Abstract
Despite the fact that the heterotrophic dinoflagellate Pfiesteria shumwayae is an organism of high interest due to alleged toxicity, its abundance in natural environments is poorly understood. To address this inadequacy, a real-time quantitative PCR assay based on mitochondrial cytochrome b (cob) and18S rRNA gene was developed and P. shumwayae abundance was investigated in several geographic locations. First, cob and its 5′-end region were isolated from a P. shumwayae culture, revealing three different copies, each consisting of an identical cob coding region and an unidentified region (X) of variable length and sequence. The unique sequences in cob and the X region were then used to develop a P. shumwayae-specific primer set. This primer set was used with reported P. shumwayae-specific 18S primers in parallel real-time PCRs to investigate P. shumwayae abundance from Maine to North Carolina along the U.S. east coast and along coasts in Chile, Hawaii, and China. Both genes generally gave similar results, indicating that this species was present, but at low abundance (mostly <10 cells · ml−1), in all the American coast locations investigated (with the exception of Long Island Sound, where which both genes gave negative results). Genetic variation was detected by use of both genes in most of the locations, and while cob consistently detected P. shumwayae or close genetic variants, some of the 18S PCR products were unrelated to P. shumwayae. We conclude that (i) the real-time PCR assay developed is useful for specific quantification of P. shumwayae, and (ii) P. shumwayae is distributed widely at the American coasts, but normally only as a minor component of plankton even in high-risk estuaries (Neuse River and the Chesapeake Bay).
Pfiesteria shumwayae Glasgow et Burkholder (9) is a heterotrophic dinoflagellate implicated in fish kill events in the Neuse River, North Carolina; Chesapeake Bay tributaries, Maryland; and some other estuaries in the U.S. east coast (9). While the toxicity of this species is controversial (4), accurate identification and quantification of this species from morphologically similar dinoflagellates is essential for understanding the global distribution of P. shumwayae and verifying association of this organism with fish kills. Molecular techniques allowing rapid and accurate identification and quantification (e.g., quantitative PCR) of this species are highly desirable (16), because traditional methods relying on thecal plate tabulation and electron microscopy are not feasible for processing a large number of samples and light microscopy is inadequate for discriminating morphologically similar species. While P. shumwayae has been detected from Florida to New York on the east coast of the United States (13, 19), in New Zealand (18), and in Norway (11), little quantitative information about this dinoflagellate on geographic and temporal scales is available, most likely due to limitations of the existing methods (but see references 15 and 21 for more recent developments). To address this inadequacy, a cob- and 18S rRNA gene-based PCR assay, similar to what has been developed for Pfiesteria piscicida (25), was developed and employed to quantify P. shumwayae cell concentration in natural environments.
Specificity and sensitivity are the two main concerns in detecting harmful algae by using molecular techniques. Given that plankton is usually composed of numerous microorganisms, cross-reaction of a single-gene species-specific PCR primer set to nontarget species is not unlikely. The use of multigene markers can provide additional safeguards against nonspecific PCR amplification. Multigene markers have proven useful in phylogeny to resolve closely related lineages (3), and the utility of dual PCR markers for species identification has recently been demonstrated in the case of P. piscicida (25; S. Lin et al., unpublished data). The small subunit of the rRNA gene (18S rRNA gene) is commonly used for quantitative PCR and has been used to design P. shumwayae-specific primers (11, 16, 18, 19). The mitochondrial cytochrome b gene (cob), which has proven useful in phylogenetic analyses and species identification for many organisms (see, e.g., references 1, 6, 7, 8, 17, and 28) because of its relatively high and even mutation rate compared to the rRNA gene (for reviews, see references 2 and 20), is a good candidate for the second gene marker. Like the 18S rRNA gene, cob offers high sensitivity as a gene marker because it exists as multiple copies in the genomes of many dinoflagellates (25; H. Zhang and S. Lin, unpublished data). In this study, we isolated this gene from P. shumwayae and demonstrated that it was a gene marker as sensitive as the 18S rRNA gene in this species. We also developed a real-time PCR assay in which P. shumwayae cob- and 18S rRNA gene-specific primer sets were used in parallel to identify and quantify this species. This technique was then used to investigate P. shumwayae abundance in areas ranging from high-risk estuaries such as the Neuse River in North Carolina and the Pocomoke River in Maryland to undocumented areas on the northeast U.S. Atlantic coast and Pacific coasts in Hawaii, Chile, and China.
MATERIALS AND METHODS
Algal cultures and sample collection.
Pfiesteria shumwayae (strain T4) was provided by P. A. Tester and R. W. Litaker (15). Pfiesteria spp. and other algae used in this study were acquired and cultured as reported previously (25). Pfiesteria spp., Cryptoperidiniopsis spp., and Karlodinium micrum (formerly Gyrodinium galatheanum) were grown in 15-practical-salinity-unit (PSU), 0.45-μm-filtered, and autoclaved seawater supplied with the cryptophyte Rhodomonas sp. (strain CCMP768) as food. Rhodomonas was grown in 15-PSU seawater amended with f/2 nutrients (10). Other algae were grown in f/2 medium prepared with 28-PSU seawater. Illumination was provided at a photon flux of approximately 100 μE m−2 s−1 under a 12-h, 12-h light-dark regimen.
Samples were collected in the exponential growth phase by centrifugation at 3,000 × g for 20 min at 4°C. For Pfiesteria spp. and other heterotrophic dinoflagellates, feeding was discontinued for 2 to 3 days to deplete the prey prior to sample collection for DNA extraction. These samples were stored at −80°C until DNA extraction.
Field sample collection.
Sampling locations are shown in Table 1. In the Neuse River, N.C., surface samples were collected from five representative stations located along the length of the estuary, weekly in the summer and weekly or biweekly in other seasons depending on the severity of the weather conditions; DNA was isolated from water samples collected in the first and third weeks and was used for further molecular analysis. In the Chesapeake Bay, samples were collected from eight stations in various tributaries. In Long Island Sound, samples were collected from six stations located along the west-east axis of the sound, where a gradient of nitrate loading occurs. Stations A1 and A2 were sampled by the New York Interstate Environmental Commission onboard R/V Natal Colosi, whereas the other four stations by were sampled by the Connecticut Department of Environment Protection onboard R/V Dempsey, biweekly in the summer, bimonthly in the winter, and monthly in the spring and fall. Water samples were taken 2 m below the surface with Niskin bottles mounted on a conductivity-temperature-density (CTD) rosette. Samples from Narragansett Bay were collected from the University of Rhode Island the Graduate School of Oceanography campus and Newport with a 1-gallon bucket and from seven additional stations near the mouth of the bay with Niskin bottles. Samples from other areas were collected near shore with a plastic bucket. Subsamples of 250 ml were fixed immediately with Utermöhl's solution (24) at a final concentration of 2%. This sample fixation scheme had been verified in the laboratory to be effective for genomic DNA isolation from P. shumwayae. Upon arrival at the laboratory, samples were examined under a microscope with a Sedgwick-Rafter counting chamber for the presence of Pfiesteria-like dinoflagellates and then stored in a cold room (4°C) until analysis.
