Abstract
In Europe, Borrelia burgdorferi genospecies causing Lyme borreliosis are mainly transmitted by the tick Ixodes ricinus. Since its discovery, B. burgdorferi has been the subject of many epidemiological studies to determine its prevalence and the distribution of the different genospecies in ticks. In the current study we systematically reviewed the literature on epidemiological studies of I. ricinus ticks infected with B. burgdorferi sensu lato. A total of 1,186 abstracts in English published from 1984 to 2003 were identified by a PubMed keyword search and from the compiled article references. A multistep filter process was used to select relevant articles; 110 articles from 24 countries contained data on the rates of infection of I. ricinus with Borrelia in Europe (112,579 ticks), and 44 articles from 21 countries included species-specific analyses (3,273 positive ticks). These data were used to evaluate the overall rate of infection of I. ricinus with Borrelia genospecies, regional distributions within Europe, and changes over time, as well as the influence of different detection methods on the infection rate. While the infection rate was significantly higher in adults (18.6%) than in nymphs (10.1%), no effect of detection method, tick gender, or collection period (1986 to 1993 versus 1994 to 2002) was found. The highest rates of infection of I. ricinus were found in countries in central Europe. B. afzelii and B. garinii are the most common Borrelia species, but the distribution of genospecies seems to vary in different regions in Europe. The most frequent coinfection by Borrelia species was found for B. garinii and B. valaisiana.
Lyme borreliosis (LB), the most frequent tick-borne disease in the northern hemisphere, is a multisystemic disorder caused by spirochetes belonging to the Borrelia burgdorferi sensu lato complex. In Europe, the principal vector of Borrelia is the tick Ixodes ricinus. The risk to humans of infection with Borrelia depends on outdoor recreational activity, on the density of tick populations, and on the infection of the ticks with Borrelia (32). Therefore, data describing the prevalence of Borrelia in ticks can be used to assess the risk of LB for public health.
B. burgdorferi sensu lato is a genetically diverse group of spirochetes. In Europe, at least the following five different genospecies belonging to the B. burgdorferi sensu lato complex have been found: B. afzelii, B. garinii, B. burgdorferi sensu stricto, B. valaisiana, and B. lusitaniae (2, 19, 29, 43, 45, 69). Different reservoir hosts seem to harbor different genospecies of B. burgdorferi sensu lato, which is explained by differential properties of the hosts’ complement systems that favor certain genospecies (73).
At least three species of the B. burgdorferi sensu lato complex (B. afzelii, B. garinii, and B. burgdorferi sensu stricto) are known to be pathogenic for humans. The pathogenicity of the other species is still unclear, although B. valaisiana and B. lusitaniae have been detected in skin biopsies of some LB patients (15, 103). There is strong evidence that infections with different genospecies of Borrelia correlate with different clinical symptoms of LB. Lyme arthritis is mainly attributed to B. burgdorferi sensu stricto, B. garinii infection is preferentially associated with neuroborreliosis, and skin manifestations are associated mainly with B. afzelii (1, 18, 121). Therefore, knowledge of the geographic distribution of different genospecies of B. burgdorferi sensu lato within their tick vector has not only ecological and epidemiological relevance but also clinical relevance.
Individual ticks can be infected with more than one genospecies of B. burgdorferi sensu lato (77, 85, 88, 97). Information on the patterns of such mixed infections may reveal important biological and ecological principles of B. burgdorferi sensu lato and also have clinical relevance, since such mixed infections have also been detected in patients (18, 103). However, mixed infections in ticks appear to be rather rare; thus, studies which further specify the mixed infections usually do not contain enough data to draw valid conclusions. A metaanalysis which merges the available data could provide more insight into mixed infections.
In this paper we describe a metaanalysis of epidemiological studies of the prevalence and distribution of genospecies of B. burgdorferi sensu lato in host-seeking ticks in various European countries based on a systematic literature review, describing (i) the distribution of B. burgdorferi sensu lato and (ii) the occurrence of different Borrelia genospecies in I. ricinus tick populations in Europe. The following questions concerning the distribution of B. burgdorferi sensu lato were examined. (i) What is the mean rate of infection of I. ricinus ticks with Borrelia in Europe? (ii) How do the rates of infection of ticks differ across Europe? (iii) Has tick infection changed during recent years? (iv) Do the methods used for detection of Borrelia in ticks influence the infection rate? In addition, the following questions concerning the occurrence of different Borrelia genospecies in I. ricinus tick populations in Europe were examined. (i) What is the predominant Borrelia species in Europe? (ii) Are there differences in the distribution of the Borrelia species in different parts of Europe? (iii) Is there a difference in Borrelia species distribution in tick nymphs and adults or in females and males? (iv) Which mixed infections involving different Borrelia species occur most often?
MATERIALS AND METHODS
We performed a computerized literature search using PubMed to identify all citations concerning rates of infection of ticks with Borrelia published from 1984 to 2003, using the keyword search terms “Ixodes” and “Borrelia”. A copy of the abstract of each identified English language citation was obtained. A multistage assessment was used to determine which articles contained relevant data. In the first step we reviewed the abstracts to determine which articles reported epidemiological data for (i) the rate of infection of I. ricinus with B. burgdorferi sensu lato or (ii) the distribution of the different Borrelia genospecies in I. ricinus. Only articles with the following criteria were included: (i) the area of tick collection was located in Europe (without the former USSR and regions with a subtropical climate), (ii) the ticks examined were I. ricinus, and (iii) the ticks were unfed, host-seeking ticks. In the second step, the articles were retrieved, and their bibliographies were screened for citations not identified in the initial step of the literature search in PubMed. In the third step of the assessment, papers with incomprehensible, incomplete, or previously published data were excluded. Data on larvae were excluded, since the infection rates have consistently been reported to be very low. If this was not possible, the entire paper was excluded. The data extracted from every paper was checked twice.
Data abstraction.
Every variable referring to area and period of tick collection, stage, gender, infection with Borrelia, and Borrelia species in the selected articles was documented. The specific variable analysis was limited to articles containing that variable, which eliminated the need to address missing data. These variables formed the foundation of the final database. Difficulties in abstracting data resulted from nonreported information or reported data that accounted for only a subset of the database. For data abstraction the following steps were carried out. The infection rates (p) of ticks examined in pools (containing at most five ticks per pool) were recalculated where possible, using the following formula: 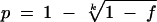 , where k is the number of specimens in each pool and f is the proportion of infected pools (17). If the number of positive ticks in the study was not given, it was calculated, if possible, based on the number of ticks examined and the reported infection rate. Papers were divided into separate records if the workers examined (i) ticks collected in different years, (ii) different ticks by different methods, (iii) ticks from different countries, or (iv) ticks from collection areas larger than 1° latitude or 2° longitude. This division was carried out only if the number of ticks examined per record was not less than 100. From papers in which the same ticks were examined with different methods leading to different results, only data obtained with PCR or an immunofluorescence assay were included. If not reported, the longitude and latitude of the sampling site of every study was approximated. If more than one collection point was given, the collection area was determined and the coordinates were averaged. Each genospecies found in a mixed infection was added to the corresponding single-infection tally. Records for species-specific analyses of pooled ticks were excluded.
, where k is the number of specimens in each pool and f is the proportion of infected pools (17). If the number of positive ticks in the study was not given, it was calculated, if possible, based on the number of ticks examined and the reported infection rate. Papers were divided into separate records if the workers examined (i) ticks collected in different years, (ii) different ticks by different methods, (iii) ticks from different countries, or (iv) ticks from collection areas larger than 1° latitude or 2° longitude. This division was carried out only if the number of ticks examined per record was not less than 100. From papers in which the same ticks were examined with different methods leading to different results, only data obtained with PCR or an immunofluorescence assay were included. If not reported, the longitude and latitude of the sampling site of every study was approximated. If more than one collection point was given, the collection area was determined and the coordinates were averaged. Each genospecies found in a mixed infection was added to the corresponding single-infection tally. Records for species-specific analyses of pooled ticks were excluded.
Literature description.
A total of 1,186 English abstracts published from 1984 to 2003 were identified in PubMed. After the abstracts were examined, 191 papers proceeded to the second stage, and their reference sections were scrutinized for missed publications, which identified another 26 articles. For analysis of infection rates 110 articles and for species-specific analysis 44 articles progressed through the third stage and to data extraction; 154 and 50 records were extracted for analysis of infection rates and species-specific analysis, respectively.
Statistics.
Statistical analyses were done using GraphPad Prism 3.0 (GraphPad Software, San Diego, Calif.). The data expressed in the bar charts are the means ± standard errors of the means; the means are indicated in scatter plots by horizontal lines. An unpaired t test with Welch's correction was performed when two groups were compared. For comparison of more than two groups, analysis of variance (one-way analysis of variance) followed by Bonferroni's multiple-comparison test was used. P values of <0.05, <0.01, and <0.001 were considered significant. Linear regression was performed to analyze the relationship between two variables.
RESULTS
Infection rates. (i) Overall infection rates.
Table 1 lists all of the records extracted for the analysis of rates of infection of B. burgdorferi sensu lato in I. ricinus ticks in Europe. The overall mean prevalence of Borrelia in ticks was 13.7% (15,423 of 112,579 ticks). Compared to nymphs (10.1%; 6,384 of 63,298 ticks), adults (18.6%; 8,051 of 43,390 ticks) had a considerably higher infection rate. There was no difference between the infection rates of females and males (18.0% [2,784 of 15,464 ticks[and 16.2% [2,321 of 14,344 ticks], respectively). For studies in which both nymphs and adults in parallel and at least 100 of each stage were examined, 28 of 64 records showed that there were at least twice as many infected adults as infected nymphs, 26 records showed that there were between one and two times more infected adults, 7 records showed that there were as many infected adults as nymphs, and the infection rate in adults was lower than that in nymphs in only 3 records.
TABLE 1.
