Abstract
Hortaea werneckii and Aureobasidium pullulans, black yeast-like fungi isolated from hypersaline waters of salterns as their natural ecological niche, have been previously defined as halophilic and halotolerant microorganisms, respectively. In the present study we assessed their growth and determined the intracellular cation concentrations of salt-adapted and non-salt-adapted cells of both species at a wide range of salinities (0 to 25% NaCl and 0 to 20% NaCl, respectively). Although 5% NaCl improved the growth of H. werneckii, even the minimal addition of NaCl to the growth medium slowed down the growth rate of A. pullulans, confirming their halophilic and halotolerant nature. Salt-adapted cells of H. werneckii and A. pullulans kept very low amounts of internal Na+ even when grown at high NaCl concentrations and can be thus considered Na+ excluders, suggesting the existence of efficient mechanisms for the regulation of ion fluxes. Based on our results, we can conclude that these organisms do not use K+ or Na+ for osmoregulation. Comparison of cation fluctuations after a hyperosmotic shock, to which nonadapted cells of both species were exposed, demonstrated better ionic homeostasis regulation of H. werneckii compared to A. pullulans. We observed small fluctuations of cation concentrations after a hyperosmotic shock in nonadapted A. pullulans similar to those in salt-adapted H.werneckii, which additionally confirmed better regulation of ionic homeostasis in the latter. These features can be expected from organisms adapted to survival within a wide range of salinities and to occasional exposure to extremely high NaCl concentrations, both characteristic for their natural environment.
Sodium is a very abundant cation in nature; nevertheless, it is toxic for most living cells, even in small concentrations. Cells living in natural saline systems, where high salt amounts cause high osmotic pressure, must maintain lower water potential than their surroundings in order to survive and proliferate and at the same time adjust to increased concentrations of sodium ions in the cells. Halophilic microorganisms have developed different strategies for counterbalancing osmotic pressure. Extremely halophilic archaea accumulate potassium up to molar levels when exposed to high external salinity (14). In contrast, eukaryotic microorganisms cannot tolerate such high intracellular ion concentrations.
In the absence of appropriate eukaryotic model organisms, mechanisms of salt tolerance have been studied mostly in salt-sensitive Saccharomyces cerevisiae (3, 4, 10, 11) and in some halotolerant fungi, such as filamentous Aspergillus nidulans, and yeasts such as Debaryomyces hansenii (1, 2, 13, 17, 19), Candida versatilis (22), and Rhodotorula mucilaginosa and Pichia guillermondii (12). The data on these fungi show that the maintenance of positive turgor pressure at high salinity is mainly due to an increased production and accumulation of glycerol, trehalose, and other organic compatible solutes. However, it is also known that in certain fungi, such as salt-tolerant D. hansenii, osmotic adjustments of the major intracellular cations also occur in response to osmotic stress (4, 20). In fact, several groups have reported that D. hansenii keeps relatively high amounts of internal sodium when grown under salt stress and from this point of view this yeast was defined as a Na+ includer organism (see reference 18 for a review).
Recently, different species of black yeasts have been isolated from hypersaline waters of solar salterns (8). These yeasts were described as a new group of eukaryotic halophiles, and they are represented by Hortaea werneckii, Phaeotheca triangularis, Trimmatostroma salinum, and halotolerant Aureobasidium pullulans (6, 8, 23). According to the two main criteria for halophily (7), H. werneckii belongs to the group of true eukaryotic halophiles. It was the dominant black yeast in the hypersaline water of all of the sampled salterns and the prevailing species at environmental salinities greater than 20% (5). The salinity range of growth for H. werneckii, defined in vitro, was from 0% to saturation (32%) NaCl with a broad optimum from 6 to 10% NaCl.
In contrast, A. pullulans is a halotolerant black yeast that is consistently isolated from salt marshes and from the water of solar salterns at lower salinities (16, 24). A. pullulans grows best without NaCl (24) but can tolerate up to 17% NaCl in the growth medium.
It has been shown previously that H. werneckii (15) and A.pullulans (9; unpublished data) accumulate glycerol when grown in saline environment. In the present study we investigated whether in halophilic H. werneckii and in halotolerant A.pullulans osmotic adjustments of sodium and potassium also occur in response to osmotic stress. We present growth and intracellular contents of cations in salt-adapted and non-salt-adapted cells of halophilic H. werneckii and halotolerant A.pullulans exposed to a wide range of salinities, as encountered in their natural ecological niche: hypersaline waters of the salterns.