TABLE 1.
Summary of sampling stations, environmental parameters, and P. shumwayae abundance estimated using PSCOB and the 18S rRNA gene
| Location and station | Geographic location | Sampling period (mo/yr or mo/day/yr) | Temp (°C) | Salinity (‰) | P. shumwayae abundance, cells ml−1, estimated using PSCOB (18S rRNA gene) |
|---|---|---|---|---|---|
| Neuse River | |||||
| Union Point Park | 35°06′14′′N, 77°02′11′′W | 7/02-7/03 | 7.4-31.5 | 0.0-13.6 | 0-3.82 (0-1.16) |
| Fairfield Harbour Marina | 35°03′53′′N, 76°58′01′′W | 7/02-7/03 | 7.6-32.0 | 0.0-18.8 | 0-6.04 (0-1.0) |
| Flanners Beach | 34°59′03′′N, 76°56′53′′W | 7/02-7/03 | 7.0-31.0 | 0.0-20.7 | 0-0.10 (0-0.5) |
| Minnesott Beach Marina | 34°58′22′′N, 76°49′14′′W | 7/02-7/03 | 4.0-33.0 | 0.0-23.0 | 0-0.63 (0-0.8) |
| Matthews Marina | 34°54′28′′N, 76°45′47′′W | 7/02-7/03 | 6.0-33.0 | 1.0-35.1 | 0 (0-0.7) |
| Chesapeake Bay | |||||
| Chicamacomico River (CCM0069) | 38°26′32′′N, 75°54′18′′W | 10/2/02 | 22.0-25.7 | 15.1-15.5 | 0 (0.55) |
| Middle (FRG0018 = MidCT1) | 39°19′59′′N, 76°24′18′′W | 9/02-10/02 | 22.4-22.8 | 9.5 | 0 (0) |
| Middle (HOK0005 = MidCT3) | 39°18′54′′N, 76°26′32′′W | 9/02-10/02 | 21.7-22.1 | 8.9-9.0 | 0 (0) |
| Middle (SUE0010 = MidCT5) | 39°17′18′′N, 76°24′56′′W | 9/02-10/02 | 22.1 | 9.59 | 0 (0) |
| Marshall Creek (MSL0011 = NewPCT1) | 38°14′14′′N, 75°15′27′′W | 9/02-10/02 | 1.8-22.8 | 0.4-18.4 | 0 (0) |
| Newport (AYR0017 = NewPCT3) | 38°17′40′′N, 75°09′47′′W | 9/02-10/02 | 3.5-23.0 | 0.0-15.0 | 0 (0) |
| Transquaking River (TRQ0146) | 38°27′55′′N, 76°00′05′′W | 10/2/02 | 23.5 | 7.0 | 0 (0.57) |
| Pocomoke River (XAK7810) | 37°57′51′′N, 75°39′04′′W | 9/02-10/02 | 16.1-27.0 | 0.3-11.2 | 0-4.83 (0-0.06) |
| Long Island Sound | |||||
| A1 | 40°48′12′′N, 73°49′36′′W | 8/02-8/03 | 12.3-22.4 | 26.0-26.5 | 0 (0) |
| A2 | 40°48′06′′N, 73°47′00′′W | 8/02-8/03 | 12.0-21.8 | 26.2-27.6 | 0 (0) |
| A4 | 40°52′21′′N, 73°44′03′′W | 8/02-9/03 | −0.6-23.5 | 24.9-27.3 | 0 (0) |
| 09 | 40°04′15′′N, 73°20′10′′W | 8/02-9/03 | −0.8-22.6 | 25.2-28.3 | 0 (0) |
| H2 | 41°10′41′′N, 72°57′38′′W | 8/02-9/03 | −0.3-25.8 | 25.2-28.3 | 0 (0) |
| K2 | 41°14′04′′N, 72°15′57′′W | 8/02-9/03 | 0.7-21.7 | 27.4-30.9 | 0 (0) |
| Narragansett Bay | |||||
| RI1 (Newport) | 41°29′24′′N, 71°18′47′′W | 8/21/03 | 21.5 | 32.5 | 0.70 (0) |
| RI2 (URI) | 41°27′00′′N, 71°27′00′′W | 3/3/04 | 5.0 | 33.0 | 0.15 (0) |
| RI3 (station 1) | 41°48′24′′N, 71°22′52′′W | 7/04 | 20.8-21.3 | 25.5-28.1 | 0-0.12 (0.16-0.32) |
| RI4 (station 2) | 41°47′17′′N, 71°22′55′′W | 7/04-8/04 | 20.9-23.3 | 27.3-27.4 | 0-0.10 (0.07-1.83) |
| RI5 (station 3) | 41°45′85′′N, 71°22′58′′W | 7/04 | 20.9-21.7 | 26.0-27.8 | 0 (0.16-4.61) |
| RI6 (station 4) | 41°44′70′′N, 71°22′20′′W | 7/04-8/04 | 20.9-22.3 | 28.3-29.3 | 0 (0.40-8.55) |
| RI7 (station 5) | 41°43′06′′N, 71°20′54′′W | 7/04 | 21.1-21.6 | 29.2-29.3 | 0 (0.82-4.64) |
| RI8 (station 6) | 41°40′29′′N, 71°21′32′′W | 7/04 | 20.8-21.6 | 29.3-29.5 | 0 (1.16-1.35) |
| RI9 (station 7) | 41°38′61′′N, 71°18′41′′W | 7/04 | 21.3-21.4 | 30.1-30.4 | 0 (1.48-1.56) |
| Boston Harbor | |||||
| BH1 | 42°19′41′′N, 70°53′24′′W | 7/03-5/04 | 10.0-20.0 | 23.5 | 0.12-1.52 (0) |
| BH2 | 42°19′41′′N, 70°53′24′′W | 7/03-5/04 | 10.0-20.0 | 25.0-26.5 | 0.20-3.41 (0) |
| BH3 | 42°19′41′′N, 70°53′24′′W | 3/04-5/04 | 5.0-10.0 | 30.0-33.0 | 0.70-1.24 (0) |
| Maine and adjacent areas | |||||
| Bar Harbor | 44°26′00′′N, 68°21′00′′W | 6/30/03 | 16.5 | 31 | 24.25a (0) |
| Bucksport River | 44°38′00′′N, 68°45′00′′W | 7/2/03 | 17.4 | 16.5 | 0.60 (0) |
| Jordon Pond | 44°19′57′′N, 68°15′15′′W | 6/30/03 | 17.5 | 0 | 0.06 (0) |
| Kennebec River | 45°15′12′′N, 69°14′00′′W | 6/29/03 | 17.0 | 2 | 3.41a (0) |
| Kittery Beach | 43°18′13′′N, 70°35′10′′W | 6/28/03 | 16.8 | 29.5 | 1.14 (0) |
| Rockland Harbor | 44°05′08′′N, 69°05′29′′W | 6/29/03 | 16.5 | 29.5 | 10.40a (0) |
| Sand Beach | 44°32′35′′N, 68°25′12′′W | 6/30/03 | 14.5 | 31.5 | 1.74a (0) |
| Seal Harbor | 44°17′57′′N, 68°14′35′′W | 7/1/03 | 16.5 | 2.5 | 0.35 (0) |
| Sheepscot River | 44°06′54′′N, 69°37′30′′W | 6/29/03 | 17.5 | 25 | 1.55 (0) |
| Southwest Harbor | 44°23′27′′N, 68°15′42′′W | 7/1/03 | 17.5 | 32.5 | 1.24 (0) |
| Northeast Harbor | 44°17′39′′N, 68°17′06′′W | 7/1/03 | 17.0 | 31 | 0.46 (0) |
| Trenton townline | 44°26′02′′N, 68°23′46′′W | 6/30/03 | 16.8 | 31 | 0.63a (0) |
| York town creek | 43°32′01′′N, 70°54′57′′W | 6/28/03 | 17.2 | 29 | 1.30a (0) |
| Hawaii | |||||
| Kahalulu Beach | 21°27′00′′N, 157°57′00′′W | 2/04 | 25.0 | 27.5 | 0 (0) |
| Hanauma Bay | 21°27′00′′N, 157°57′00′′W | 2/04 | 25.0 | 35.5 | 0 (0) |
| Chile | |||||
| i-Mar | 41°30′00′′S, 72°58′48′′W | 12/12/03 | 17.0 | 17.0 | 0.50 (0) |
| Pelluco 1 | 41°30′00′′S, 72°58′48′′W | 12/12/03 | 19.0 | 32.0 | 0.72 (0) |
| Pelluco 2 | 41°30′00′′S, 72°58′48′′W | 12/12/03 | 17.0 | 15.0 | 0.18 (0) |
| Puerto Mont | 41°25′59′′S, 73°06′00′′W | 12/12/03 | 17.0 | 14.0 | 0.34a (0) |
| Jiaozhou Bay, China | |||||
| Station 1 | 36°22′00′′N, 120°15′00′′E | 12/29/03 | 8.0 | 31 | 0 (0) |