Records with data on rates of infection of I. ricinus ticks with B. burgdorferi sensu lato in Europea
| Country | Reference | Year(s) | Method(s) | Infection rate (%) | No. of ticks
|
||
|---|---|---|---|---|---|---|---|
| Total | Nymphs | Adults | |||||
| Austria | 117 | 1997 | DFM | 20.8 | 1,163 | 853 | 310 |
| 53 | 1989-2002 | DFM | 23.2 | 422 | 211 | 211 | |
| Belgium | 85 | 1996 | PCR | 23 | 489 | 444 | 45 |
| Bulgaria | 9 | 2000 | PCR | 32.7 | 202 | 90 | 112 |
| 8 | NG | BSK | 17 | 47 | 47 | ||
| Croatia | 100 | 1995 | PCR | 45 | 124 | 34 | 90 |
| Czech Republic | 4 | 1995-1998 | PCR | 4.5 | 779 | 572 | 207 |
| 48 | 1988-1996 | DFM | 20.7 | 8,339 | 3,546 | 4,793 | |
| 128 | 1987-1991 | IFA | 15.3 | 5,104 | 4,417 | 687 | |
| 50 | 1992 | DFM | 27 | 163 | 34 | 129 | |
| 20 | 2000 | PCR | 20.3 | 370 | 261 | 109 | |
| 49 | 1995 | DFM | 24.3 | 350 | 150 | 200 | |
| 49 | 1996 | DFM | 22 | 350 | 150 | 200 | |
| 49 | 1997 | DFM | 26 | 350 | 150 | 200 | |
| 49 | 1998 | DFM | 24.9 | 350 | 150 | 200 | |
| 49 | 1999 | DFM | 17.7 | 350 | 150 | 200 | |
| 49 | 2000 | DFM | 17.7 | 350 | 150 | 200 | |
| 49 | 2001 | DFM | 24 | 350 | 150 | 200 | |
| 53 | 1989-2002 | DFM | 21.8 | 1,094 | 586 | 508 | |
| 51 | 1991 | DFM | 15.2 | 350 | 150 | 200 | |
| 51 | 1992 | DFM | 23 | 350 | 150 | 200 | |
| 51 | 1993 | DFM | 22.3 | 350 | 150 | 200 | |
| 51 | 1994 | DFM | 19.8 | 350 | 150 | 200 | |
| 52 | 1988 | GS | 8.5 | 378 | 378 | ||
| 47 | 1988-1989 | DFM | 20.2 | 460 | 209 | 251 | |
| 68 | NG | DFM | 7.9 | 570 | NG | NG | |
| 114 | 1997 | DFM | 5.4 | 555 | 145 | 410 | |
| 114 | 1998 | DFM | 11 | 163 | 13 | 150 | |
| Denmark | 74 | 1990-1991 | IFA | 18.6 | 317 | 202 | 115 |
| 59 | 1995 | IFA | 5.3 | 396 | 396 | ||
| 59 | 1996 | IFA | 3.1 | 829 | 829 | ||
| 59 | 1997 | IFA | 2.4 | 327 | 327 | ||
| 60 | 1996 | IFA | 5.1 | 1,151 | 1,045 | 106 | |
| Estonia | 79 | 2000 | PCR | 15 | 100 | 100 | |
| Finland | 62 | 1996 | PCR, DFM, BSK | 32.2 | 726 | 303 | 423 |
| 63 | 1992 | BSK | 5.5 | 1210 | 392 | 818 | |
| 79 | 2000 | PCR | 5.1 | 454 | 343 | 111 | |
| France | 94 | 1994 | PCR | 12 | 249 | 249 | |
| 95 | 1995 | PCR | 8.2 | 461 | 461 | ||
| 31 | 1992 | IFA | 9.8 | 1,556 | 1,014 | 542 | |
| 31 | 1992 | IFA | 6.4 | 1,822 | 1,422 | 400 | |
| 31 | 1992 | IFA | 4.7 | 1,295 | 811 | 484 | |
| 129 | 1991 | IFA | 10.7 | 383 | 314 | 69 | |
| 96 | 1994-1995, 1998 | PCR | 14.3 | 638 | 419 | 219 | |
| Germany | 25 | 1985-1986 | IFA | 14.6 | 2,369 | 1,157 | 1,212 |
| 26 | 1994 | IFA | 19.1 | 472 | 157 | 315 | |
| 27 | 1997 | PCR | 36.2 | 492 | 91 | 401 | |
| 97 | 1999-2000 | PCR | 35.2 | 1,055 | 507 | 548 | |
| 38 | 1992-1993 | DFM | 10.4 | 279 | 68 | 211 | |
| 38 | 1992-1992 | DFM | 4.7 | 1,185 | 1,185 | ||
| 38 | 1992-1993 | BSK | 8.4 | 598 | 485 | 113 | |
| 38 | 1992-1993 | IFA | 12.4 | 194 | 147 | 47 | |
| 64 | 1991 | IFA | 18.4 | 414 | 414 | ||
| 126 | 1992 | DFM | 16 | 100 | 100 | ||
| 126 | 1993 | BSK | 18 | 100 | 100 | ||
| 126 | 1994 | PCR | 22 | 100 | 100 | ||
| 111 | 1996 | PCR | 9 | 3,926 | 3,520 | 406 | |
| 69 | NG | PCR | 18.1 | 226 | 226 | ||
| 71 | 1987-1988 | IFA | 6.3 | 1,954 | 1,954 | ||
| 88 | 1998-2000 | PCR | 15.8 | 3,138 | NG | NG | |
| 44 | 1999-2000 | PCR | 11.1 | 305 | 243 | 62 | |
| 65 | 1986 | BSK | 5.8 | 173 | 49 | 124 | |
| 98 | 1994 | PCR | 9 | 112 | 112 | ||
| 90 | NG | PCR | 31.5 | 165 | NG | NG | |
| 125 | 1993-1994 | BSK | 16.3 | 559 | 559 | ||
| 81 | 1991 | DFM, IFA | 17.9 | 756 | 531 | 225 | |
| Ireland | 36 | 1997-1998 | PCR | 12 | 183 | 164 | 19 |
| 37 | NG | IFA | 12 | 100 | 100 | ||
| 66 | 1995 | PCR | 14.9 | 915 | 686 | 229 | |
| 34 | 1992-1993 | IFA | 2.6 | 2,587 | 2,518 | 69 | |
| 33 | 1990-1991 | IFA | 6.5 | 612 | 509 | 103 | |
| 67 | 1995 | PCR | 18.4 | 512 | 411 | 101 | |
| Italy | 13 | 1994 | PCR | 40 | 227 | 218 | 9 |
| 11 | NG | PCR | 4.4 | 420 | NG | NG | |
| 105 | 1997-1998 | PCR | 2.9 | 1,503 | 1,475 | 28 | |
| 12 | NG | PCR | 19.8 | 86 | 84 | 2 | |
| 24 | 1998 | PCR | 11.4 | 55 | 55 | ||
| 106 | NG | PCR | 4.3 | 141 | 125 | 16 | |
| 80 | 1995 | BSK | 0 | 80 | 80 | ||
| 80 | 1995 | PCR | 0 | 110 | 110 | ||
| Latvia | 69 | NG | PCR | 31.3 | 300 | 300 | |
| 23 | 1999 | PCR | 31.2 | 205 | 55 | 150 | |
| 23 | 2000 | PCR | 25.7 | 300 | 150 | 150 | |
| Lithuania | 86 | 1988-1991 | DFM | 10.1 | 3,820 | 287 | 3,533 |
| The Netherlands | 121 | NG | BSK | 10.5 | 248 | 176 | 72 |
| 102 | 1994 | PCR | 15.6 | 96 | 39 | 57 | |
| 102 | 1994 | IFA | 19.5 | 210 | 120 | 90 | |
| 101 | 1988-1993 | IFA | 24.6 | 463 | 341 | 122 | |
| 17 | 1991 | DFM | 12.5 | 522 | 476 | 46 | |
| Norway | 58 | 1999 | PCR | 15.8 | 341 | 185 | 156 |
| Poland | 110 | 1997-1998 | PCR | 21.6 | 616 | 48 | 568 |
| 113 | 2000 | PCR | 11.6 | 424 | 424 | ||
| 108 | 1999 | PCR | 7.8 | 1,710 | 1,160 | 550 | |
| 122 | 1993 | IFA | 11.5 | 1,666 | 1,070 | 596 | |
| 7 | 2000 | IFA | 11.3 | 1,387 | 1,264 | 123 | |
| 7 | 2001 | IFA | 9.6 | 993 | 931 | 62 | |
| 93 | 1996 | DFM, BSK | 20.7 | 92 | 6 | 86 | |
| 93 | 1996 | DFM, BSK | 12.8 | 815 | 110 | 705 | |
| 83 | 1998 | IFA | 18.6 | 587 | 538 | 49 | |
| 83 | 1998 | IFA | 13.6 | 536 | 464 | 72 | |
| 107 | 1999 | PCR | 18.9 | 514 | 234 | 280 | |
| 127 | 1998 | PCR | 4 | 1,262 | 835 | 427 | |
| 127 | 1999 | PCR | 5.7 | 1,379 | 1,006 | 373 | |
| 127 | 2000 | PCR | 4.8 | 1,356 | 955 | 401 | |
| 127 | 2001 | PCR | 3.3 | 1,439 | 894 | 545 | |
| 14 | 2000 | DFM | 8.8 | 114 | 50 | 64 | |
| 14 | 2001 | PCR | 5.3 | 549 | 265 | 284 | |
| 112 | 1996 | IFA | 8.1 | 1333 | 554 | 779 | |
| 123 | 1994 | IFA | 8.8 | 3,958 | 2,395 | 1,563 | |
| Portugal | 18 | 1998 | PCR | 75 | 55 | 55 | |
| 69 | NG | PCR | 14.7 | 217 | 217 | ||
| Slovakia | 22 | 1991 | DFM | 18.5 | 2,857 | 2,857 | |
| 29 | 1998 | IFA | 49.1 | 114 | 114 | ||
| 115 | 1994 | DFM | 4.8 | 809 | 149 | 660 | |
| 115 | 1995 | DFM | 17.2 | 805 | 215 | 590 | |
| 115 | 1996 | DFM | 15.6 | 805 | 155 | 650 | |
| 115 | 1996 | DFM | 14.2 | 399 | 113 | 286 | |
| 69 | NG | PCR | 40.5 | 585 | 585 | ||
| 21 | 2002 | PCR | 43.3 | 60 | 20 | 40 | |
| 42 | 2000 | PCR | 28.2 | 177 | 93 | 84 | |
| 43 | 1999 | PCR | 32.2 | 966 | 382 | 584 | |
| 43 | 2000 | PCR | 33.2 | 328 | 174 | 154 | |
| Slovenia | 104 | 1990 | IFA | 7.7 | 496 | 411 | 85 |
| 116 | NG | BSK | 19 | 363 | 206 | 157 | |
| Spain | 3 | 1992-1993 | PCR | 1.5 | 134 | 134 | |
| Sweden | 40 | 1988-1991 | IFA | 14.4 | 2974 | 397 | 2,577 |
| 119 | 1994-1995 | PCM | 13 | 1,908 | 1,908 | ||
| 28 | 1999 | PCR | 11 | 301 | 301 | ||
| 120 | 1991 | PCM | 15.4 | 408 | 408 | ||
| 120 | 1992 | PCM | 20.6 | 471 | 471 | ||
| 120 | 1993 | PCM | 24.1 | 551 | 551 | ||
| 82 | 1988 | PCM | 12.8 | 539 | 459 | 80 | |
| 82 | 1989 | PCM | 8.6 | 1,005 | 946 | 59 | |
| 5 | 1991 | IFA | 20.8 | 149 | 92 | 57 | |
| 56 | 1991 | PCM | 9.1 | 396 | 396 | ||
| 6 | 1989 | PCM | 2 | 42 | 42 | ||
| 57 | 1992 | PCM | 12.6 | 381 | 381 | ||
| 57 | 1993 | PCM | 11 | 581 | 581 | ||
| 57 | 1994 | PCM | 14.2 | 604 | 604 | ||
| 39 | NG | IFA | 29.6 | 54 | 16 | 38 | |
| Switzerland | 92 | 1987-1993 | IFA, DFM, BSK | 30.4 | 727 | 727 | |
| 54 | 1993 | BSK | 13.7 | 95 | 22 | 73 | |
| 84 | 1987 | IFA | 30.8 | 182 | 94 | 88 | |
| 75 | 1991 | BSK | 16.5 | 133 | 133 | ||
| 76 | NG | PCR | 49 | 100 | 100 | ||
| 61 | 1999-2001 | IFA | 10.9 | 460 | 415 | 45 | |
| 55 | 1993-1994 | BSK | 19.1 | 235 | 93 | 142 | |
| 109 | NG | BSK | 13.6 | 118 | NG | NG | |
| 30 | 1990 | DFM | 21.4 | 56 | 56 | ||
| United Kingdom | 16 | 1997 | BSK | 8.5 | 128 | NG | NG |
| 72 | 1995-1996 | PCR | 4.6 | 680 | 580 | 100 | |
| 41 | NG | PCR | 7.7 | 65 | 65 | ||
| 89 | 1994 | IFA or PCR | 8.3 | 109 | 73 | 36 | |
| 89 | 1995 | IFA or PCR | 3.9 | 333 | 221 | 112 | |
The total infection rate is indicated. The number of nymphs and adults examined shows the stage composition of all ticks examined (total) for each record. BSK, cultivation in BSK medium; DFM, dark-field microscopy; GS, Giemsa-stained smears; IFA, immunofluorescence assay; PCM, phase-contrast microscopy; NG, not given. The average infection rate was 13.7%, and a total of 112,579 ticks were examined.