MATERIALS AND METHODS
Strains, media, and growth conditions.
The strains used in the study were H.werneckii (B-736) and A. pullulans (B-802), black yeasts from the ascomycetous order Dothideales, which were obtained from the Culture Collection of the National Institute of Chemistry (MZKI), Ljubljana, Slovenia. These strains were isolated from the hypersaline water of a crystallizer in the solar saltern Sečovlje, at the eastern coast of the Adriatic Sea (8, 24). Parallel control experiments for the determination of intracellular cation concentrations were performed with the type strain D. hansenii PYCC2968 (CBS767).
The cultures were grown on solid malt extract medium amended with increasing concentrations of NaCl for determination of colony size. They were inoculated in triplicates for each of the NaCl concentrations and grown for 3 months at 22°C.
For determination of growth rates and for cation measurements, the cultures were grown in three parallels in a defined liquid yeast nitrogen base medium (YNB) with Casamino Acids (CSM) (both from Qbiogene) as follows: YNB, 1.6 g liter−1; CSM, 0.8 g liter−1; (NH4)2SO4, 5 g liter−1; and glucose, 20 g liter−1. Where required, media were supplemented with NaCl, as mentioned in the legends for the individual figures. Incubation was performed at 27 ± 1°C on a rotary shaker at 180 rpm. Inoculum cultures were grown in 50 ml of liquid YNB with appropriate NaCl concentrations to mid-exponential phase. A total of 1 ml of the inoculum was added to 100 ml of YNB (with the same NaCl concentration) in 500-ml Erlenmeyer flasks for sample cultures. Growth was monitored spectrophotometrically by measuring optical density of the cultures at 600 nm.
The cultures for the hyperosmotic-shock experiments were grown in YNB or in YNB with 10% NaCl as described above. At the beginning of the experiment, 10% NaCl was added to the culture in mid-exponential phase. The samples (1 ml) for cation measurements were taken periodically during the first 60 min after the shock (t = 0, 1, 5, 10, 15, 20, 25, 30, and 60 min).
Determination of dry weight.
For growth rate calculations, the samples of cultures grown in the liquid YNB medium were filtered through paper filters (Black ribbon) and dried at 80°C to constant weight.
For dry weight determination, the samples of cultures (2 by 6 ml) grown in liquid YNB medium amended with different concentrations of NaCl were filtered through Millipore membrane filters (0.8-μm pore size) and dried at 80°C to constant weight. The duplicates differed mostly by ±5%.
Determination of intracellular cation concentrations.
Cation contents of the cells were determined in the mid-exponential growth phase by a modified procedure of Ramos et al. (21). Millipore membrane filters used for filtration of samples were prewashed with 2% HCl and three times with MilliQ water. Samples of cells (1 ml) grown in YNB media of various salinities were filtered through prewashed Millipore membrane filters (0.8-μm pore size) and washed with ice-cold 20 mM MgCl2 and an isosmotic concentration of sorbitol. The washing procedure usually took less than one minute (in all cases less than 3min). The cells collected on the filter were washed out onto a new filter and washed again in the same manner. The cells on the filters were treated overnight with acid (4% HCl), and the cations were analyzed by atomic absorption spectrophotometry (17).
RESULTS
Growth of H. werneckii and A. pullulans at different NaCl concentrations.
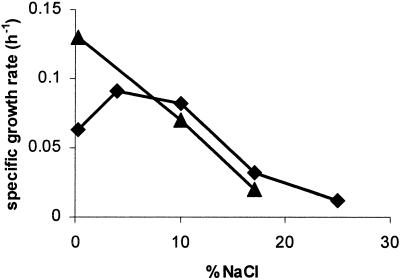
H. werneckii and A. pullulans were isolated from solar salterns as their natural ecological niche (5, 8). We determined their in vitro growth as measured by the maximal colony size on salt-amended solid media after 3 months. Growth of A. pullulans was slowed down even in the presence of low concentrations of NaCl, whereas in H. werneckii the colony size was not affected at up to 15% NaCl (not shown). The halophilic character of H. werneckii was further confirmed by growth in liquid media (Fig. 1). Five percent of NaCl stimulated the growth of H. werneckii in comparison with medium without added salt and was capable of growth on a wide range of NaCl concentrations (up to 25% NaCl). In comparison, A.pullulans responded as a halotolerant yeast, since it was capable of growth on media with up to 17% NaCl, but it grew considerably better on media without NaCl (Fig. 1).