| Station 2 | 36°22′00′′N, 120°15′00′′E | 12/29/03 | 6.5 | 20.5 | 0 (0) |
| Xiamen Harbor, China | |||||
| ET90 | 24°26′46′′N, 118°04′04′′E | 12/29/03 | 17.2 | 32.0 | 0 (0) |
| ST2106 | 24°26′46′′N, 118°04′04′′E | 12/29/03 | 17.4 | 33.0 | 0 (0) |
PCR product was subcloned and sequenced.
Temperature and salinity were measured with a multisensor YSI sonde (Neuse River), CTD instrument (Chesapeake Bay, Long Island Sound, and Narragansett Bay), or thermometer and refractometer (other locations). Chlorophyll a concentration was measured fluorometrically based on acetone extraction (Neuse River and Chesapeake Bay) or in vivo fluorescence (Narragansett Bay). These parameters are available online for Long Island Sound (http://www.ctdep.us.gov) and Chesapeake Bay tributaries (http://mddnr.chesapeakebay.net/newmonthech/contmon/).
Prey addition experiment.
To examine whether P. shumwayae was limited by prey availability, water samples were incubated with prey added. Replicate water samples from each Neuse River station were sieved through 20-μm mesh to remove larger predators, fed Rhodomonas sp. strain CCMP767 (grown in the same way as strain CCMP768) at 5 × 103 to 10 × 103 cells · ml−1 thereafter, and kept in an incubator until being shipped overnight on ice to our laboratory at Avery Point, Groton, Conn. Upon arrival, samples were examined microscopically as described above, incubated at 20°C for 1 day, and then fixed in 2% Utermöhl's solution for DNA extraction (first- and third-week samples) or stored in the cold room (4°C).
DNA extraction.
DNA from the Utermöhl's solution-preserved field samples or from culture-derived samples was extracted essentially as described by Zhang and Lin (25) with slightly modifications. Briefly, 30 ml of field samples was centrifuged at 3,000 × g for 20 min at 4°C and supernatant was carefully removed. Pellets were then resuspended in 100 μl DNA extraction buffer (containing 0.1 M EDTA, 1% sodium dodecyl sulfate, and 20 μg proteinase K [Invitrogen]) and incubated at 55°C for 16 h. DNA was then isolated by adding 16.5 μl each of 5 M NaCl and 10% cetyltrimethylammonium bromide (Sigma) in 0.7 M NaCl and incubating at 55°C for 10 min, followed by one chloroform extraction and one phenol-chloroform extraction. DNA was then purified by being passed twice through DNA Clean and Concentrator columns (Zymo Research, Orange, CA). DNA from the 30-ml subsample was dissolved in 30 μl of distilled and deionized water and stored at −20°C until PCR was performed. The DNA recovery rate in this procedure had been examined in preliminary experiments. Laboratory-grown P. piscicida cells (30, 300, 3,000, 30,000, and 3 × 106 cells) were added to 30 ml of the randomly selected water samples collected in this study, and DNA was purified with the protocol mentioned above. Real-time PCR results for these DNA samples showed that the recovery rate was consistently about 50% for samples containing 30 to 30,000 P. piscicida cells and decreased to 30% for the sample spiked with 3 × 106 P. piscicida cells, most likely because the amount of DNA exceeded the binding capacity of the column (5 μg). Results of PCR for P. piscicida cob verified that the quality of the DNA was good (Zhang and Lin, unpublished data). In this study, however, as a further quality safeguard, DNA quality was verified by PCR with universal 18S rRNA gene primers when P. shumwayae-specific PCR yielded no result, as was previously done for P. piscicida (25).
Known numbers of P. shumwayae cells grown in the laboratory were added to the 0.45-μm-filtered and autoclaved seawater collected from Avery Point, Long Island Sound, where no P. shumwayae had been detected (nor was it detected in this study with PCR assays as well as a microscopic examination), and DNA was extracted following the same procedure as for field samples, including passing through the DNA Clean and Concentrator column twice. The DNA was then used as the standard in real-time PCR.
cob cloning and sequencing.
The cob coding region in P. shumwayae has been isolated (27). However, in search of variable regions useful for development of P. shumwayae-specific primers, the 5′-end and upstream regions of the P. shumwayae cob coding region needed to be analyzed. DNA was extracted essentially following a previously reported protocol (25). The 5′-end and upstream regions of the P. shumwayae cob coding region (including the mitochondrial cytochrome c oxidase subunit 3 gene, cox3) were amplified using 50 ng of P. shumwayae genomic DNA as the template and the primer set PPCOX3-PPCOB (previously designed for cloning the same regions from P. piscicida [25]). The PCR product was then diluted 50 times and used as the template for nested PCR using primer set PPCOX3-PSCOBR1 (Table 2). Amplification was carried out with 1 min at 95°C once, followed by 35 cycles of 20 s at 94°C, 30 s at 52°C, and 1 min at 72°C. PCR products were subcloned and sequenced (14).