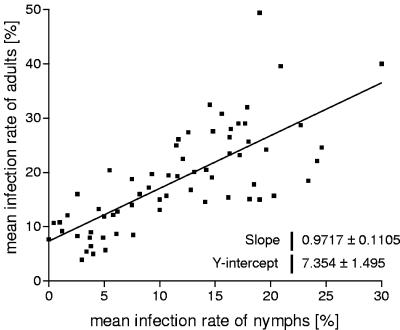
Correlating the rates of infection of nymphs with those of adults enabled a linear regression (Fig. 1), resulting in the following formula: IN = 0.97 × IA − 7.35, where IN and IA are the mean rates of infection of nymphs and adults, respectively.
FIG. 1.
Regression of mean rates of infection of Borrelia in nymphs and adults. Only studies in which both nymphs and adults and at least 100 individuals of each stage were examined were included. Each data point represents one record.
(ii) Influence of detection methods.
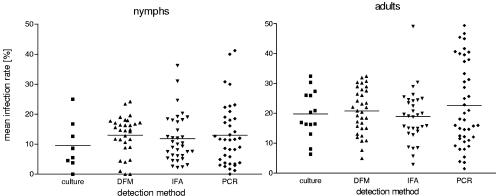
The methods generally used for detection of Borrelia in ticks are cultivation in BSK medium, dark-field microscopy, an immunofluorescence assay, and PCR. A comparison of the mean infection rates for studies in which at least one of these methods was used for detection of Borrelia in ticks revealed no significant difference in either nymphs or adults (Fig. 2). It was noteworthy that the highest infection rates (nymphs, >30%; adults, >35%) were obtained almost exclusively with PCR in Bulgaria, Croatia, southern Germany, Latvia, and Slovakia. In the analysis described below, no distinction between detection methods was made.
FIG. 2.
Influence of detection method on infection rates. DFM, dark-field microscopy; IFA, immunofluorescence assay.
(iii) Infection rates in different regions of Europe.
The rates of infection of ticks with Borrelia were correlated with the latitude or longitude of the sampling site in every study. For this purpose, the coordinates of the sites of tick collection were determined. In studies with a large collection area, the means of latitude and longitude were calculated. Coordinates were transformed to decimal values. Negative longitudes represent the zone west of Greenwich, England, and positive longitudes represent the zone east of Greenwich.
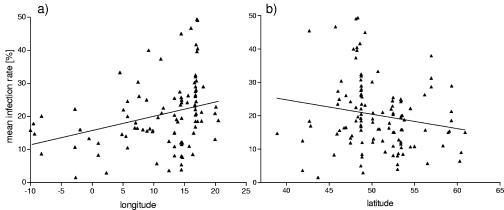
Regression analyses of the mean infection rates with the corresponding longitudes or latitudes showed that there was a significant increase in the infection rate in adult ticks from western Europe to eastern Europe (14 to 24%, as calculated from the linear regression; P < 0.05) (Fig. 3a), whereas no such trend was seen for nymphs (data not shown). Latitude had no effect on the prevalence of tick infection either in nymphs (data not shown) or in adults (Fig. 3b). The effect of longitude on the infection rates did not change if only studies in which at least 100 nymphs or adults were examined were included. However, this correction did lead to a significant increase in the infection rates in adult ticks from northern Europe to southern Europe (12 to 23%, as calculated from the linear regression; P < 0.01; r2 = 0.092) (data not shown).
FIG. 3.
Regression analysis of the mean rates of infection in adults with the corresponding longitude (P < 0.05; r2 = 0.102) (a) or latitude (P > 0.05; r2 = 0.031) (b).
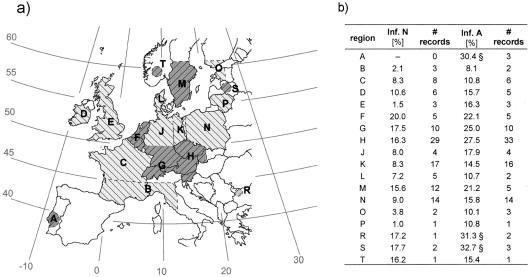
To calculate the infection rates in several regions in Europe, the means of the infection rates for all studies in each region were determined for nymphs and adults. To define the regions, the following criteria were taken into account. (i) The regions were defined so that they were large enough to include the studies with large collection areas completely; and (ii) geographic conditions were taken into consideration. The available data are summarized and mapped in Fig. 4. We distinguished regions with low infection rates (nymphs, ≤11%; adults, ≤20%) and high infection rates (nymphs, >11%; adults, >20%). In three regions (regions A, R, and S) the mean rate of infection of adults was extremely high (>30%) (Fig. 4b).
FIG. 4.
(a) Map of the defined regions. Areas with low infection rates (nymphs, ≤11%; adults, ≤20%) are indicated by light gray; areas with higher infection rates are indicated by dark gray. (b) Regions and means of the rates of infection of nymphs (Inf. N) and adults (Inf. A). The number of records used for every region is indicated. A section sign indicates that the infection rates were extremely high (>30%).
The limitation to studies in which at least 100 adults or 100 nymphs were examined led to considerably different results for four of the regions. In regions A and M the rates of infection of adults decreased from 30 to 8% and from 21 to 11%, respectively. In region R the rate of infection of adults increased from 31 to 46% and for nymphs no data could be used. In region S, an increase from 17 to 23% in the rate of infection of nymphs occurred.
(iv) Years of tick collection.
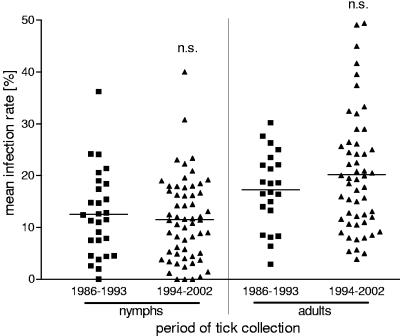
To compare the course of infection rates over the years, studies with a collection period longer than 1 year were excluded unless the data could be separated into years. As the rates of infection of ticks in several regions in Europe vary significantly (Fig. 4), a comparison of the rates of infection per year requires a representative profile of regions with high and low infection rates in each year. Since such data were not available, data for ranges of years (1986 to 1993 and 1994 to 2001) were merged. Data for regions with extremely high infection rates (regions A, R, and S) were excluded to obtain similar proportions of areas with high and low infection rates in both periods. A comparison of the two periods revealed no significant difference in the means of the rates of infection of nymphs or adults (Fig. 5).
FIG. 5.
Comparison of rates of infection of nymphs and adults in two collecting periods. Each data point represents one record. To obtain a similar proportion of areas with high and low infection rates in both periods, data for extremely high infection rates (>30%) were excluded. n.s., not significant.
Species-specific analysis. (i) Overall ratio of Borrelia species in Europe.
The data from studies in which there were species-specific analyses of B. burgdorferi sensu lato in I. ricinus ticks are summarized in Tables 2 and 3. The following problems were noticed. (i) The number of ticks examined per study was extremely low in some cases (3 of 50 records included less than 10 ticks). Therefore, we first checked in every analysis to determine whether these studies distorted the results. If this was the case, the studies were excluded, as mentioned below. (ii) The stage and gender of the ticks examined were not stated in every study, which reduced the number of records considerably. In Table 2 each species found in a mixed infection reported separately was added to the appropriate single-infection tally in order to harmonize results (therefore, the sum of the percentages may result in values greater than 100%). To avoid overrepresentation of single studies in which high numbers of ticks were examined, the percentages of positive ticks for each species (combination) of every single study were used for the analyses (Fig. 6 to 8).
TABLE 2.
Records extracted for analysis of B. burgdorferi sensu lato genospecies in I. ricinus ticks in Europea
| Country | Reference | Total no. | No. of Borrelia-positive ticks
|
|||||
|---|---|---|---|---|---|---|---|---|
| Nontypeable | B. afzelii | B. garinii | B. burgdorferi sensu stricto | B. valaisiana | B. lusitaniae | |||
| Austria | 117 | 29 | 0 | 11 | 17 | 2 | ||
| 118 | 50 | 0 | 25 | 21 | 5 | |||
| Belgium | 85 | 114 | 17 | 14 | 79 | 56 | ||
| Bulgaria | 9 | 24 | 1 | 18 | 2 | 5 | 2 | 1 |
| 8 | 8 | 0 | 0 | 4 | 4 | |||
| 10 | 46 | 6 | 26 | 3 | 15 | 3 | 1 | |
| 10 | 22 | 2 | 11 | 5 | 0 | 5 | 0 | |
| Croatia | 100 | 56 | 8 | 37 | 5 | 2 | 15 | |
| Czech Republic | 4 | 34 | 0 | 15 | 21 | 0 | ||
| 20 | 76 | 1 | 45 | 16 | 8 | 9 | ||
| 114 | 9 | 0 | 2 | 3 | 7 | |||
| Estonia | 79 | 15 | 0 | 12 | 3 | 0 | 0 | 0 |
| 62 | 142 | 5 | 101 | 38 | 0 | |||
| Finland | 79 | 23 | 0 | 12 | 2 | 9 | 0 | 0 |
| France | 94 | 25 | 0 | 19 | 9 | 3 | ||
| 95 | 38 | 12 | 17 | 9 | 3 | |||
| 129 | 3 | 0 | 0 | 1 | 2 | |||
| 96 | 91 | 5 | 36 | 29 | 5 | 27 | 0 | |
| Germany | 97 | 371 | 5 | 261 | 126 | 46 | ||
| 69 | 41 | 3 | 18 | 15 | 0 | 6 | 0 | |
| 124 | 67 | 0 | 6 | 26 | 27 | 8 | ||
| 78 | 17 | 1 | 3 | 13 | 0 | |||
| 44 | 34 | 0 | 6 | 19 | 11 | 2 | ||
| 98 | 10 | 0 | 4 | 5 | 1 | 0 | 0 | |
| Ireland | 36 | 22 | 4 | 1 | 10 | 0 | 8 | |
| 66 | 136 | 8 | 9 | 47 | 27 | 64 | ||
| 67 | 94 | 2 | 16 | 26 | 21 | 47 | ||
| Italy | 13 | 91 | 21 | 8 | 42 | 56 | 1 | |
| Latvia | 69 | 94 | 1 | 39 | 34 | 5 | 19 | 0 |
| 23 | 64 | 1 | 20 | 29 | 3 | 15 | 0 | |
| 23 | 77 | 8 | 35 | 20 | 10 | 10 | 0 | |
| The Netherlands | 121 | 26 | 0 | 26 | 0 | 0 | ||
| 102 | 15 | 0 | 6 | 7 | 0 | 5 | ||
| 87 | 63 | 13 | 2 | 19 | 29 | |||
| Norway | 58 | 54 | 0 | 49 | 1 | 14 | ||
| Poland | 110 | 121 | 14 | 57 | 45 | 48 | ||
| Portugal | 18 | 41 | 0 | 0 | 0 | 0 | 0 | 41 |
| 69 | 32 | 5 | 1 | 14 | 0 | 18 | 0 | |
| Slovakia | 29 | 37 | 0 | 25 | 5 | 0 | 5 | 2 |
| 69 | 237 | 26 | 129 | 59 | 5 | 42 | 0 | |
| 42 | 50 | 0 | 21 | 5 | 0 | 23 | 1 | |
| 43 | 311 | 0 | 186 | 79 | 7 | 68 | 6 | |
| 43 | 109 | 0 | 45 | 8 | 0 | 53 | 3 | |
| Slovenia | 116 | 60 | 0 | 32 | 20 | 8 | ||
| Sweden | 28 | 32 | 2 | 14 | 10 | 4 | 2 | |
| Switzerland | 92 | 50 | 0 | 3 | 19 | 26 | 2 | |
| 61 | 20 | 2 | 0 | 13 | 0 | 3 | 3 | |
| 55 | 41 | 2 | 14 | 8 | 21 | 0 | 0 | |
| 109 | 28 | 0 | 6 | 12 | 8 | 2 | ||
| United Kingdom | 72 | 23 | 2 | 0 | 15 | 2 | 7 | |
The numbers of B. afzelii-, B. garinii-, B. burgdorferi sensu stricto-, B. valaisiana-, and B. lusitaniae-positive ticks and the total numbers of ticks examined are shown. Each species found in a coinfection reported separately was added to the corresponding single-infection tally.