FIG. 1.
Growth rates of halophilic H. werneckii (♦) and of halotolerant A. pullulans (▴) in media of various salinities. The cultures were grown in liquid YNB medium amended with various concentrations of NaCl at 27 ± 1°C on a rotary shaker, harvested in different growth phases, and dried to constant weight. Growth rates were calculated from doubling times obtained from the logarithmic curves of dry biomass in the exponential growth phase. The experiments were repeated three times.
Intracellular potassium and sodium ions in H. werneckii and A. pullulans grown at different constant NaCl concentrations.
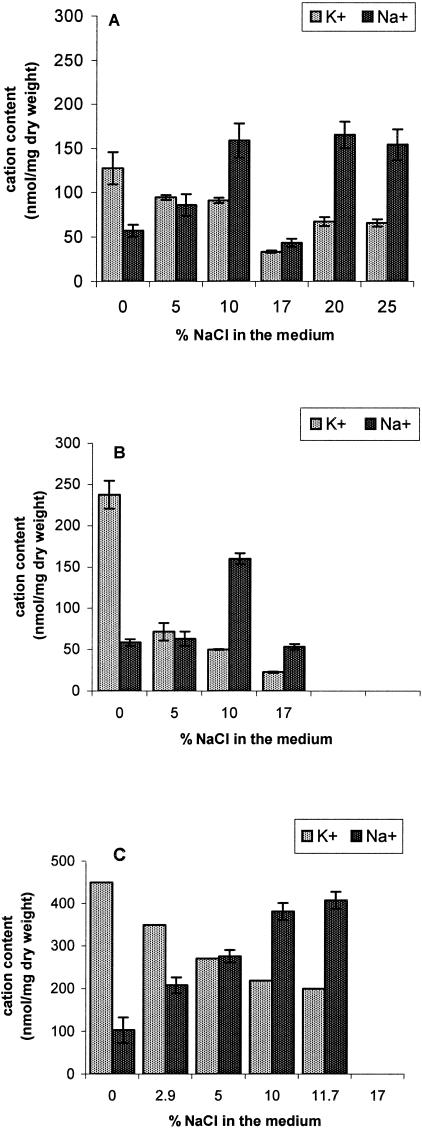
Cells of H. werneckii were grown in liquid media at different salinities, ranging from 0 to 25% NaCl. The measurements of cation contents in cells grown at constant salt concentration have shown that the amounts of K+ and Na+ in H. werneckii were changing according to the NaCl concentration of the medium (Fig. 2A). When H. werneckii was grown in a medium without added NaCl, it accumulated a relatively high amount of K+ and a very low amount of Na+. With the increasing NaCl concentration of the medium, the amounts of K+ decreased, whereas the Na+ content increased and in the end reached a higher value than the initial K+ content. Interestingly, the amounts of both cations deviated considerably from this trend at 17% NaCl, where both ions reached their minimal values. The ratios between potassium and sodium are presented in Table 1. The ratio was the highest in cells grown in the medium without added NaCl, and it decreased with increasing NaCl concentrations, being the lowest at 20% NaCl.
FIG. 2.
Amounts of K+ and Na+ in cells of H. werneckii (A), A.pullulans (B), and D. hansenii (C) grown at constant salt concentrations. The fungi were grown in defined NaCl-amended YNB liquid medium in three parallel flasks at 27 ± 1°C on a rotary shaker to mid-exponential growth phase. Five samples from each of the flasks were taken for cation measurements. The values shown are means ± the standard errors of the mean (n = 15).
TABLE 1.
Ratios of intracellular K+ and Na+ in H. werneckii, A. pullulans, and D. hansenii grown at different constant NaCl concentrations
| NaCl concn in the medium (%) | K+/Na+
|
||
|---|---|---|---|
| H. werneckii | A. pullulans | D. hansenii | |
| 0 | 2.24 | 4.04 | 4.37 |
| 2.9 | NPa | NP | 1.68 |
| 5 | 1.10 | 1.14 | 0.98 |
| 10 | 0.57 | 0.31 | 0.57 |
| 11.7 | NP | NP | 0.49 |
| 17 | 0.77 | 0.43 | NP |
| 20 | 0.41 | NP | NP |
| 25 | 0.42 | NP | NP |
NP, experiment not performed.