TABLE 2.
Primers used in this study
| Primer | Sequence (5′→3′) | Reference or source |
|---|---|---|
| PPCOB | GATGTGATAAAGAAGAGACCCCGAAATGAGTTTC | 25 |
| PPCOX3 | CCTCCAGAAGGTTTCTATCTTCCAGATCCTTG | 25 |
| PSMTF1 | AGATATGACCGTGAGGAAGATGCT | This study |
| PSMTF2 | TGACTTTCTAACTTCTAACTTCTTTACATC | This study |
| PSMTF3 | TTCATTCCTATCAACCGTGAGATC | This study |
| PSMTR1 | AGAAAGGGAGAGACCGTTGATAAGA | This study |
| PSMTR2 | GCATCTTCCTCACGGTCATATCTTG | This study |
| PSCOBR1 | AACACCATCCATAGAATATTTCTCTCATG | This study |
| PS18SF1 | ACAGTTTTAGTGTATTTGATGATCG | 16 |
| PS18SR1 | TCGAAAGCTGATAGGTCAGAATC | 16 |
| 18SCOMF | TGCATGGCCGTTCTTAGTTGGTGG | 25 |
| 18SCOMR | CACCTACGGAAACCTTGTTACGAC | 25 |
Development of specific primers and quantitative real-time PCR.
Based on the alignment of available the dinoflagellate cob sequence and its 5′-end noncoding sequences, variable regions were identified. P. shumwayae-specific primer sets were designed from these regions by using Beacon Designer 3.0. These primer sets were tested against 20 cultures, including P. piscicida, Cryptoperidinium sp., Karlodinium micrum, and other dinoflagellates, as well as Rhodomonas sp (25). The most specific primer set, PSMTF2-PSCOBR1 (product size, 326 bp, containing 196 bp of the P. shumwayae cob coding region and 130 bp of upstream region; for convenience, this mitochondrial DNA [mtDNA] fragment is called PSCOB hereafter), was selected for further field sample analyses. The P. shumwayae-specific 18S rRNA gene primer set PS18SF1-PS18SR1 was also designed based on the reported primer sequences (product size, 221 bp; called PS18S hereafter; essentially equal to species B forward and reverse primers in reference 16). P. shumwayae in water samples was quantified by real-time PCR on the DNA extracted from field samples, using the primer sets PSMTF2-PSCOBR1 (PSCOB) and PS18SF1-PS18SR1 (PS18S) in separate PCRs in the same multiwell plate. Real-time PCR was carried out with SYBR Green Supermix on an iCycler iQ real-time PCR detection system (Bio-Rad Laboratories, Inc., Hercules, CA). Amplification conditions were 3 min at 95°C followed by 50 cycles of 20 s at 94°C, 30 s at 57°C, and 20 s at 72°C. One microliter of DNA solution was used in all reactions.
Quality assurance.
The real-time PCR assay from each of the two genes was used to compare the results and verify the specificity of one set of primers with the other. This technique was expected to prevent errors caused by false-positive results that could occur with single-gene PCR. In tests prior to analysis of the field samples, both PSCOB and PS18S produced positive results only for established P. shumwayae cultures, and both genes yielded negative results for known non-P. shumwayae cultures (e.g., CCMP1827, CCMP1828, and P. piscicida). However, in field application of the technique, the common presence of inhibitory compounds in estuaries may cause false-negative results for both genes. To distinguish true- from false-negative results, an additional PCR was run with a set of universal 18S rRNA gene primers that had been demonstrated to react with a wide range of algae (25; Zhang and Lin, unpublished data). If a PCR product of the expected size was generated from the DNA sample, the negative results for P. shumwayae-specific PCR were considered a genuine representation of the absence of P. shumwayae. Otherwise, the negative results from the P. shumwayae-specific PCR were attributed to poor DNA quality, and the result was discarded. In the present study, all of the field DNA samples gave positive results for universal 18S rRNA gene amplification. All positive real-time PCR products were further analyzed by agarose gel (2%) electrophoresis to conform the molecular sizes. If one gene gave positive a result and the other gave a negative result, PCR products were subcloned and sequenced to verify the identity in most of the cases. Finally, even if results were positive for both genes, random clones were sequenced for samples from new locations to verify the identity.
Sequencing and phylogenetic analyses of real-time PCR products from field samples.
PCR products of PSCOB and PS18S from field samples were purified by ethanol precipitation and subcloned (see Tables 1, 3, and 4), and four to eight of the resulting clones were sequenced (14).
TABLE 3.
P. shumwayae abundance in Chesapeake Bay tributaries estimated using PSCOB and PS18S
| Station |
P. shumwayae abundance, cells ml−1, estimated using PSCOB (PS18S)
|
||
|---|---|---|---|
| 9/23-26/02 | 10/1-10/02 | 10/21-31/02 | |
| CCM0069 | 0 (0.55) | ||
| MidCT1 | 0 (0) | 0 (0) | 0 (0) |
| MidCT3 | 0 (0) | 0 (0) | 0 (0) |
| MidCT5 | 0 (0) | 0 (0) | 0 (0) |
| NewPCT1 | 0 (0) | 0 (0) | |
| NewPCT3 | 0 (0) | 0 (0) | |
| TRQ0146 | 0 (0.57) | ||
| XAK7810 | 0 (0) | 4.83 (0.06) | |
TABLE 4.
P. shumwayae cell concentration in Narragansett Bay estimated using PSCOB and PS18S
| Station |
P. shumwayae cell concn, cells ml−1, estimated using PSCOB (P18S)
|
||||
|---|---|---|---|---|---|
| 8/21/03 | 3/3/04 | 7/13/04 | 7/28/04 | 8/10/04 | |
| RI1 (Newport) | 0.70a (0) | ||||
| RI2 (URI) | 0.15a (0) | ||||
| RI3 (station 1) | 0 (0.16a) | 0 (0.32) | |||
| RI4 (station 2) | 0 (1.83) | 0 (0.25) | 0.12a (0.07a) | ||
| RI5 (station 3) | 0 (4.61) | 0.10a (0.16a) | |||
| RI6 (station 4) | 0 (3.24a) | 0 (0.40) | 0 (8.55) | ||
| RI7 (station 5) | 0 (4.64a) | 0 (0.82) | |||
| RI8 (station 6) | 0 (1.16a) | 0 (1.35) | |||
| RI9 (station 7) | 0 (1.56a) | 0 (1.48) | |||
PCR product was subcloned and sequenced.
