordinate, fluorescence intensity (in arbitrary units) per milligram of protein; abscissa, time (in minutes).
Acriflavine accumulated in X-1 cells to a steady-state level about two times higher than that in ATCC 33277 cells (Fig. 2), a result indicating that xepB encodes an efflux pump functioning in vivo. The accumulation level increased in both strains immediately after CCCP addition and reached almost the same level (Fig. 2). These results suggest that a proton motive force drives the XepB pump.
Sequence analysis.
XepB was found to contain all four conserved motifs of the RND transporters (12), and the hydropathy profile of XepB showed 12 transmembrane regions and the two characteristic, large periplasmic loops (data not shown) (8). Evaluation with the BLASTP program showed that XepB is homologous to RND exporters of E. coli, YegN (E value [1], 1 × 10−113), AcrD (1 × 10−79), YhiV (4 × 10−77), AcrB (4 × 10−76), and AcrF (4 × 10−75). AcrD pumps out aminoglycosides (15), SDS, deoxycholate, and novobiocin (11). The effect of XepB on aminoglycoside MICs could not be ascertained because of the characteristic intrinsic resistance of obligate anaerobes to this class of compounds, but the inactivation of xep genes did produce hypersusceptibility to SDS (Table 2). YhiV extrudes basic dyes such as ethidium bromide, crystal violet, and rhodamine 6G, in addition to SDS and deoxycholate (11), a substrate specificity somewhat similar to that of XepB.
XepA is similar to members of the MFP family of E. coli K-12 (YegM, YhiU, YbjY, CusB, AcrA, and AcrE), with E values ranging from 2 × 10−14 to 2 × 10−9. All of these except CusB (5) are involved in multidrug efflux (11). In addition, the XepA protein, like many members of the MFP family, contains the lipoprotein signal cleavage site LTSC (7) 20 amino acids downstream from the second methionine of the ORF, which was predicted as the initiation codon here (21).
Among the proteins in the sequenced bacterial genomes, XepC was most similar to TolC of Vibrio cholerae (E value, 1.9 × 10−16). Other homologs included OprM of Pseudomonas aeruginosa (10), CzcC of Ralstonia sp. strain CH34 (6), AprF of P. aeruginosa (3), and TolC of E. coli (E value, 8.3 × 10−10).
The DNA sequence downstream from the xepCAB locus includes a hairpin structure 321 bp after the stop codon of xepB, followed by the sequence UUUUUU, suggestive of a rho-independent terminator. Although the ORF PG0541 is annotated as a hypothetical protein in this 321-bp sequence, this ORF is not homologous to any gene related to drug efflux. There was a 4-base overlap between the xepA and the xepB genes. The interval between xepC and xepA was only 43 bases and did not contain a transcription terminator or a Shine-Dalgarno sequence. These three genes are likely located in a single operon. The disruption of xepC or xepA probably causes the polar effect on the expression of downstream genes.
Conclusions.
We showed that the XepCAB proteins in P. gingivalis constitute an active multidrug efflux pump. This is, as far as we are aware, the first report of a functional RND multidrug pump in an obligate anaerobe. Interestingly, this pump is functioning even in a species that is intrinsically susceptible to most antibiotics. With the increased use of antibiotics in periodontal practice (18, 20), there is danger that mutant strains overexpressing these and other multidrug pumps may become selected for in the future.
Acknowledgments
We thank H. Nikaido for suggesting initial experiments and O. Ueda and S. Aoyama for technical assistance. Thanks are also due to S. Nishiyama for critically reading the manuscript. We also thank The Institute for Genomic Research and Forsyth Dental Center for making the P. gingivalis genome sequence available.
This work was supported by a grants-in-aid for Scientific Research (C) (to T. I.) from the Japan Society for the Promotion of Science (grant 13671926) and for Scientific Frontier Promoted Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (grant 1006).
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dinh, T., I. T. Paulsen, and M. H. Saier, Jr. 1994. A family of extracytoplasmic proteins that allow transport of large molecules across the outer membranes of gram-negative bacteria. J. Bacteriol. 176:3825-3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duong, F., A. Lazdunski, B. Cami, and M. Murgier. 1992. Sequence of a cluster of genes controlling synthesis and secretion of alkaline protease in Pseudomonas aeruginosa: relationships to other secretory pathways. Gene 121:47-54. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher, H. M., H. A. Schenkein, R. M. Morgan, K. A. Bailey, C. R. Berry, and F. L. Macrina. 1995. Virulence of a Porphyromonas gingivalis W83 mutant defective in the prtH gene. Infect. Immun. 63:1521-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grass, G., and C. Rensing. 2001. Genes involved in copper homeostasis in Escherichia coli. J. Bacteriol. 183:2145-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Groβe, C., G. Grass, A. Anton, S. Franke, A. N. Santos, B. Lawley, N. L. Brown, and D. H. Nies. 1999. Transcriptional organization of the czc heavy-metal homeostasis determinant from Alcaligenes eutrophus. J. Bacteriol. 181:2385-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayashi, S., and H. C. Wu. 1990. Lipoproteins in bacteria. J. Bioenerg. Biomembr. 22:451-471. [DOI] [PubMed] [Google Scholar]
- 8.Ma, D., D. N. Cook, M. Alberti, N. G. Pon, H. Nikaido, and J. E. Hearst. 1993. Molecular cloning and characterization of acrA and acrE genes of Escherichia coli. J. Bacteriol. 175:6299-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyamae, S., H. Nikaido, Y. Tanaka, and F. Yoshimura. 1998. Active efflux of norfloxacin by Bacteroides fragilis. Antimicrob. Agents Chemother. 42:2119-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nikaido, H. 1996. Multidrug efflux pumps of gram-negative bacteria. J. Bacteriol. 20:5853-5859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishino, K., and A. Yamaguchi. 2001. Analysis of complete library of putative drug transporter genes in Escherichia coli. J. Bacteriol. 183:5803-5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paulsen, I. T., M. H. Brown, and R. A. Skurray. 1996. Proton-dependent multidrug efflux systems. Microbiol. Rev. 60:575-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Putman, M., H. W. van Veen, and W. N. Konings. 2000. Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 64:672-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricci, V., and L. J. V. Piddock. 2000. Accumulation of norfloxacin by Bacteroides fragilis. Antimicrob. Agents Chemother. 44:2361-2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenberg, E. Y., D. Ma, and H. Nikaido. 2000. AcrD of Escherichia coli is an aminoglycoside efflux pump. J. Bacteriol. 182:1754-1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saier, M. H., Jr., R. Tam, A. Reizer, and J. Reizer. 1994. Two novel families of bacterial membrane proteins concerned with nodulation, cell division and transport. Mol. Microbiol. 11:841-847. [DOI] [PubMed] [Google Scholar]
- 17.Sambrook, J., F. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd edition. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 18.Slots, J. 2000. Primer for antimicrobial periodontal therapy. J. Periodontal Res. 35:108-114. [DOI] [PubMed] [Google Scholar]
- 19.Smith, C. J., T. K. Bennett, and A. C. Parker. 1994. Molecular and genetic analysis of the Bacteroides uniformis cephalosporinase gene, cblA, encoding the species-specific β-lactamase. Antimicrob. Agents Chemother. 38:1711-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Winkelhoff, A. J., T. E. Rams, and J. Slots. 1996. Systemic antibiotic therapy in periodontitis. Periodontology 2000 10:45-78. [DOI] [PubMed] [Google Scholar]
- 21.von Heijne, G. 1985. Signal sequences. The limits of variations. J. Mol. Biol. 184:99-105. [DOI] [PubMed] [Google Scholar]