TABLE 3.
Records of mixed infections with B. burgdorferi sensu lato genospecies
| Country | Reference | Total | No. of ticks infected with:
|
Triple infection | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| B. afzelii and B. garinii | B. afzelii and B. burgdorferi sensu stricto | B. garinii and B. burgdorferi sensu stricto | B. afzelii and B. valaisiana | B. garinii and B. valaisiana | B. burgdorferi sensu stricto and B. valaisiana | B. garinii and B. lusitaniae | ||||
| Austria | 117 | 1 | 1 | 0 | 0 | 0 | ||||
| 118 | 1 | 1 | 0 | 0 | 0 | |||||
| Bulgaria | 9 | 5 | 1 | 2 | 0 | 1 | 0 | 0 | 1 | 0 |
| Croatia | 100 | 11 | 0 | 1 | 0 | 10 | 0 | 0 | 0 | |
| Czech Republic | 4 | 2 | 2 | 0 | 0 | 0 | ||||
| 20 | 4 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | ||
| 114 | 3 | 2 | 0 | 1 | 0 | |||||
| Finland | 62 | 2 | 2 | 0 | 0 | 0 | ||||
| France | 94 | 6 | 3 | 1 | 2 | 0 | ||||
| 95 | 3 | 2 | 1 | 0 | 0 | |||||
| 96 | 11 | 2 | 1 | 0 | 2 | 6 | 0 | 0 | 0 | |
| Germany | 97 | 66 | 59 | 6 | 0 | 1 | ||||
| 44 | 4 | 0 | 0 | 4 | 0 | 0 | 0 | 0 | ||
| 69 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| Ireland | 66 | 18 | 0 | 1 | 0 | 2 | 14 | 0 | 1 | |
| 67 | 17 | 0 | 3 | 0 | 3 | 10 | 0 | 1 | ||
| 36 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | ||
| Italy | 13 | 31 | 0 | 1 | 23 | 0 | 1 | 0 | 6 | |
| 106 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Latvia | 69 | 4 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | 0 |
| 23 | 4 | 0 | 1 | 0 | 0 | 2 | 1 | 0 | 0 | |
| 23 | 6 | 0 | 3 | 0 | 0 | 3 | 0 | 0 | 0 | |
| The Netherlands | 102 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | |
| Norway | 58 | 10 | 0 | 10 | 0 | 0 | ||||
| Poland | 110 | 41 | 11 | 17 | 11 | 2 | ||||
| Portugal | 69 | 6 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 0 |
| Slovakia | 69 | 24 | 2 | 2 | 0 | 0 | 20 | 0 | 0 | 0 |
| 43 | 34 | 2 | 2 | 0 | 2 | 26 | 0 | 0 | 2 | |
| Switzerland | 55 | 3 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 |
| 61 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| United Kingdom | 72 | 3 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | |
FIG. 6.
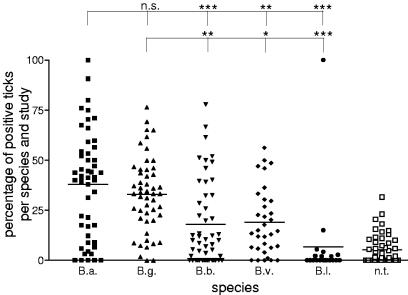
Overall ratio of the Borrelia species B. afzelii (B.a.), B. garinii (B.g.), B. burgdorferi sensu stricto (B.b.), B. valaisiana (B.v.), and B. lusitaniae (B.l.) in I. ricinus ticks in Europe. Fifty records with 3,273 positive ticks were included. The percentages of positive ticks per species for every single study are given. n.t., nontypeable; n.s., not significant; one asterisk, P < 0.05; two asterisks, P < 0.01; three asterisks, P < 0.001.
FIG. 8.
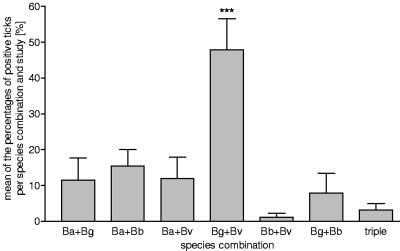
Distribution of mixed infections in ticks. Only records in which B. afzelii (Ba), B. garinii (Bg), B. burgdorferi sensu stricto (Bb), and B. valaisiana (Bv) were examined were included (21 records). The average of the percentages of positive ticks per species combination for every single study is given. Three asterisks, P < 0.001.
Figure 6 shows the overall ratio of Borrelia species in Europe. No distinction was made between nymphs and adults. The mean percentages of B. afzelii-, B. garinii-, B. burgdorferi sensu stricto-, B. valaisiana-, and B. lusitaniae-infected ticks were 38, 33, 18, 19, and 7%, respectively. Five percent of the borreliae were untypeable, and 13% of the ticks had a mixed infection. There was no significant difference between B. afzelii and B. garinii, but the means for both species were significantly different from the means for the other three species.
Since all the studies distinguished between the genospecies B. afzelii, B. garinii, and B. burgdorferi sensu stricto (50 records), but not every study tested for B. valaisiana (33 records) and only a few studies tested for for B. lusitaniae (20 records), the data for the latter two species are weaker. Also, in records which did not determine B. valaisiana and B. lusitaniae, these species might be recognized as “nontypeable” or be falsely recognized as one of the other species. For example, we reported this problem in a study employing real-time PCR which distinguished species by different numbers of mismatches with a fluorescent probe. The PCR product of B. valaisiana had the same melting point as that of B. afzelii and was therefore recognized as B. afzelii (97).
To check if studies containing B. lusitaniae- and nontypeable Borrelia-positive ticks influenced the results, we repeated the analysis after these ticks were excluded. The mean percentages of B. afzelii, B. garinii, B. burgdorferi sensu stricto, and B. valaisiana positive ticks remained similar to those shown in Fig. 6 (36, 34, 15, and 21%, respectively).
(ii) Stage- and gender-dependent distribution of Borrelia species in Europe.
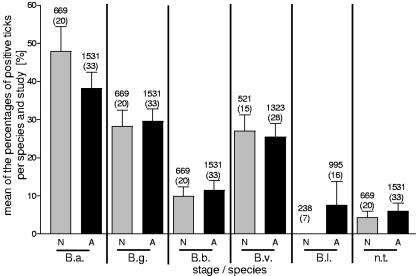
No significant difference was seen when the prevalence of each Borrelia species in nymphs was compared to that in adults (Fig. 7). This result did not change when we included only studies which distinguished between the genospecies B. afzelii, B. garinii, B. burgdorferi sensu stricto, and B. valaisiana.
FIG. 7.
Distribution of the Borrelia genospecies B. afzelii (B.a.), B. garinii (B.g.), B. burgdorferi sensu stricto (B.b.), B. valaisiana (B.v.), and B. lusitaniae (B.l.) and nontypeable Borrelia (n.t.) in nymphs (N) and adults (A). The average of the percentages of positive ticks per species for every single study is given. The numbers of infected ticks examined are indicated above the bars, and the numbers of records are indicated in parentheses.
Only 11 records for a total of 156 female and 97 male ticks were available for a direct gender-based comparison of Borrelia species distribution. Since no information on gender was given for the ticks with mixed infections, we were not able to add each species in a mixed infection to the appropriate single-infection tally. Hence, for gender-specific comparisons, only infections with a single species were included. Data were extracted from 11 records (156 females and 97 males). There was no significant difference in the mean percentage between females and males for any species (data not shown). Insufficient data were available for analysis with B. valaisiana and B. lusitaniae.
(iii) Mixed infections.
The data for the 13% mixed infections are shown in Table 3, which indicates the summarized frequencies for the combinations reported. The occurrence of mixed infections in nymphs (12.1%) was not statistically different from that in adults (13.6%). The distribution of combinations of mixed infections is shown in Fig. 8. If the analysis was restricted to studies which distinguished between B. afzelii, B. garinii, B. burgdorferi sensu stricto, and B. valaisiana (21 records), the combination of B. garinii and B. valaisiana occurred 51% more often than all other species combinations.
A mixed infection with B. lusitaniae was described only once in combination with B. garinii (9). Combinations of three species occurred only rarely.
A direct comparison of species combinations for nymphs and adults revealed no significant differences (data not shown), although only a few records were available (10 records for nymphs and 14 records for adults).
(iv) Borrelia genospecies distribution in different parts of Europe.
To compare the distributions of Borrelia species in various parts of Europe, we merged the data from different countries. For this purpose, the following inclusion criteria were taken into account. (i) The countries had to be adjacent (except group 3). (ii) The ratios of genospecies in the records were similar. (iii) There had to be at least four records for each group. Based on these criteria, seven groups were defined (Table 4). Because of the low number of records, studies in which less than 10 ticks were examined were excluded to avoid distortion. Since only a few data are available for B. lusitaniae, this organism was also excluded from this analysis. The results clearly revealed diverse patterns of species distribution in the different areas of Europe. In groups 1, 2, and 3 significantly more ticks were infected with B. afzelii than with B. garinii, while in groups 4 and 5 B. garinii seemed to predominate. In group 4, ticks seemed to be equally frequently infected with B. valaisiana and B. garinii. The data for groups 6 and 7 revealed that there was no significant difference between B. afzelii and B. garinii.
TABLE 4.
Distribution of Borrelia genospecies in different parts of Europea
| Group | Countries | % of positive ticks (mean ± SEM)
|
No. of records for B. afzelii, B. garinii, B. burgdorferi sensu stricto, and nontypeable Borrelia | No. of records for B. valaisiana | ||||
|---|---|---|---|---|---|---|---|---|
| B. afzelii | B. garinii | B. burgdorferi sensu stricto | B. valaisiana | Nontypeable Borrelia | ||||
| 1 | Southern Germany, Czech Republic, Slovakia | 55 ± 4 | 25 ± 6b | 3 ± 2b | 27 ± 7c | 2 ± 1 | 8 | 6 |
| 2 | Norway, Sweden, Finland, Estonia | 68 ± 9 | 18 ± 5b | 16 ± 8b | 2 ± 2 | 2 ± 1 | 5 | 3 |
| 3 | Bulgaria, Croatia, Slovenia | 60 ± 5 | 16 ± 5b | 14 ± 6b | 16 ± 5b | 8 ± 3 | 5 | 4 |
| 4 | United Kingdom, Ireland | 7 ± 4c | 43 ± 8 | 13 ± 5c | 41 ± 5 | 9 ± 3 | 4 | 4 |
| 5 | Central and northern Germany | 26 ± 7 | 52 ± 7 | 17 ± 8c | 8 ± 3c | 3 ± 2 | 5 | 4 |
| 6 | Austria, Switzerland | 25 ± 8 | 44 ± 7 | 25 ± 9 | 7 ± 3 | 3 ± 2 | 6 | 4 |
| 7 | The Netherlands, Belgium, northern France | 46 ± 15 | 34 ± 9 | 19 ± 9 | 33 | 11 ± 5 | 6 | 1 |
Seven different groups were defined as described in the text. The P values are the P values for the species with the highest mean percentage (for groups 1 to 3, B.afzelii, for groups 4 and 5, B. garinii).
P < 0.001.
P < 0.01.
As mentioned above, some studies distinguished only between the genospecies B. afzelii, B. garinii, and B. burgdorferi sensu stricto. Since B. valaisiana- and untypeable Borrelia-infected ticks are also included in Table 4, this may have resulted in a disproportion in the ratio of the first three species. Due to the low number of records it was not possible to compare only studies in which all four species were examined. Therefore, we repeated the analysis and excluded untypeable Borrelia- and B. valaisiana-positive ticks, which allowed direct comparison of B. afzelii, B. garinii, and B. burgdorferi sensu stricto. There was no significant difference in the results compared to the results shown in Table 4 (data not shown).