Sequences were aligned using the CLUSTAL W (1.8) server at the DNA Data Bank of Japan (DDBJ) (http://www.ddbj.nig.ac.jp/Welcome-e.html), using the default values. The alignment was then manually adjusted using the SeaView program to maintain codon integrity prior to analysis with the Phylo_Win package. To assess the degree of variation of the PCR clones, phylogenetic trees based on nucleotide sequences were constructed using the neighbor-joining method (22). Pairwise distances were corrected using the Kimura two-parameter model (12), and support for nodes was tested with bootstrap analyses (2,000 replications).
Nucleotide sequence accession numbers.
The sequences of clones Pscox3-cob1, Pscox3-cob2, and Pscox3-cob3 have been deposited in GenBank under accession numbers AY746979 to AY746981, respectively.
RESULTS
Cloning of the 5′ upstream region of the P. shumwayae cob coding region.
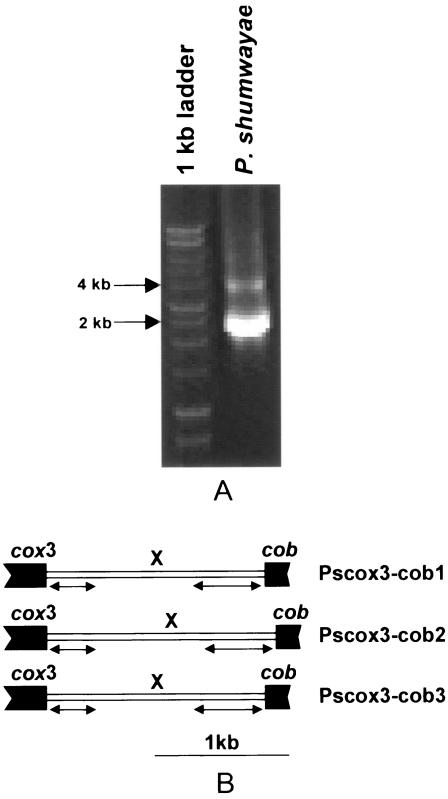
No clear DNA band was obtained with primer set PPCOX3-PPCOB. However, nested PCR with primer set PPCOX3-PSCOBR1 generated a strong band of 2 to 2.5 kb and a weak band of 4 kb. The strong band was subcloned, and three clones with different lengths of DNA fragments were obtained, each containing cox3, cob, and an unidentified sequence (X region) flanking the 5′ end of the P. shumwayae cob coding region (Fig. 1). The first clone, Pscox3-cob1 (2,157 bp) contained 321 bp of the 3′-end coding region of cox3 and 196 bp of the 5′-end coding region of the P. shumwayae cob coding region interrupted by a 1,640-bp X region. The second clone, Pscox3-cob2 (2,399 bp), and the third clone, Pscox3-cob3 (2,174 bp), contained the same cox3 and cob regions as Pscox3-cob1 but with 1,882-bp and 1,657-bp X regions, respectively. The coding regions of cox3 and cob in the three clones were identical in sequence, whereas in the X region, only 369 bp at its 5′ end and 495 bp at the 3′were identical in all three clones.
FIG. 1.
PCR amplification of mitochondrial cytochrome b and its 5′ flanking region of P. shumwayae and organization of the gene fragments. (A) PCR amplification using primers PPCOX3 and PSCOBR1 (Table 2). Arrows on the left indicate the molecular sizes of two of the bands in the l-kb DNA ladder. (B) Organization of the three PCR-amplified gene fragments. cox3, cytochrome c oxidase subunit 3; cob, cytochrome b; X, sequence that does not show similarity to known genes in GenBank DNA databases; bidirectional arrows, regions that are identical in all three copies. Coding regions are shown as solid boxes.
Phylogenetic analysis.
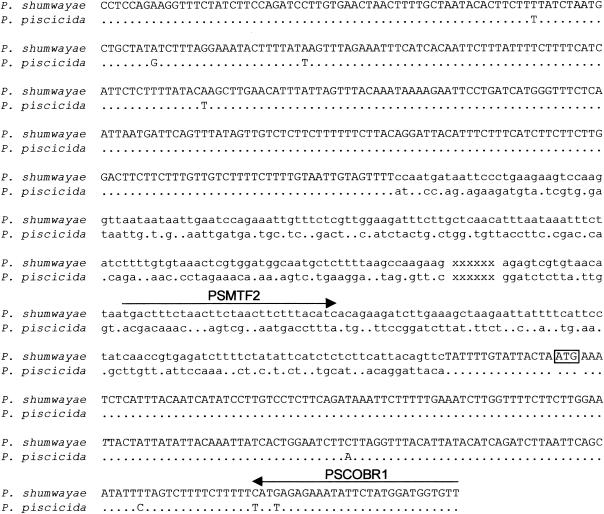
Comparison of the P. shumwayae cob coding region and its 5′ upstream region with counterparts from P. piscicida indicated that these two species shared 98 to 99% nucleotide identity in the coding regions of cox3 and cob. However, no similarity was found between these two Pfiesteria species in the noncoding X region, rendering it potentially useful in designing species-specific primers (Fig. 2). The detailed phylogenetic analyses of the P. shumwayae cob coding region and the counterparts from other dinoflagellates and other organisms are described elsewhere (27) but in general show a clear distinction between P. shumwayae and P. piscicida.
FIG. 2.
Alignment of the mitochondrial gene fragments that contain (5′ to 3′) cox3 (uppercase), the X region (lowercase), and cob (uppercase) in the two Pfiesteria species. Dots indicate nucleotides in P. piscicida that are identical to corresponding position in P. shumwayae. xxxxxx, X region (nucleotide sequence not shown); arrows, regions used to design primers for real-time PCR analysis. The potential start codon ATG of cob is boxed.
P. shumwayae-specific primers.
All primers used in this study are listed in Table 2. Based on multiple alignments of cob and upstream sequences of P. shumwayae with those of the other organisms, P. shumwayae-specific primers sets were designed (Table 2). For comparison, P. shumwayae-specific primers for 18S rRNA were also designed based upon the reported sequence (16).
The primer sets showed slight variation in specificity and sensitivity. PSMTF2-PSCOBR1 appeared to be most specific, while PSMTF1-PSMTR1 was most sensitive (not shown). Since specificity is more important, PSMTF2-PSCOBR1 was selected for further tests. P. shumwayae-specific 18S rRNA gene primer set PS18SF1-PS18SR1 also appeared to be specific to P. shumwayae and as sensitive as PSMTF2-PSCOBR1 by real-time PCR (see below).
Both PSCOB and PS18S primers yielded no DNA product with a blank control (H2O), DNA of Rhodomonas sp., Pfiesteria piscicida, the Pfiesteria-like dinoflagellates (CCMP1827, CCMP1828, and CCMP1835), and other dinoflagellates (not shown). The absence of PCR product in these cases was not due to poor quality of DNA, as the same DNA templates yielded abundant PCR products when the universal 18S rRNA gene primers (18SCOMF and 18SCOMR) were used (25).