Halotolerant A. pullulans grew at lower maximal salinities (up to 17% NaCl). Similarly to H. werneckii, the cells of A.pullulans grown at constant salt concentration had the highest amount of K+ and the lowest amount of Na+, when grown without added NaCl (Fig. 2B). With a rising external NaCl concentration, the amount of K+ decreased, and the amount of Na+ gradually increased by up to 10% NaCl in the medium and then decreased again at 17% NaCl. Accordingly, the ratio of K+/Na+ was the highest in the cells grown without added NaCl in the medium, and it reached its lowest values at 10% NaCl (Table 1).
In parallel control experiments the intracellular K+ and Na+ content of the salt-tolerant Na+ includer yeast D. hansenii was determined (Fig. 2C and Table 1). This yeast kept significantly higher amounts of sodium than H. werneckii or A. pullulans at all conditions studied.
Intracellular potassium and sodium ions in the cells after hyperosmotic shock.
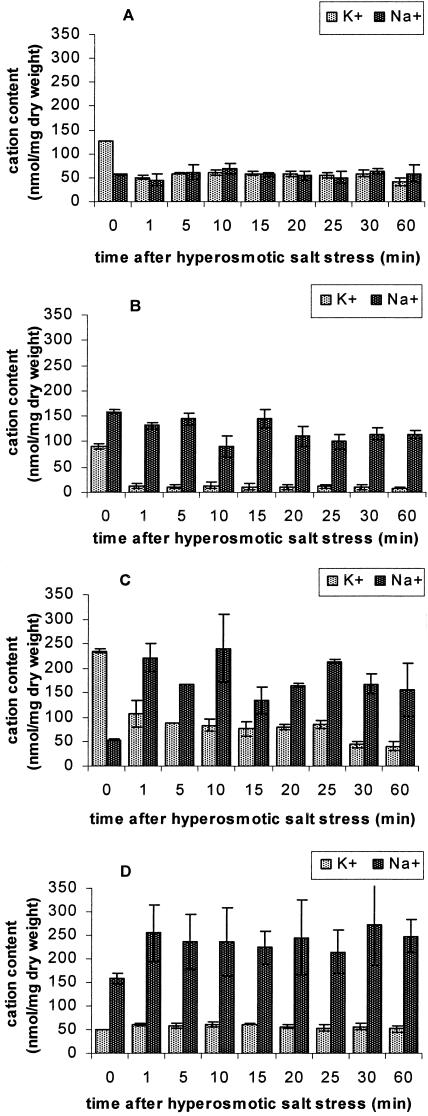
Nonadapted and salt-adapted cells of H.werneckii and A. pullulans were subjected to hyperosmotic shocks by sudden increase in salt concentration from 0 to 10% NaCl and from 10 to 20% NaCl, respectively. The intracellular cation contents were measured during 60 min after the shock as seen in Fig. 3.
FIG. 3.
Amounts of K+ and Na+ in nonadapted and in salt-adapted cells of H. werneckii and A. pullulans after a hyperosmotic shock. (A) Nonadapted H. werneckii; (B) salt-adapted H. werneckii, grown at 10% NaCl; (C) nonadapted A. pullulans; (D) salt-adapted A.pullulans, grown at 10% NaCl. The fungi were grown in YNB (A and C) or NaCl-amended YNB (B and D) liquid medium at 27 ± 1°C on a rotary shaker to mid-exponential growth phase, when 10% NaCl was added to the cultures. The samples for cation measurements were taken during the first 60 min after the shock. Each experiment was performed consecutively three times. The values shown are means of three separate experiments ± the standard errors of the mean (n = 3).
When nonadapted H. werneckii was shocked with the addition of 10% NaCl, we observed an immediate drop in the amount of K+, and no significant changes in intracellular Na+. One hour after the shock, the ratio between K+ and Na+ reached almost the same value as measured in salt-adapted cells (compare values in Fig. 2A and 3A). Within 1 h, the fluctuations in ion concentrations measured at the indicated time intervals were very low. When the salt-adapted cells grown at 10% NaCl were shocked with additional 10% NaCl, this caused a drastic drop of K+ content, which remained unchanged within 1 h. In contrast, the shock did not cause any drastic changes of Na+ amount. After 1 h, the final ratio between K+ and Na+ was even lower than in salt-adapted cells (compare Fig. 2A and 3B).