DISCUSSION
A metaanalysis provides a versatile alternative to the more traditional review methods and allows quantitative conclusions to be drawn. The prevalence of Borrelia infection in ticks is one of the most essential components of risk assessment for LB. In recent years, many studies of the rate of infection of ticks with Borrelia have been reported. Analysis of data from 155 records of studies conducted in Europe showed that the overall mean infection rate was 13.6% and that the rate of infection of adult ticks was significantly higher than that of nymphs, which was observed in the majority of the studies and is explained by the fact that host-seeking adult ticks had had two blood meals on different hosts. These data are in line with the data reviewed by other workers (35, 46). Recently, a B. miyamotoi-like Borrelia species was detected in I. ricinus ticks in Europe (28, 99). Therefore, the rate of infection of ticks with spirochetes belonging to the B. burgdorferi sensu lato complex may be overestimated by some detection methods.
The correlation of rates of infection of adult ticks with the coordinates of sampling sites showed a significant trend, with the rates increasing from western Europe to eastern Europe. No effect was seen for nymphal ticks. The effect of longitude on the rates of B. burgdorferi sensu lato infection of unfed I. ricinus ticks was also examined by Gray et al. in 1998. These authors reported that the rates of infection for both nymphal and adult I. ricinus ticks were significantly higher in eastern Europe than in the west (35). In contrast to our analyses, they restricted the area to the coordinates from 5°W to 20°E. Indeed, a restriction of our data to this area also led to a significant increase in the rates of infection of nymphs (7 to 15%, calculated from regression; P < 0.05; r2 = 0.037) from western Europe to eastern Europe. The map in Fig. 4 shows that the regions with the highest infection rates are located in central Europe (Austria, Czech Republic, southern Germany, Switzerland, Slovakia, and Slovenia). The high infection rates in central Europe combined with the large number of studies carried out in these regions probably led to the overall trend from western Europe to eastern regions of Europe.
Including only studies in which at least 100 adults and/or nymphs were examined led to different results in only four of the regions, indicating that most of the values are robust. Since in these four regions only a few records were available, they are vulnerable to outliers. In region A, for example, the decrease in the infection rate is based on the loss of a single record with an infection rate of 75%. Therefore, more studies with a higher number of ticks examined are required in such regions to obtain more robust results.
The available data did not allow analysis of the tick infection rate by years. Therefore, we compared two time periods (1986 to 1993 versus 1994 to 2001). In the first of the two periods, ticks were examined by a immunofluorescence assay, dark-field microscopy, or culture. From 1994 on, PCR, which is generally considered to be the most sensitive method, was the most commonly used detection method. Nevertheless, this did not lead to any differences in reported infection rates between the two periods. It was noteworthy that the highest infection rates reported for nymphs (>25%) and adults (>30%) were found only in the period from 1997 to 2001 and were almost all detected with PCR. Not enough data were available to break down the data by year and method, but exclusion of PCR from the analysis made no difference (data not shown). Taken together, there is no indication that there was an increase in the tick infection rates over time or due to introduction of the more sensitive PCR method. For future analyses, and if a sufficient sampling population is available, it would be interesting to study the effect that a significant seasonal event can have on the rate of infection of I. ricinus.
The fact that the isolated variables are interconnected should be taken into account. A solution to this problem would be a multiple linear or logistic regression, but the heterogeneity of data reporting precluded such an approach.
In Europe there are at least three species that are known to be pathogenic for humans, B. afzelii, B. garinii, and B. burgdorferi sensu stricto, the last of which is the only species found in the United States (2). As the different Borrelia species appear to be associated with different clinical manifestations, accurate knowledge of the distribution of these species in Europe might be helpful. Analyses of data from 53 records revealed a distinct pattern of Borrelia species distribution in Europe: B. afzelii and B. garinii are the most common species, followed by B. valaisiana and B. burgdorferi sensu stricto. B. lusitaniae was tested in only a few studies but seems to be rather rare or nonexistent in most regions, since it was found in only 8 of the 20 records, which showed that B. lusitaniae was present at mostly a low frequency. The occurrence of B. bissettii was described once in combination with B. afzelii and B. garinii (43) in Slovakia. The prevalence of Borrelia genospecies in I. ricinus ticks seems to vary in different parts of Europe. This could be explained by the prevalence of different reservoir hosts, although considerably more data are necessary before robust patterns can be determined and a correlation between ecological data and clinical treatment manifestations can be carried out. Not enough data were available to break down the species differences by method of detection. Therefore, the possibility that the differences in species distribution by region may be determined in part by the different accuracies of the methods used by the different investigators in each region could not be ruled out.
Various studies showed that there are specific associations between Borrelia species and reservoir hosts. B. afzelii is preferentially transmitted by small rodents, and B. valaisiana and B. garinii are mainly associated with birds, although the latter genospecies is very heterogeneous and some strains preferentially infect ticks via rodents. B. burgdorferi sensu stricto seems to occur in both birds and rodents (42, 43, 55, 70, 72, 91). This host association can be explained by the specific effect of the host's complement system on each B. burgdorferi sensu lato genospecies (73). Direct comparison of questing nymphs and adults showed no significant difference in the Borrelia species distribution, suggesting that the infected hosts for larvae and nymphs are similar.
Thirteen percent of Borrelia-positive I. ricinus ticks showed infection with multiple genospecies. Such mixed infections in individual ticks can be explained by (i) superinfection of ticks that are already infected transovarially, (ii) cotransmission of multiple Borrelia species from an infected tick to an uninfected tick feeding on the same host, (iii) cotransmission of several strains from a host infected by more than one Borrelia species, or (iv) consecutive infectious blood meals. In contrast to a questing nymph, which has had only one previous blood meal as a larva, an adult has fed on two different hosts. Therefore, it appeared likely that adults would exhibit more mixed infections than nymphs, although this was not the case. A possible explanation for this could be that the effect of complement may occur not only in the host, which selects for Borrelia species, but also in the midgut of the tick by complement taken up during the blood meal, as discussed by Kurtenbach et al. (69, 73). In fact, the most frequent combination of Borrelia species found in Europe was B. garinii and B. valaisiana, both of which occur most commonly in birds.
In conclusion, the prevalence of different B. burgdorferi sensu lato genospecies was deduced for various regions in Europe. While the infection rate was about twofold higher in adult ticks than in nymphs, no effect of detection method, tick gender, or collection period (1986 to 1993 versus 1994 to 2002) was found.
Acknowledgments
We thank Isabel Diterich and Markus Müller for helpful discussions, Sebastian Hoffmann for statistical help, and Sonja von Aulock for revision of the manuscript.
REFERENCES
- 1.Balmelli, T., and J. C. Piffaretti. 1995. Association between different clinical manifestations of Lyme disease and different species of Borrelia burgdorferi sensu lato. Res. Microbiol. 146:329-340. [DOI] [PubMed] [Google Scholar]
- 2.Baranton, G., D. Postic, I. Saint Girons, P. Boerlin, J. C. Piffaretti, M. Assous, and P. A. Grimont. 1992. Delineation of Borrelia burgdorferi sensu stricto, Borrelia garinii sp. nov., and group VS461 associated with Lyme borreliosis. Int J. Syst. Bacteriol. 42:378-383. [DOI] [PubMed] [Google Scholar]
- 3.Barral, M., A. L. Garcia-Perez, R. A. Juste, A. Hurtado, R. Escudero, R. E. Sellek, and P. Anda. 2002. Distribution of Borrelia burgdorferi sensu lato in Ixodes ricinus (Acari: Ixodidae) ticks from the Basque country, Spain. J. Med. Entomol. 39:177-184. [DOI] [PubMed] [Google Scholar]
- 4.Basta, J., J. Plch, D. Hulinska, and M. Daniel. 1999. Incidence of Borrelia garinii and Borrelia afzelii in Ixodes ricinus ticks in an urban environment, Prague, Czech Republic, between 1995 and 1998. Eur. J. Clin. Microbiol. Infect. Dis. 18:515-517. [DOI] [PubMed] [Google Scholar]
- 5.Berglund, J., and R. Eitrem. 1993. Tick-borne borreliosis in the archipelago of southern Sweden. Scand. J. Infect. Dis. 25:67-72. [PubMed] [Google Scholar]
- 6.Bergstrom, S., B. Olsen, N. Burman, L. Gothefors, T. G. Jaenson, M. Jonsson, and H. A. Mejlon. 1992. Molecular characterization of Borrelia burgdorferi isolated from Ixodes ricinus in northern Sweden. Scand. J. Infect. Dis. 24:181-188. [DOI] [PubMed] [Google Scholar]
- 7.Bukowska, K., D. Kosik-Bogacka, and W. Kuzna-Grygiel. 2003. The occurrence of Borrelia burgdorferi sensu lato in the populations of Ixodes ricinus in forest areas of Szczecin during 2000-2001. Ann. Agric. Environ. Med. 10:5-8. [PubMed] [Google Scholar]
- 8.Christova, I., S. Hohenberger, C. Zehetmeier, and B. Wilske. 1998. First characterization of Borrelia burgdorferi sensu lato from ticks and skin biopsy in Bulgaria. Med. Microbiol. Immunol. (Berl.) 186:171-175. [DOI] [PubMed] [Google Scholar]