Real-time PCR to determine quantitation capability.
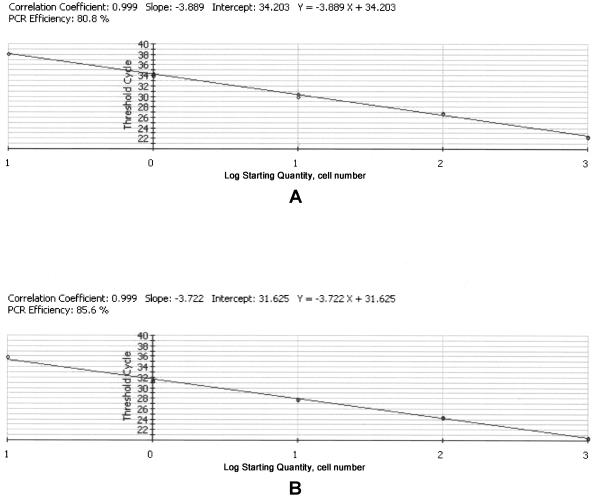
To test the utility of PSCOB primers for quantifying P. shumwayae, 5, 50, 500, 5,000, and 50,000 cells were added to 50 ml autoclaved seawater from Avery Point, Long Island Sound, where no P. shumwayae was detected. DNA was isolated as described above and dissolved in 50 μl H2O to make a dilution series of 0.1, 1, 10, 100, and 1,000 cells/μl. This series was used as the standard to test the PSMTF2-PSCOBR1 and PS18SF1-PS18SR1 primer sets for quantitation capability.
A strong linear correlation between the log of cell number and threshold cycle number was obtained for both primer sets (Fig. 3), indicating a consistent recovery rate with the Zymo column for a range of DNA quantities derived from 5 to 50,000 P. shumwayae cells. Based on the correlation within the range of cell concentrations in the standard, 0.1 cell per reaction (equivalent to three P. shumwayae cells in a 30-ml water sample) can be detected for both PSMTF2-PSCOBR1 and PS18SF1-PS18SR1. However, in practice, <0.1 cell could be detected based on the extended regression line of the standard curve (Table 1).
FIG. 3.
Real-time quantitative PCR standard curves of P. shumwayae, using primer sets PSMTF2-PSCOBR1 (A) and PS18SF1-PS18SR1 (B). P. shumwayae genomic DNAs equivalent to 1,000, 100, 10, 1, and 0.1 cells were used as templates. The standard curve was constructed as log of P. shumwayae cell number versus number of threshold cycles. Shown are results of duplicates (two data points overlap in cases where only one circle is shown).
P. shumwayae abundance in natural environments.
Under the light microscope with a Sedgwick-Rafter counting chamber (1 ml), few if any cells resembling Pfiesteria were observed in the natural water samples. With the primer sets developed and the real-time PCR conditions described above, P. shumwayae was detected, albeit in low abundance, in all areas investigated except Long Island Sound, Hawaii, and the Chinese coasts (Jiaozhou Bay and Xiamen Harbor).
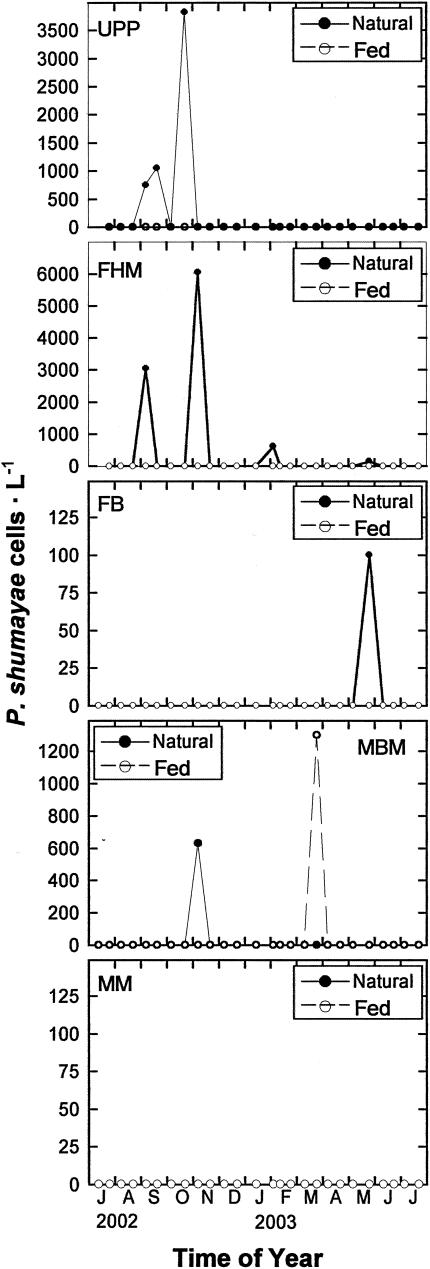
(i) Neuse River.
P. shumwayae was found in three samples from Union Point Park and three from Fairfield Harbour Marina (Fig. 4). Most of the positive results at these two stations occurred in late autumn. In contrast, Flanners Beach and Minnesott Beach Marina each showed a positive result in May and November, respectively (Fig. 4). No P. shumwayae was detected in Matthews Marina. Throughout the study period, only one sample from Minnesott Beach Marina (20 March 2003) exhibited growth of P. shumwayae in the prey-amended incubation.
FIG. 4.
Temporal variation in P. shumwayae abundance at the five stations in Neuse River, N.C. P. shumwayae cell concentration was measured with PSCOB primers for natural seawater before (Natural) and after (Fed) fed incubation using Rhodomonas sp. as prey. UPP, FB, FHM, MBM, and MM, Union Point Park, Flanners Beach, Fairfield Harbour Marina, Minnesott Beach Marina, and Matthews Marina stations, respectively.
(ii) Chesapeake Bay tributaries.
Most of the water samples analyzed were negative, except at three stations. At station XAK7810 in late October 2002, both PSCOB and PS18Sgave positive results, with an estimate of 4.83 (PSCOB) and 0.06 (PS18S) cells · ml−1, respectively. In early October 2002, at TRQ0146 and CCM0069, PS18S estimated 0.55 and 0.57 cell · ml−1, respectively, while PSCOB yielded no results (Tables 1 and 3).
(iii) Narragansett Bay.
Water samples from the Graduate School of Oceanography campus of Rhode Island and from Newport both contained very low number of P. shumwayae cells (Tables 1 and 4). In August 2003 and March 2004, about 0.70 and 0.15 cell · ml−1, respectively, were detected in Newport by PSCOB, while PS18S yielded no positive results. All water samples collected in July to August 2004 from the seven stations near the mouth of Narragansett estuary showed positive results from PS18S(0.16 to 4.64 cell · ml−1); however, only two stations at two time points gave positive results with PSCOB (0.12 and 0.10 cell · ml−1, respectively) (Table 4). Further analyses revealed that the PS18S-only positive sequences were unrelated to P. shumwayae (See below).