In nonadapted halotolerant A. pullulans, growing on a medium without NaCl, the addition of 10% NaCl caused an immediate drop of K+ and an increase of Na+. This trend continued during half an hour and remained unchanged thereafter. After 30 min the values of Na+ and K+ already reached the steady state and compared well to the amounts measured in salt-adapted A. pullulans grown at 10% NaCl (compare Fig. 2B and 3C). When salt-adapted cells were stressed with additional 10% NaCl, the amount of K+ did not change significantly, whereas the amount of Na+ increased immediately and after slight fluctuations the increased values remained unchanged (Fig. 3D).
DISCUSSION
Black yeasts are a group of rare extremophilic eukaryotes. Different species inhabit various natural extreme niches, among them hypersaline waters of the solar salterns. According to the newly introduced definition of halophily for fungi (8), H. werneckii and A. pullulans are halophilic and halotolerant species, respectively. Growth on solid and in liquid media in the presence or absence of increasing concentrations of NaCl were quantified for both H. werneckii and A. pullulans. Although low NaCl concentrations stimulated growth of H. werneckii, this was not observed in A. pullulans, which grew best without NaCl. Thus, the halophilic character of H. werneckii and the halotolerance of A. pullulans were confirmed.
When microbial cells are exposed to high salt concentrations, a hyperosmotic shock is followed by a rapid osmotic adjustment, resulting in loss of turgor and volume in nonadapted cells, while adapted fungal cells selectively accumulate compatible solutes (4, 16). It has already been shown that in contrast to extremely halophilic Archaea, which accumulate high amounts of ions at high environmental salinities, halotolerant fungi do not accumulate high internal ion concentrations when grown at hypersaline conditions but rather counterbalance the osmotic imbalance by the accumulation of polyols. D. hansenii seems to be an exception since it accumulates both, ions and polyols (18). Previous studies have shown that both H. werneckii (15) and A. pullulans (9; unpublished data) accumulate glycerol as the main compatible solute to counterbalance the increase of external salinity. In addition, preliminary data on the analysis of proteins from the whole-cell lysates of H. werneckii show that proteins are relatively acidic (16). Acidic proteins are characteristic for extremely halophilic archaea, which accumulate high amounts of potassium. In the case of halophilic H. werneckii it turned out that the plasma membrane proteins contributed importantly to the acidity of the proteins. Nevertheless, we speculated that due to the high ratio of acidic proteins in the cell lysates, halophilic H. werneckii might accumulate high amounts of cations besides glycerol. Surprisingly, H. werneckii and A. pullulans kept very low intracellular amounts of potassium and sodium even when the cells were grown in the presence of high NaCl concentrations, which indicates the sodium excluder character of both fungi. The mean values were significantly below those measured in D. hansenii, known as a sodium includer yeast, and thus far the best-described halotolerant eukaryotic representative in relation to the ion accumulation mechanism. In the present study, we performed parallel control experiments with D. hansenii in order to compare internal cation contents among the three fungal species. Our results were similar to those previously reported from other groups (see reference 18 for a recent review) proving the accuracy of our analytical methods.
Interestingly, in H. werneckii and in A. pullulans the amounts of K+ and Na+ were the lowest in the cells grown at 17% NaCl. H. werneckii still grows well at this medium salinity, but 17% NaCl most probably represents a turning point for this organism, as indicated by restricted colony size of H. werneckii, slower growth rate, and characteristic changes of physiological behavior (16). We have no definitive explanation for this, although we believe that it might be related to some critical changes in the cells under these conditions. On the other hand, 17% NaCl represents the upper limit for growth in A. pullulans, again indicating the difference in halotolerance between the two species.
In both halophilic H. werneckii and halotolerant A. pullulans, the ratio between potassium and sodium was highest in the cells grown without added NaCl in the medium and it decreased with the increasing concentration of NaCl, being lowest at 20 and 10% NaCl, respectively. This ratio was similar as in D. hansenii, although absolute values were higher in the latter.