- 9.Christova, I., L. Schouls, I. van De Pol, J. Park, S. Panayotov, V. Lefterova, T. Kantardjiev, and J. S. Dumler. 2001. High prevalence of granulocytic ehrlichiae and Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from Bulgaria. J. Clin. Microbiol. 39:4172-4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christova, I., J. Van De Pol, S. Yazar, E. Velo, and L. Schouls. 2003. Identification of Borrelia burgdorferi sensu lato, Anaplasma and Ehrlichia species, and spotted fever group rickettsiae in ticks from southeastern Europe. Eur. J. Clin. Microbiol. Infect. Dis. 22:535-542. [DOI] [PubMed] [Google Scholar]
- 11.Cinco, M., D. Padovan, R. Murgia, L. Frusteri, M. Maroli, I. van de Pol, N. Verbeek-De Kruif, S. Rijpkema, and F. Taggi. 1998. Prevalence of Borrelia burgdorferi infection in Ixodes ricinus in central Italy. Eur. J. Clin. Microbiol. Infect. Dis. 17:134-135. [DOI] [PubMed] [Google Scholar]
- 12.Cinco, M., D. Padovan, R. Murgia, M. Maroli, L. Frusteri, M. Heldtander, K. E. Johansson, and E. O. Engvall. 1997. Coexistence of Ehrlichia phagocytophila and Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from Italy as determined by 16S rRNA gene sequencing. J. Clin. Microbiol. 35:3365-3366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cinco, M., D. Padovan, R. Murgia, L. Poldini, L. Frusteri, I. van de Pol, N. Verbeek-De Kruif, S. Rijpkema, and M. Maroli. 1998. Rate of infection of Ixodes ricinus ticks with Borrelia burgdorferi sensu stricto, Borrelia garinii, Borrelia afzelii and group VS116 in an endemic focus of Lyme disease in Italy. Eur. J. Clin. Microbiol. Infect. Dis. 17:90-94. [DOI] [PubMed] [Google Scholar]
- 14.Cisak, E., J. Chmielewska-Badora, B. Rajtar, J. Zwolinski, L. Jablonski, and J. Dutkiewicz. 2002. Study on the occurrence of Borrelia burgdorferi sensu lato and tick-borne encephalitis virus (TBEV) in ticks collected in Lublin region (eastern Poland). Ann. Agric. Environ. Med. 9:105-110. [PubMed] [Google Scholar]
- 15.Collares-Pereira, M., S. Couceiro, I. Franca, K. Kurtenbach, S. M. Schafer, L. Vitorino, L. Goncalves, S. Baptista, M. L. Vieira, and C. Cunha. 2004. First isolation of Borrelia lusitaniae from a human patient. J. Clin. Microbiol. 42:1316-1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davidson, M. M., R. Evans, C. L. Ling, A. D. Wiseman, A. W. Joss, and D. O. Ho-Yen. 1999. Isolation of Borrelia burgdorferi from ticks in the Highlands of Scotland. J. Med. Microbiol. 48:59-65. [DOI] [PubMed] [Google Scholar]
- 17.De Boer, R., K. E. Hovius, M. K. Nohlmans, and J. S. Gray. 1993. The woodmouse (Apodemus sylvaticus) as a reservoir of tick-transmitted spirochetes (Borrelia burgdorferi) in The Netherlands. Zentralbl. Bakteriol. 279:404-416. [DOI] [PubMed] [Google Scholar]
- 18.Demaerschalck, I., A. BenMessaoud, M. De Kesel, B. Hoyois, Y. Lobet, P. Hoet, G. Bigaignon, A. Bollen, and E. Godfroid. 1995. Simultaneous presence of different Borrelia burgdorferi genospecies in biological fluids of Lyme disease patients. J. Clin. Microbiol. 33:602-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Michelis, S., H. S. Sewell, M. Collares-Pereira, M. Santos-Reis, L. M. Schouls, V. Benes, E. C. Holmes, and K. Kurtenbach. 2000. Genetic diversity of Borrelia burgdorferi sensu lato in ticks from mainland Portugal. J. Clin. Microbiol. 38:2128-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Derdakova, M., L. Beati, B. Pet'ko, M. Stanko, and D. Fish. 2003. Genetic variability within Borrelia burgdorferi sensu lato genospecies established by PCR-single-strand conformation polymorphism analysis of the rrfA-rrlB intergenic spacer in Ixodes ricinus ticks from the Czech Republic. Appl. Environ Microbiol. 69:509-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derdakova, M., M. Halanova, M. Stanko, A. Stefancikova, L. Cislakova, and B. Pet'ko. 2003. Molecular evidence for Anaplasma phagocytophilum and Borrelia burgdorferi sensu lato in Ixodes ricinus ticks from eastern Slovakia. Ann. Agric. Environ. Med. 10:269-271. [PubMed] [Google Scholar]
- 22.Drgonova, M., and J. Rehacek. 1995. Prevalence of Lyme borrelia in ticks in Bratislava, Slovak Republic. Cent. Eur. J. Public Health 3:134-137. [PubMed] [Google Scholar]
- 23.Etti, S., R. Hails, S. M. Schafer, S. De Michelis, H. S. Sewell, A. Bormane, M. Donaghy, and K. Kurtenbach. 2003. Habitat-specific diversity of Borrelia burgdorferi sensu lato in Europe, exemplified by data from Latvia. Appl. Environ Microbiol. 69:3008-3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Favia, G., G. Cancrini, A. Carfi, D. Grazioli, E. Lillini, and A. Iori. 2001. Molecular identification of Borrelia valaisiana and HGE-like Ehrlichia in Ixodes ricinus ticks sampled in north-eastern Italy: first report in Veneto region. Parassitologia (Rome) 43:143-146. [PubMed] [Google Scholar]
- 25.Fingerle, V. 1994. Prevalence of Borrelia burgdorferi sensu lato in Ixodes ricinus in southern Germany. J. Spirochetal Tick-Borne Dis. 1:41-45. [Google Scholar]
- 26.Fingerle, V., U. Hauser, G. Liegl, B. Petko, V. Preac-Mursic, and B. Wilske. 1995. Expression of outer surface proteins A and C of Borrelia burgdorferi in Ixodes ricinus. J. Clin. Microbiol. 33:1867-1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fingerle, V., U. G. Munderloh, G. Liegl, and B. Wilske. 1999. Coexistence of ehrlichiae of the phagocytophila group with Borrelia burgdorferi in Ixodes ricinus from southern Germany. Med. Microbiol. Immunol. (Berl.) 188:145-149. [DOI] [PubMed] [Google Scholar]
- 28.Fraenkel, C. J., U. Garpmo, and J. Berglund. 2002. Determination of novel Borrelia genospecies in Swedish Ixodes ricinus ticks. J. Clin. Microbiol. 40:3308-3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gern, L., C. M. Hu, E. Kocianova, V. Vyrostekova, and J. Rehacek. 1999. Genetic diversity of Borrelia burgdorferi sensu lato isolates obtained from Ixodes ricinus ticks collected in Slovakia. Eur. J. Epidemiol. 15:665-669. [DOI] [PubMed] [Google Scholar]
- 30.Gern, L., N. Lebet, and J. Moret. 1996. Dynamics of Borrelia burgdorferi infection in nymphal Ixodes ricinus ticks during feeding. Exp. Appl. Acarol. 20:649-658. [DOI] [PubMed] [Google Scholar]
- 31.Gilot, B., B. Degeilh, J. Pichot, B. Doche, and C. Guiguen. 1996. Prevalence of Borrelia burgdorferi (sensu lato) in Ixodes ricinus (L.) populations in France, according to a phytoecological zoning of the territory. Eur. J. Epidemiol. 12:395-401. [DOI] [PubMed] [Google Scholar]
- 32.Gray, J. 1999. Risk assessment in Lyme borreliosis. Wien Klin. Wochenschr. 111:990-993. [PubMed] [Google Scholar]
- 33.Gray, J. S., O. Kahl, C. Janetzki, and J. Stein. 1992. Studies on the ecology of Lyme disease in a deer forest in County Galway, Ireland. J. Med. Entomol. 29:915-920. [DOI] [PubMed] [Google Scholar]
- 34.Gray, J. S., O. Kahl, C. Janetzki, J. Stein, and E. Guy. 1995. The spatial distribution of Borrelia burgdorferi-infected Ixodes ricinus in the Connemara region of County Galway, Ireland. Exp. Appl. Acarol. 19:163-172. [DOI] [PubMed] [Google Scholar]
- 35.Gray, J. S., O. Kahl, J. N. Robertson, M. Daniel, A. Estrada-Pena, G. Gettinby, T. G. Jaenson, P. Jensen, F. Jongejan, E. Korenberg, K. Kurtenbach, and P. Zeman. 1998. Lyme borreliosis habitat assessment. Zentralbl. Bakteriol. 287:211-228. [DOI] [PubMed] [Google Scholar]
- 36.Gray, J. S., J. N. Robertson, and S. Key. 2000. Limited role of rodents as reservoirs of Borrelia burgdorferi sensu lato in Ireland. Eur. J. Epidemiol. 16:101-103. [DOI] [PubMed] [Google Scholar]
- 37.Gray, J. S., A. Schonberg, D. Postic, J. Belfaiza, and I. Saint-Girons. 1996. First isolation and characterisation of Borrelia garinii, agent of Lyme borreliosis, from Irish ticks. Ir. J. Med. Sci. 165:24-26. [DOI] [PubMed] [Google Scholar]
- 38.Gupta, S. K., A. Schonberg, and T. Hiepe. 1995. Prevalence of ticks in relation to their role as vector of Borrelia burgdorferi under autochthone conditions. Appl. Parasitol. 36:97-106. [PubMed] [Google Scholar]
- 39.Gustafson, R., A. Gardulf, and B. Svenungsson. 1989. Comparison of culture, indirect immunofluorescence and dark-field microscopy for detection of spirochetes from Ixodes ricinus ticks. Eur. J. Clin. Microbiol. Infect. Dis. 8:570-572. [DOI] [PubMed] [Google Scholar]
- 40.Gustafson, R., T. G. Jaenson, A. Gardulf, H. Mejlon, and B. Svenungsson. 1995. Prevalence of Borrelia burgdorferi sensu lato infection in Ixodes ricinus in Sweden. Scand. J. Infect. Dis. 27:597-601. [DOI] [PubMed] [Google Scholar]
- 41.Guy, E. C., and R. G. Farquhar. 1991. Borrelia burgdorferi in urban parks. Lancet 338:253. [DOI] [PubMed] [Google Scholar]
- 42.Hanincova, K., S. M. Schafer, S. Etti, H. S. Sewell, V. Taragelova, D. Ziak, M. Labuda, and K. Kurtenbach. 2003. Association of Borrelia afzelii with rodents in Europe. Parasitology 126:11-20. [DOI] [PubMed] [Google Scholar]
- 43.Hanincova, K., V. Taragelova, J. Koci, S. M. Schafer, R. Hails, A. J. Ullmann, J. Piesman, M. Labuda, and K. Kurtenbach. 2003. Association of Borrelia garinii and B. valaisiana with songbirds in Slovakia. Appl. Environ. Microbiol. 69:2825-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hildebrandt, A., K. H. Schmidt, B. Wilske, W. Dorn, E. Straube, and V. Fingerle. 2003. Prevalence of four species of Borrelia burgdorferi sensu lato and coinfection with Anaplasma phagocytophila in Ixodes ricinus ticks in central Germany. Eur. J. Clin. Microbiol. Infect. Dis. 22:364-367. [DOI] [PubMed] [Google Scholar]
- 45.Hubalek, Z., and J. Halouzka. 1997. Distribution of Borrelia burgdorferi sensu lato genomic groups in Europe, a review. Eur. J. Epidemiol. 13: 951-957. [DOI] [PubMed] [Google Scholar]
- 46.Hubalek, Z., and J. Halouzka. 1998. Prevalence rates of Borrelia burgdorferi sensu lato in host-seeking Ixodes ricinus ticks in Europe. Parasitol. Res. 84:167-172. [DOI] [PubMed] [Google Scholar]
- 47.Hubalek, Z., J. Halouzka, and Z. Juricova. 1991. A comparison of the occurrence of borreliae in nymphal and adult Ixodes ricinus ticks. Zentralbl. Bakteriol. 275:133-137. [DOI] [PubMed] [Google Scholar]
- 48.Hubalek, Z., J. Halouzka, and Z. Juricova. 1998. Investigation of haematophagous arthropods for borreliae—summarized data, 1988-1996. Folia Parasitol. (Prague) 45:67-72. [PubMed] [Google Scholar]
- 49.Hubalek, Z., J. Halouzka, and Z. Juricova. 2003. Longitudinal surveillance of the tick Ixodes ricinus for borreliae. Med. Vet. Entomol. 17:46-51. [DOI] [PubMed] [Google Scholar]