(iv) Boston Harbor.
No sample exhibited positive results with PS18S; however, PSCOB showed positive results for all samples (Table 5). The cell concentration ranged from 0.12 to 4.12 cells · ml−1.
TABLE 5.
P. shumwayae cell concentration in Boston Harbor estimated using PSCOB and PS18S
| Station |
P. shumwayae cell concn, cells ml−1, estimated using PSCOB (PS18S)
|
||
|---|---|---|---|
| 7/2/03 | 3/13/04 | 5/31/04 | |
| BH1 | 0.12a (0.00) | 1.52a (0.00) | 0.81a (0.00) |
| BH2 | 3.41 (0.00) | 4.12 (0.00) | 0.20 (0.00) |
| BH3 | 0.70 (0.00) | 1.24 (0.00) | |
PCR product was subcloned and sequenced.
(v) Maine embayments.
No positive results were yielded with PS18S, but an appreciable abundance (0.06 to 24.30 cells · ml−1) of P. shumwayae was detected with PSCOB (Table 1).
(vi) Chile.
No positive results were yielded with PS18S, but low cell concentrations were detected with PSCOB (Table 1).
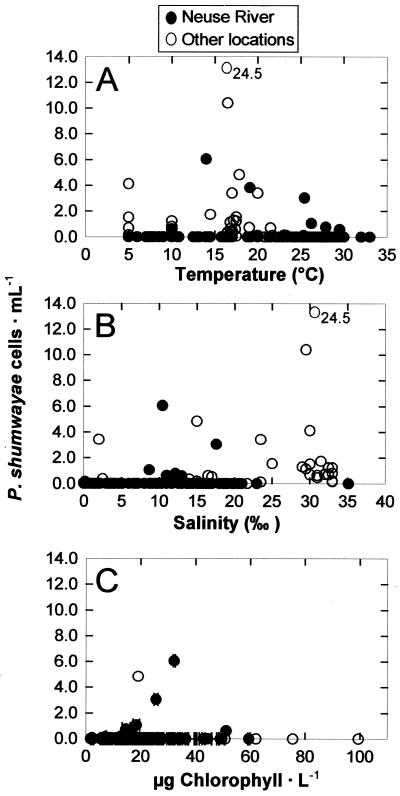
The limited number of positive samples and low cell concentrations detected preclude the possibility of identifying the environmental determinant of P. shumwayae population dynamics. An attempt to find environmental factors conducive to P. shumwayae growth indicated some trend in the Neuse River. In this estuary, the temperature ranged from 4 to 33°C, the salinity ranged from 0 to 35‰, and the chlorophyll concentration ranged from 1.7 to 100 μg · liter−1, with strong seasonal variations. P. shumwayae appeared to occur more frequently and slightly more abundantly when the temperature was between 15.0 and 26.0°C (Fig. 5A), when the salinity was 10.0 to 20.0‰ (Fig. 5B), or when the chlorophyll concentration was 20.0 to 40.0 μg liter−1 (Fig. 5C), but the difference was not statistically significant (P > 0.05 by the F test). For instance, the occurrence frequency and average cell concentration of P. shumwayae were 12.9% and 0.2 ± 0.9 cell · ml−1, respectively, within this temperature range, in comparison to 6.8% and 0.1 ± 0.7 cell · ml−1outside the range. Environmental data from other locations were more limited and did not allow analysis for each location. When data from those locations were pooled, the pattern was weakened and seemed to extend the apparent favorable salinity to 30‰. In general, no correlation between P. shumwayae abundance and either temperature, salinity, or chlorophyll concentration can be formulated.
FIG. 5.
Relationship between P. shumwayae abundance (quantified with PSCOB primers) and temperature (A), salinity (B), and chlorophyll concentration (C) in the Neuse River alone and other locations (pooled). A high cell concentration is out of the scale and indicated by the number.
Genetic diversity in natural environments.
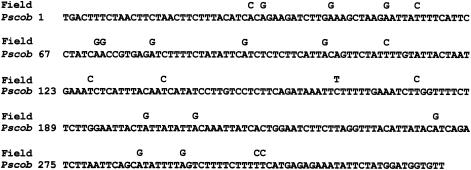
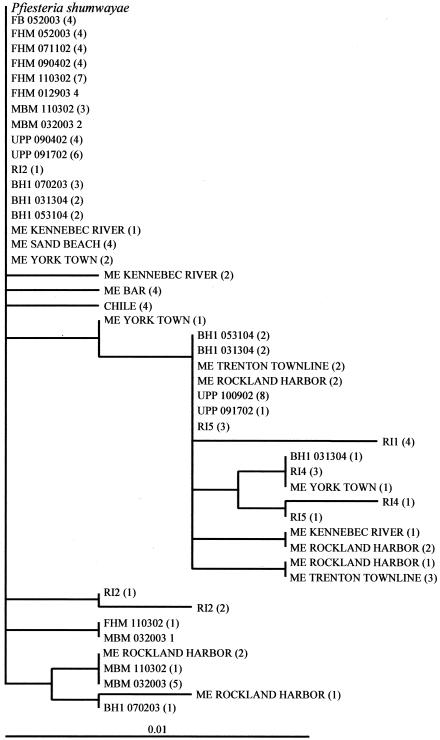
PSCOB amplicons for 25 water samples from 16 stations (Tables 1, 4 and 5) were analyzed by randomly subcloning and sequencing the PCR products. The results revealed that most of the PSCOB clones were identical to the documented P. shumwayae sequence but some were slightly different. The sequence variation ranged from 1 to 4 nucleotide substitutions (0.3 to 1.3%) in each sample, totaling 22 substitution sites for all samples (Fig. 6). Most of these substitutions (20 out of 22 sites) were A-to-G or T-to-C transitions (Fig. 6), and the same nucleotide substitution was usually found in multiple clones from the same or different sampling locations (Fig. 7). Therefore, these variations cannot be attributed merely to PCR-cloning error, which usually occurs randomly. The majority of different clones were found in Narragansett Bay and northward, although some diversity was also found in Chile, Neuse River, and Chesapeake Bay samples (Fig. 7).
FIG. 6.
Polymorphism of the clones obtained for field samples. The complete sequence shown is the 326-bp PSCOB fragment (Pscob) in the documented P. shumwayae mtDNA sequence (accession numbers AY746979 to AY746981). Numbers on the left indicate positions of the first nucleotide of each line. All possible nucleotide substitutions (19) found in all the field samples (Field) are shown above the corresponding sites in the P. shumwayae sequence. Blank spaces in Field indicate nucleotide identical to those in Pscob. Typically, 0 to 4 of these substitutions occur in one sample.
FIG. 7.