When cells of H. werneckii and A. pullulans were exposed to hyperosmotic shock, they again showed characteristically different responses. In general, the ion contents of H. werneckii subjected to hyperosmotic shock were much lower than in A. pullulans. The nonadapted cells of both species, grown in the medium without NaCl and shocked by the addition of 10% NaCl to the medium, reacted by an immediate drop in the amount of K+. In contrast, an additional increase of salinity from 10 to 20% NaCl caused a drop in K+ amount only in H. werneckii. Hyperosmotic shock by the addition of 10% NaCl caused almost no increase of Na+ in H. werneckii, whereas in A. pullulans we observed a significant increase. With the second shock, caused by an additional 10% NaCl, sodium values increased only in halophilic H. werneckii but remained high and unchanged in halotolerant A. pullulans.
The observed pattern of ion fluctuations after hyperosmotic shock is in accordance with growth characteristics of both black yeasts under these conditions. Twenty percent NaCl slowed down the growth rate of H. werneckii, whereas in the case of A. pullulans the addition of 10% NaCl was clearly already inhibitory. Hyperosmotic shocks apparently affect the ionic homeostasis differently, as shown by the fluctuations of Na+ contents, which in halotolerant A. pullulans are observed immediately after salt stress, while in halophilic H. werneckii this pattern is seen only at higher salinities (above 10%NaCl). Our results nevertheless indicate that both species most probably have efficient transport systems for sodium exclusion, in accordance with their halophilic and halotolerant characters, respectively. This assumption is supported by the recent identification of ENA genes, coding for sodium pumps, in H. werneckii (unpublished data).
In conclusion, we observed particularly low values of internal potassium at most of tested saline conditions and only a slight increase in intracellular amount of sodium in both studied organisms. In contrast to D. hansenii, black yeasts are thus sodium excluder yeasts, although they live in natural hypersaline environments. We can therefore assume that in black yeasts ions probably do not contribute significantly to osmoadaptation and that compatible solutes rather than ions accumulate in these organisms to counterbalance the osmotic imbalance. This assumption is supported by our recent data which show that in H. werneckii other compatible solutes besides glycerol accumulate as a response to increased salinity.
Acknowledgments
The study was supported by a young researcher's grant from the Ministry of Education, Science, and Sports of the Republic of Slovenia (T.K.), by grant BMC 2002-04011-C05-01 from the Spanish Ministerio de Ciencia y Tecnología (J.R.), and by Slovenian-Spanish bilateral collaboration (Proyecto Conjunto de Cooperación Bilateral Hispano-Eslovena 04-05)
We thank C. Casanova Muñoz for valuable technical assistance in performing the atomic absorption spectrophotometry determinations and T. Račnik for growth curve determinations.
REFERENCES
- 1.Almagro, A., C. Prista, C. Quintas, A. Madeira Lopes, J. Ramos, and M. C. Loureiro-Dias. 2000. Effects of salts on Debaryomyces hansenii and Saccharomyces cerevisiae under stress conditions. Int. J. Food Microbiol. 56:191-197. [DOI] [PubMed] [Google Scholar]
- 2.Andre, L., A. Nilsson, and L. Adler. 1988. The role of glycerol in osmotolerance of the yeast Debaryomyces hansenii. J. Gen. Microbiol. 134:669-677. [Google Scholar]
- 3.Blomberg, A. 2000. Metabolic surprises in Saccharomyces cerevisiae during adaptation to saline conditions; questions, some answers, and a model. FEMS Microbiol. Lett. 182:1-8. [DOI] [PubMed] [Google Scholar]
- 4.Blomberg, A., and L. Adler. 1992. Physiology of osmotolerance in fungi. Adv. Microb. Physiol. 33:145-212. [DOI] [PubMed] [Google Scholar]
- 5.Butinar, L., S. Sonjak, P. Zalar, A. Plemenitaš, and N. Gunde-Cimerman. 2005. Melanized halophilic fungi are eukaryotic members of microbial communities in hypersaline waters of solar salterns. Botanica Marina 48:73-79. [Google Scholar]
- 6.De Hoog, G. S., P. Zalar, C. Urzi, F. de Leo, N. A. Yurlova, and K. Sterflinger. 1999. Relationships of dothideaceous black yeasts and meristematic fungi based on 5.8S and ITS2 rDNA sequence comparison. Studies Mycol. 43:31-37. [Google Scholar]
- 7.Gunde-Cimerman, N., J. C. Frisvad, P. Zalar, and A. Plemenitaš. 2005. Halotolerant and halophilic fungi, p. 69-127. In S. K. Deshmukh and M. K. Rai (ed.), The biodiversity of fungi: their role in human life. Science Publishers, Inc., New Delhi, India.