- 50.Hubalek, Z., J. Halouzka, and Z. Juricova. 1993. Prevalence of borreliae in Ixodes ricinus ticks from urban parks. Folia Parasitol. (Prague) 40:236. [PubMed] [Google Scholar]
- 51.Hubalek, Z., J. Halouzka, and Z. Juricova. 1996. A simple method of transmission risk assessment in enzootic foci of Lyme borreliosis. Eur. J. Epidemiol. 12:331-333. [DOI] [PubMed] [Google Scholar]
- 52.Hubalek, Z., E. I. Korenberg, Z. Juricova, V. Kovalevski Yu, J. Halouzka, and S. V. Shcherbakov. 1990. Prevalence of borreliae in Ixodes ricinus ticks from southern Moravia, Czechoslovakia. Folia Parasitol. (Prague) 37: 359-362. [PubMed] [Google Scholar]
- 53.Hubalek, Z., D. Stunzner, J. Halouzka, W. Sixl, I. Wendelin, Z. Juricova, and Y. O. Sanogo. 2003. Prevalence of borreliae in ixodid ticks from a floodplain forest ecosystem. Wien Klin. Wochenschr. 115:121-124. [DOI] [PubMed] [Google Scholar]
- 54.Humair, P. F., O. Peter, R. Wallich, and L. Gern. 1995. Strain variation of Lyme disease spirochetes isolated from Ixodes ricinus ticks and rodents collected in two endemic areas in Switzerland. J. Med. Entomol. 32: 433-438. [DOI] [PubMed] [Google Scholar]
- 55.Humair, P. F., O. Rais, and L. Gern. 1999. Transmission of Borrelia afzelii from Apodemus mice and Clethrionomys voles to Ixodes ricinus ticks: differential transmission pattern and overwintering maintenance. Parasitology 118:33-42. [DOI] [PubMed] [Google Scholar]
- 56.Jaenson, T. G., and L. Talleklint. 1992. Incompetence of roe deer as reservoirs of the Lyme borreliosis spirochete. J. Med. Entomol. 29:813-817. [DOI] [PubMed] [Google Scholar]
- 57.Jaenson, T. G., and L. Talleklint. 1996. Lyme borreliosis spirochetes in Ixodes ricinus (Acari: Ixodidae) and the varying hare on isolated islands in the Baltic Sea. J. Med. Entomol. 33:339-343. [DOI] [PubMed] [Google Scholar]
- 58.Jenkins, A., B. E. Kristiansen, A. G. Allum, R. K. Aakre, L. Strand, E. J. Kleveland, I. van de Pol, and L. Schouls. 2001. Borrelia burgdorferi sensu lato and Ehrlichia spp. in Ixodes ticks from southern Norway. J. Clin. Microbiol. 39:3666-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jensen, P. M., and F. Frandsen. 2000. Temporal risk assessment for Lyme borreliosis in Denmark. Scand. J. Infect. Dis. 32:539-544. [DOI] [PubMed] [Google Scholar]
- 60.Jensen, P. M., H. Hansen, and F. Frandsen. 2000. Spatial risk assessment for Lyme borreliosis in Denmark. Scand. J. Infect. Dis. 32:545-550. [DOI] [PubMed] [Google Scholar]
- 61.Jouda, F., M. Crippa, J. L. Perret, and L. Gern. 2003. Distribution and prevalence of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks of canton Ticino (Switzerland). Eur. J. Epidemiol. 18:907-912. [DOI] [PubMed] [Google Scholar]
- 62.Junttila, J., M. Peltomaa, H. Soini, M. Marjamaki, and M. K. Viljanen. 1999. Prevalence of Borrelia burgdorferi in Ixodes ricinus ticks in urban recreational areas of Helsinki. J. Clin. Microbiol. 37:1361-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Junttila, J., R. Tanskanen, and J. Tuomi. 1994. Prevalence of Borrelia burgdorferi in selected tick populations in Finland. Scand. J. Infect. Dis. 26:349-355. [DOI] [PubMed] [Google Scholar]
- 64.Kahl, O., C. Janetzki, J. S. Gray, J. Stein, and R. J. Bauch. 1992. Tick infection rates with Borrelia: Ixodes ricinus versus Haemaphysalis concinna and Dermacentor reticulatus in two locations in eastern Germany. Med. Vet. Entomol. 6:363-366. [DOI] [PubMed] [Google Scholar]
- 65.Kahl, O., K. Schmidt, A. Schonberg, U. Laukamm-Josten, W. Knulle, and U. Bienzle. 1989. Prevalence of Borrelia burgdorferi in Ixodes ricinus ticks in Berlin (West). Zentbl. Bakteriol. Mikrobiol. Hyg. Ser. A 270:434-440. [DOI] [PubMed] [Google Scholar]
- 66.Kirstein, F., S. Rijpkema, M. Molkenboer, and J. S. Gray. 1997. The distribution and prevalence of B. burgdorferi genomospecies in Ixodes ricinus ticks in Ireland. Eur. J. Epidemiol. 13:67-72. [DOI] [PubMed] [Google Scholar]
- 67.Kirstein, F., S. Rijpkema, M. Molkenboer, and J. S. Gray. 1997. Local variations in the distribution and prevalence of Borrelia burgdorferi sensu lato genomospecies in Ixodes ricinus ticks. Appl. Environ. Microbiol. 63:1102-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kmety, E., J. Rehacek, and V. Vyrostekova. 1987. Investigations of ticks for the presence of Borrelia in Czechoslovakia. Zentbl. Bakteriol. Mikrobiol. Hyg. Ser. A 263:468-470. [DOI] [PubMed] [Google Scholar]
- 69.Kurtenbach, K., S. De Michelis, H. S. Sewell, S. Etti, S. M. Schafer, R. Hails, M. Collares-Pereira, M. Santos-Reis, K. Hanincova, M. Labuda, A. Bormane, and M. Donaghy. 2001. Distinct combinations of Borrelia burgdorferi sensu lato genospecies found in individual questing ticks from Europe. Appl. Environ. Microbiol. 67:4926-4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kurtenbach, K., S. De Michelis, H. S. Sewell, S. Etti, S. M. Schafer, E. Holmes, R. Hails, M. Collares-Pereira, M. Santos-Reis, K. Hanincova, M. Labuda, A. Bormane, and M. Donaghy. 2002. The key roles of selection and migration in the ecology of Lyme borreliosis. Int. J. Med. Microbiol. 291(Suppl. 33):152-154. [DOI] [PubMed] [Google Scholar]
- 71.Kurtenbach, K., H. Kampen, A. Dizij, S. Arndt, H. M. Seitz, U. E. Schaible, and M. M. Simon. 1995. Infestation of rodents with larval Ixodes ricinus (Acari: Ixodidae) is an important factor in the transmission cycle of Borrelia burgdorferi s.l. in German woodlands. J. Med. Entomol. 32:807-817. [DOI] [PubMed] [Google Scholar]
- 72.Kurtenbach, K., M. Peacey, S. G. Rijpkema, A. N. Hoodless, P. A. Nuttall, and S. E. Randolph. 1998. Differential transmission of the genospecies of Borrelia burgdorferi sensu lato by game birds and small rodents in England. Appl. Environ. Microbiol. 64:1169-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kurtenbach, K., H. S. Sewell, N. H. Ogden, S. E. Randolph, and P. A. Nuttall. 1998. Serum complement sensitivity as a key factor in Lyme disease ecology. Infect. Immun. 66:1248-1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Landbo, A. S., and P. T. Flong. 1992. Borrelia burgdorferi infection in Ixodes ricinus from habitats in Denmark. Med. Vet. Entomol. 6:165-167. [DOI] [PubMed] [Google Scholar]
- 75.Leuba-Garcia, S., M. D. Kramer, R. Wallich, and L. Gern. 1994. Characterization of Borrelia burgdorferi isolated from different organs of Ixodes ricinus ticks collected in nature. Zentbl. Bakteriol. 280:468-475. [DOI] [PubMed] [Google Scholar]
- 76.Leutenegger, C. M., N. Pusterla, C. N. Mislin, R. Weber, and H. Lutz. 1999. Molecular evidence of coinfection of ticks with Borrelia burgdorferi sensu lato and the human granulocytic ehrlichiosis agent in Switzerland. J. Clin. Microbiol. 37:3390-3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liebisch, G., B. Sohns, and W. Bautsch. 1998. Detection and typing of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks attached to human skin by PCR. J. Clin. Microbiol. 36:3355-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lottmann, H., B. Wilske, and H. Herrmann. 1996. Characterization of Borrelia burgdorferi sensu lato strains isolated from Ixodes ricinus in Mecklenburg-Vorpommern, Germany. Med. Microbiol. Immunol. (Berl.) 184:181-184. [PubMed] [Google Scholar]
- 79.Makinen, J., I. Vuorinen, J. Oksi, M. Peltomaa, Q. He, M. Marjamaki, and M. K. Viljanen. 2003. Prevalence of granulocytic Ehrlichia and Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected from southwestern Finland and from Vormsi Island in Estonia. APMIS 111:355-362. [DOI] [PubMed] [Google Scholar]
- 80.Mannelli, A., D. Cerri, L. Buffrini, S. Rossi, S. Rosati, T. Arata, M. Innocenti, M. C. Grignolo, G. Bianchi, A. Iori, and F. Tolari. 1999. Low risk of Lyme borreliosis in a protected area on the Tyrrhenian coast, in central Italy. Eur. J. Epidemiol. 15:371-377. [DOI] [PubMed] [Google Scholar]
- 81.Matuschka, F. R., M. Heiler, H. Eiffert, P. Fischer, H. Lotter, and A. Spielman. 1993. Diversionary role of hoofed game in the transmission of Lyme disease spirochetes. Am. J. Trop. Med. Hyg. 48:693-699. [DOI] [PubMed] [Google Scholar]
- 82.Mejlon, H. A., and T. G. Jaenson. 1993. Seasonal prevalence of Borrelia burgdorferi in Ixodes ricinus in different vegetation types in Sweden. Scand. J. Infect. Dis. 25:449-456. [DOI] [PubMed] [Google Scholar]
- 83.Michalik, J., T. Hofman, A. Buczek, M. Skoracki, and B. Sikora. 2003. Borrelia burgdorferi s.l. in Ixodes ricinus (Acari: Ixodidae) ticks collected from vegetation and small rodents in recreational areas of the city of Poznan. J. Med. Entomol. 40:690-697. [DOI] [PubMed] [Google Scholar]
- 84.Miserez, V., L. Gern, and A. Aeschlimann. 1990. Borrelia burgdorferi in ticks of the Canton Tessin (Switzerland). Parassitologia (Rome) 32:293-299. [PubMed] [Google Scholar]
- 85.Misonne, M. C., G. Van Impe, and P. P. Hoet. 1998. Genetic heterogeneity of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in Belgium. J. Clin. Microbiol. 36:3352-3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Motiejunas, L., J. Bunikis, A. G. Barbour, and A. Sadziene. 1994. Lyme borreliosis in Lithuania. Scand. J. Infect. Dis. 26:149-155. [DOI] [PubMed] [Google Scholar]
- 87.Nohlmans, L. M., R. de Boer, A. E. van den Bogaard, and C. P. van Boven. 1995. Genotypic and phenotypic analysis of Borrelia burgdorferi isolates from The Netherlands. J. Clin. Microbiol. 33:119-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oehme, R., K. Hartelt, H. Backe, S. Brockmann, and P. Kimmig. 2002. Foci of tick-borne diseases in southwest Germany. Int. J. Med. Microbiol. 291(Suppl. 33):22-29. [DOI] [PubMed] [Google Scholar]
- 89.Ogden, N. H., P. A. Nuttall, and S. E. Randolph. 1997. Natural Lyme disease cycles maintained via sheep by co-feeding ticks. Parasitology 115:591-599. [DOI] [PubMed] [Google Scholar]
- 90.Ohlenbusch, A., F. R. Matuschka, D. Richter, H. J. Christen, R. Thomssen, A. Spielman, and H. Eiffert. 1996. Etiology of the acrodermatitis chronica atrophicans lesion in Lyme disease. J. Infect. Dis. 174:421-423. [DOI] [PubMed] [Google Scholar]
- 91.Olsen, B., T. G. Jaenson, and S. Bergstrom. 1995. Prevalence of Borrelia burgdorferi sensu lato-infected ticks on migrating birds. Appl. Environ. Microbiol. 61:3082-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Peter, O., A. G. Bretz, and D. Bee. 1995. Occurrence of different genospecies of Borrelia burgdorferi sensu lato in ixodid ticks of Valais, Switzerland. Eur. J. Epidemiol. 11:463-467. [DOI] [PubMed] [Google Scholar]