- 8.Gunde-Cimerman, N., P. Zalar, G. S. de Hoog, and A. Plemenitaš. 2000. Hypersaline waters in salterns: natural ecological niches for halophilic black yeasts. FEMS Microbiol. Ecol. 32:235-240. [DOI] [PubMed] [Google Scholar]
- 9.Hernández-Saavedra, N. Y., J. L. Ochoa, and R. Vazquez-Dulhalt. 1995. Osmotic adjustment in marine yeast. J. Plankton Res. 17:59-69. [Google Scholar]
- 10.Hohmann, S. 2002. Osmotic stress signaling and osmoadaptation in yeasts. Microbiol. Mol. Biol. Rev. 66:300-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hohmann, S., and P. W. H. Mager (ed.). 2003. Topics in current genetics, vol. 1. Springer-Verlag, Berlin, Germany.
- 12.Lahav, R., P. Fareleira, A. Nejidat, and A. Abeliovich. 2002. The identification and characterisation of osmotolerant yeast isolates from chemical wastewater evaporation ponds. Microb. Ecol. 43:388-396. [DOI] [PubMed] [Google Scholar]
- 13.Larsson, C., C. Morales, L. Gustafsson, and L. Adler. 1990. Osmoregulation of the salt-tolerant yeast Debaryomyces hansenii grown in a chemostat at different salinities. J. Bacteriol. 172:1769-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oren, A. 1999. Bioenergetic aspects of halophilism. Microbiol. Mol. Biol. Rev. 63:334-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrovič, U., N. Gunde-Cimerman, and A. Plemenitaš. 2002. Cellular responses to environmental salinity in the halophilic black yeast Hortaea werneckii. Mol. Microbiol. 45:665-672. [DOI] [PubMed] [Google Scholar]
- 16.Plemenitaš, A., and N. Gunde-Cimerman. 2005. Cellular responses in the halophilic black yeast Hortaea werneckii to high environmental salinity, p. 453-470. In N. Gunde-Cimerman, A. Oren, and A. Plemenitaš (ed.), Adaptation to life at high salt concentrations in Archaea, Bacteria, and Eukarya. Springer-Verlag, Berlin, Germany.
- 17.Prista, C., A. Almagro, M. C. Loureiro-Dias, and J. Ramos. 1997. Physiological basis for the high salt tolerance of Debaryomyces hansenii. Appl. Environ. Microbiol. 63:4005-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prista, C., M. C. Loureiro-Dias, V. Montiel, R. García, and J. Ramos. 2005. Mechanisms underlying the halotolerant way of Debaryomyces hansenii. FEMS Yeast Res. 5:693-701. [DOI] [PubMed] [Google Scholar]
- 19.Ramos, J. 1999. Contrasting salt tolerance mechanisms in Saccharomyces cerevisiae and Debaryomyces hansenii, p. 377-390. In S. G. Pandalai (ed.), Recent research developments in microbiology, vol. 3. Research Signpost, Trivandrum, India. [Google Scholar]
- 20.Ramos, J. 2005. Introducing Debaryomyces hansenii, a salt loving yeast, p. 441-452. In N. Gunde-Cimerman, A. Oren, and A. Plemenitaš (ed.), Adaptation to life at high salt concentrations in Archaea, Bacteria, and Eukarya. Springer-Verlag, Berlin, Germany.
- 21.Ramos, J., R. Haro, and A. Rodríguez-Navarro. 1990. Regulation of potassium fluxes in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1029: 211-217. [DOI] [PubMed] [Google Scholar]
- 22.Silva-Graça, M., and C. Lucas. 2003. Physiological studies on long-term adaptation to salt stress in the extremely halotolerant yeast Candida versatilis CBS 4019 (syn. C. halophila). FEMS Yeast Res. 3:247-260. [DOI] [PubMed] [Google Scholar]
- 23.Sterflinger, K., G. S. de Hoog, and G. Haase. 1999. Phylogeny and ecology of meristematic ascomycetes. Studies Mycol. 43:5-22. [Google Scholar]
- 24.Zalar, P., G. S. de Hoog, and N. Gunde-Cimerman. 1999. Ecology of halotolerant dothideaceous black yeasts. Studies Mycol. 43:38-48. [Google Scholar]