- 93.Pet'ko, B. 1997. Borrelia burgdorferi sensu lato in the Ixodes ricinus ticks in southern Poland. Ann. Agric. Environ. Med. 4:263-269. [PubMed] [Google Scholar]
- 94.Pichon, B., E. Godfroid, B. Hoyois, A. Bollen, F. Rodhain, and C. Perez-Eid. 1995. Simultaneous infection of Ixodes ricinus nymphs by two Borrelia burgdorferi sensu lato species: possible implications for clinical manifestations. Emerg. Infect. Dis. 1:89-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Pichon, B., L. Mousson, C. Figureau, F. Rodhain, and C. Perez-Eid. 1999. Density of deer in relation to the prevalence of Borrelia burgdorferi s.l. in Ixodes ricinus nymphs in Rambouillet forest, France. Exp. Appl. Acarol. 23:267-275. [DOI] [PubMed] [Google Scholar]
- 96.Quessada, T., F. Martial-Convert, S. Arnaud, H. Leudet De La Vallee, B. Gilot, and J. Pichot. 2003. Prevalence of Borrelia burgdorferi species and identification of Borrelia valaisiana in questing Ixodes ricinus in the Lyon region of France as determined by polymerase chain reaction-restriction fragment length polymorphism. Eur. J. Clin. Microbiol. Infect. Dis. 22: 165-173. [DOI] [PubMed] [Google Scholar]
- 97.Rauter, C., R. Oehme, I. Diterich, M. Engele, and T. Hartung. 2002. Distribution of clinically relevant Borrelia genospecies in ticks assessed by a novel, single-run, real-time PCR. J. Clin. Microbiol. 40:36-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Richter, D., S. Endepols, A. Ohlenbusch, H. Eiffert, A. Spielman, and F. R. Matuschka. 1999. Genospecies diversity of Lyme disease spirochetes in rodent reservoirs. Emerg. Infect. Dis. 5:291-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Richter, D., D. B. Schlee, and F. R. Matuschka. 2003. Relapsing fever-like spirochetes infecting European vector tick of Lyme disease agent. Emerg. Infect. Dis. 9:697-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rijpkema, S., D. Golubic, M. Molkenboer, N. Verbeek-De Kruif, and J. Schellekens. 1996. Identification of four genomic groups of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in a Lyme borreliosis endemic region of northern Croatia. Exp. Appl. Acarol. 20:23-30. [DOI] [PubMed] [Google Scholar]
- 101.Rijpkema, S., J. Nieuwenhuijs, F. F. Franssen, and F. Jongejan. 1994. Infection rates of Borrelia burgdorferi in different instars of Ixodes ricinus ticks from the Dutch North Sea island of Ameland. Exp. Appl. Acarol. 18:531-542. [DOI] [PubMed] [Google Scholar]
- 102.Rijpkema, S. G., M. J. Molkenboer, L. M. Schouls, F. Jongejan, and J. F. Schellekens. 1995. Simultaneous detection and genotyping of three genomic groups of Borrelia burgdorferi sensu lato in Dutch Ixodes ricinus ticks by characterization of the amplified intergenic spacer region between 5S and 23S rRNA genes. J. Clin. Microbiol. 33:3091-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rijpkema, S. G. T., D. J. Tazelaar, M. J. C. H. Molkenboer, et al. 1997. Detection of Borrelia afzelii, Borrelia burgdorferi sensu stricto, Borrelia garinii and group VS116 by PCR in skin biopsies of patients with erythema migrans and acrodermatitis chronica atrophicans. Clin. Microbiol. Infect. 3:109-116. [DOI] [PubMed] [Google Scholar]
- 104.Ruzic-Sablijic, E., F. Strle, and J. Cimperman. 1993. The Ixodes ricinus tick as a vector of Borrelia burgdorferi in Slovenia. Eur. J. Epidemiol. 9:396-400. [DOI] [PubMed] [Google Scholar]
- 105.Santino, I., M. del Piano, R. Sessa, G. Favia, and A. Iori. 2002. Detection of four Borrelia burgdorferi genospecies and first report of human granulocytic ehrlichiosis agent in Ixodes ricinus ticks collected in central Italy. Epidemiol. Infect. 129:93-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Santino, I., A. Iori, M. Nicoletti, S. Valletta, C. Cimmino, G. L. Scoarughi, D. Santapaola, R. Sessa, and M. Del Piano. 2003. Prevalence of Borrelia burgdorferi sensu lato genomospecies and of the human granulocytic ehrlichiosis (HGE) agent in Ixodes ricinus ticks collected in the area of Monti Lepini, Italy. Int. J. Immunopathol. Pharmacol. 16:105-108. [DOI] [PubMed] [Google Scholar]
- 107.Skotarczak, B., and B. Wodecka. 2003. Molecular evidence of the presence of Borrelia burgdorferi sensu lato in blood samples taken from dogs in Poland. Ann. Agric. Environ Med. 10:113-115. [PubMed] [Google Scholar]
- 108.Skotarczak, B., B. Wodecka, and A. Cichocka. 2002. Coexistence of DNA of Borrelia burgdorferi sensu lato and Babesia microti in Ixodes ricinus ticks from north-western Poland. Ann. Agric. Environ. Med. 9:25-28. [PubMed] [Google Scholar]
- 109.Speck, S., K. Failing, B. Reiner, and M. M. Wittenbrink. 2002. Evaluation of different media and a BGM cell culture assay for isolation of Borrelia burgdorferi sensu lato from ticks and dogs. Vet. Microbiol. 89:291-302. [DOI] [PubMed] [Google Scholar]
- 110.Stanczak, J., B. Kubica-Biernat, M. Racewicz, W. Kruminis-Lozowska, and J. Kur. 2000. Detection of three genospecies of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks collected in different regions of Poland. Int. J. Med. Microbiol. 290:559-566. [DOI] [PubMed] [Google Scholar]
- 111.Stanczak, J., G. Okroy-Rysop, M. Racewicz, B. Kubica-Biernat, and W. Kruminis-Lozowska. 2002. Prevalence of Borrelia burgdorferi sensu lato in the selected Ixodes ricinus (Acari: Ixodidae) population in Weilburg forests, Hesse, Germany. Int. J. Med. Microbiol. 291(Suppl. 33):206-209. [DOI] [PubMed] [Google Scholar]
- 112.Stanczak, J., M. Racewicz, W. Kubica-Biernat, W. Kruminis-Lozowska, J. Dabrowski, A. Adamczyk, and M. Markowska. 1999. Prevalence of Borrelia burgdorferi sensu lato in Ixodes ricinus ticks (Acari, Ixodidae) in different Polish woodlands. Ann. Agric. Environ. Med. 6:127-132. [PubMed] [Google Scholar]
- 113.Stanczak, J., M. Racewicz, W. Kruminis-Lozowska, and B. Kubica-Biernat. 2002. Coinfection of Ixodes ricinus (Acari: Ixodidae) in northern Poland with the agents of Lyme borreliosis (LB) and human granulocytic ehrlichiosis (HGE). Int. J. Med. Microbiol. 291(Suppl. 33):198-201. [DOI] [PubMed] [Google Scholar]
- 114.Stepanova-Tresova, G., J. Kopecky, and M. Kuthejlova. 2000. Identification of Borrelia burgdorferi sensu stricto, Borrelia garinii and Borrelia afzelii in Ixodes ricinus ticks from southern Bohemia using monoclonal antibodies. Zentbl. Bakteriol. 289:797-806. [DOI] [PubMed] [Google Scholar]
- 115.Stepanova-Tresova, G., B. Pet'ko, A. Stefancikova, and D. Nadzamova. 2000. Occurrence of Borrelia burgdorferi sensu stricto, Borrelia garinii and Borrelia afzelii in the Ixodes ricinus ticks from eastern Slovakia. Eur. J. Epidemiol. 16:105-109. [DOI] [PubMed] [Google Scholar]
- 116.Strle, F., Y. Cheng, J. A. Nelson, M. M. Picken, J. K. Bouseman, and R. N. Picken. 1995. Infection rate of Ixodes ricinus ticks with Borrelia afzelii, Borrelia garinii, and Borrelia burgdorferi sensu stricto in Slovenia. Eur. J. Clin. Microbiol. Infect. Dis. 14:994-1001. [DOI] [PubMed] [Google Scholar]
- 117.Stünzner, D., Z. Hubalek, J. Halouzka, D. Postic, K. Pierer, and E. Marth. 1998. Prevalence of Borrelia burgdorferi s.l. in Ixodes ricinus ticks from Styria (Austria) and species identification by PCR-RFLP analysis. Zentbl. Bakteriol. 288:471-478. [DOI] [PubMed] [Google Scholar]
- 118.Stunzner, D., K. Pierer, Z. Hubalek, J. Halouzka, E. Aberer, M. M. Millner, and E. Marth. 1999. Species identification of Borrelia burgdorferi sensu lato from tick and human isolates in Styria (Austria) by PCR-RFLP analysis. Wien Klin. Wochenschr. 111:994-996. [PubMed] [Google Scholar]
- 119.Talleklint, L., and T. G. Jaenson. 1996. Relationship between Ixodes ricinus density and prevalence of infection with Borrelia-like spirochetes and density of infected ticks. J. Med. Entomol. 33:805-811. [DOI] [PubMed] [Google Scholar]
- 120.Talleklint, L., and T. G. Jaenson. 1996. Seasonal variations in density of questing Ixodes ricinus (Acari: Ixodidae) nymphs and prevalence of infection with B. burgdorferi s.l. in south central Sweden. J. Med. Entomol. 33:592-597. [DOI] [PubMed] [Google Scholar]
- 121.van Dam, H. K., K. Vos, A. Widjojokusumo, B. M. de Jongh, L. Spanjaard, A. C. P. Ramselaar, M. D. Kramer, and J. Dankert. 1993. Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin. Infect. Dis. 17:708-717. [DOI] [PubMed] [Google Scholar]
- 122.Wegner, Z., J. Stanczak, M. Racewicz, W. Kruminis-Lozowska, and B. Kubica-Biernat. 1993. Occurrence of Borrelia spirochaetes in ticks (Acari, Ixodidae) collected in the forest areas in Olsztyn province (north central Poland). Bull. Inst. Mar. Trop. Med. Gdynia 44-45:51-59. [PubMed] [Google Scholar]
- 123.Wegner, Z., J. Stanczak, M. Racewicz, B. Kubica-Biernat, and W. Kruminis-Lozowska. 1997. The etiological agent of Lyme disease, Borrelia burgdorferi, in ticks (Acari: Ixodidae) from eastern Poland. Zentbl. Bakteriol. 286:93-106. [DOI] [PubMed] [Google Scholar]
- 124.Wittenbrink, M. M., C. Reuter, K. Baumeister, H. Schutze, and H. Krauss. 1998. Identification of group VS116 strains among Borrelia burgdorferi sensu lato grown from the hard tick, Ixodes ricinus (Linnaeus, 1758) by off-coupled restriction fragment length polymorphism analysis. Zentbl. Bakteriol. 288:45-57. [DOI] [PubMed] [Google Scholar]
- 125.Wittenbrink, M. M., C. Reuter, M. L. Manz, and H. Krauss. 1996. Primary culture of Borrelia burgdorferi from Ixodes ricinus ticks. Zentbl. Bakteriol. 285:20-28. [DOI] [PubMed] [Google Scholar]
- 126.Wittenbrink, M. M., D. Thiele, and H. Krauss. 1994. Comparison of dark-field microscopy, culture, and polymerase chain reaction (PCR) for detection of Borrelia burgdorferi in field-collected Ixodes ricinus ticks. Zentbl. Bakteriol. 281:183-191. [DOI] [PubMed] [Google Scholar]
- 127.Wodecka, B. 2003. Detection of Borrelia burgdorferi sensu lato DNA in Ixodes ricinus ticks in northwestern Poland. Ann. Agric. Environ. Med. 10:171-178. [PubMed] [Google Scholar]
- 128.Zeman, P. 1998. Borrelia-infection rates in tick and insect vectors accompanying human risk of acquiring Lyme borreliosis in a highly endemic region in central Europe. Folia Parasitol. (Prague) 45:319-325. [PubMed] [Google Scholar]
- 129.Zhioua, E., D. Postic, F. Rodhain, and C. Perez-Eid. 1996. Infection of Ixodes ricinus (Acari: Ixodidae) by Borrelia burgdorferi in Ile de France. J. Med. Entomol. 33:694-697. [DOI] [PubMed] [Google Scholar]