Unrooted neighbor-joining tree of P. shumwayae mtDNA clones (PSCOB, 326 bp) from natural environments. For each sampling station, four to eight of the resulting clones were sequenced; numbers in parentheses indicate the number of clones with identical sequence. Clone codes: Pfiesteria shumwayae, sequence in the P. shumwayae cob coding region 5′ upstream region, which is identical in all three clones (Pscox3-cob1, Pscox3-cob2, and Pscox3-cob3); UPP, FB, FHM, and MBM, Union Point Park, Flanners Beach, Fairfield Harbour Marina, and Minnesott Beach Marina stations in Neuse River, N.C., respectively (Table 1); RI, Narragansett Bay in Rhode Island; BH, Boston Harbor in Massachusetts; ME, embayment in Maine; CHILE, Puerto Mont, Chile (see Table 1). In some cases, the name of the sampling station is followed by a number indicating the substation and/or a string of numbers indicating the month, day, and year of the sampling event (e.g., BH1 070203, Boston Harbor sampling station 1 sampled on 2 July 2003).
The 326-bp PSCOB-only positive clones from Narragansett Bay were either identical to or slightly different from (<1.3%) P. shumwayae cob (Fig. 7). Conversely, sequences of the 221-bp PS18S-only positive clones showed that none of them were P. shumwayae 18S but were either Gyrodinium-like dinoflagellates (4 of the 28 clones analyzed [14%]) or unknown eukaryotes (24 clones [86%]). In the latter case, 20 clones shared an identical sequence that was only 81% identical to the reported P. shumwayae 18S rRNA gene and 80 to 90% identical to other dinoflagellate 18S sequences in GenBank. Using the combination of PS18SF1 with 18SCOMR (Table 2), a nearly full-length fragment of this 18S rRNA gene from these samples was found (GenBank accession no. AY788914). Phylogenetic analyses for this 18S rRNA gene sequence further indicated that these were likely undocumented organisms (not shown).
In the Boston Harbor and Maine embayments, where PS18S did not produce positive results, PSCOB also revealed some genetic variation (Fig. 7). In Maine samples, seven clones from three locations were identical to P. shumwayae (Fig. 7). Another 29 clones belonged to nine distinct variants, each with <1% difference from the P. shumwayae sequence.
DISCUSSION
Sequence and organization of cob and adjacent genes.
Similar to the case for P. piscicida (25), multiple copies of cob exist in the P. shumwayae genome. In this study we have cloned three copies of mtDNA fragments from P. shumwayae containing cox3, cob, and an unidentified X region flanking the 5′ end of the P. shumwayae cob coding region (Fig. 1). These clones are identical in coding regions but only partially identical in the X region. Comparison of these sequences between the two Pfiesteria species shows that these two species share 98 to 99% nucleotide identity in coding regions but their X regions show no nucleotide similarity. The lack of similarity renders this noncoding region of mtDNA useful for development of species-specific primers. It will be of interest to find out if this region occurs in other dinoflagellates and whether species-specific probes can be developed based on its unique sequence.
PSCOB-PS18S real-time PCR assay.
Utility of the developed real-time PCR for laboratory cultures as well as field samples was demonstrated. The primer sets designed (PSMTF2-PSCOBR1 and PS18SF1-PS18SR1) proved to be specific and sensitive. The sensitivity of the primers was demonstrated by the low detection limit, which allowed detection of P. shumwayae at such low abundances that detection failed by microscopic analysis. The specificity of the assay was shown by a series of analyses. PCR with PSCOB and PS18S primers yielded no products for non-P. shumwayae species, and both consistently produced positive results for cultured P. shumwayae. However, more positive results were obtained in the real-time PCR assay for PSCOB in some field samples and for PS18S in others. In the former cases (Neuse River, Chesapeake Bay, Boston Harbor, Maine, and adjacent embayments), agarose gel analyses indicated that the PCR products of both genes had the correct fragment sizes most of the time, although occasionally (<1% of all samples analyzed) PSCOB gave obviously incorrect results (amplicons with different sizes [not shown]). Sequencing of the PSCOB-only positive PCR fragments indicated that in most of the cases, these mt fragments had the same nucleotide sequence as P. shumwayae, although some variations were observed (Fig. 6). It is not clear why PS18S gave fewer positive results than PSCOB, even though both gene markers appeared to be equally sensitive (Fig. 3). One possibility is that those field samples contained P. shumwayae-like species that had mt gene sequences similar to PSCOB but more distinct 18S rRNA genes. This contrasts with the case of P. piscicida, in which the 18S rRNA gene usually gives more positive results than the mt gene in field samples (Lin et al., unpublished data). Interestingly, in Narragansett Bay, PS18S gave more positive results than PSCOB, and none of the clones obtained from PS18S-only positive PCR products were genuine P. shumwayae (Table 4) but were related to other dinoflagellates or unknown eukaryotes. This result strongly suggests that caution is required in detecting or quantifying P. shumwayae when using single-gene PCR, especially based solely on the 18S rRNA gene. In natural assemblages, there probably are many unrecognized organisms similar to the group detected in this study. It is quite possible that a “specific” single-gene primer set designed based on the known sequences can cross-react with some of these unrecognized species. Therefore, products from a single-gene PCR must be further analyzed (e.g., by sequencing) to determine whether the positive result is genuine. More variable DNA such as internal transcribed spacers or nontranscribed spacers (15, 21) will be better gene markers to reduce the chance of nonspecific amplification. To further prevent false conclusions in critical cases such as P. shumwayae that involve health or environmental concerns, dual PCR with a proper quality assurance procedure as used in this study is desirable.
In this study, we used SYBR Green Supermix in real-time PCR, as had been widely used. In addition to its low cost, which is highly desirable when processing a large number of samples, the system (without a fluorochrome on the primer or use of additional probes) makes it easier to subclone the PCR product when needed. The SYBR Green real-time PCR detection system has facilitated quantification of gene copy numbers and cell concentrations for other species (26, Zhang and Lin, unpublished data). Since SYBR Green is a generic stain for all DNA, it is not possible to distinguish products from the 18S rRNA gene and cob primer sets if they are used in the same PCR. In the future, however, duplex PCR can be conducted if the two specific primer sets are labeled with different fluorochromes that emit different colors of fluorescence. Alternatively, molecular beacons can be designed and labeled with different fluorochromes that would specifically hybridize to target PCR products. If interference of two primer sets with each other in PCR proves to be minimal, the duplex PCR can be used to reduce interreaction variation, number of reactions, and cost of reagents.
P. shumwayae distribution and abundance.
P. shumwayae seems to be a global organism. Previously, it has been found in the North Atlantic (Florida to New York) (13, 19), Norway) (11), and the South Pacific (New Zealand) (18). In this study, the map of P. shumwayae distribution is extended to Maine in the northeast of the North Atlantic and to Chile in the South Pacific. More importantly, this study has provided estimates of the abundance of this species and indicates that this species generally occurs at low cell concentration in the natural environment, at least in the areas and time periods investigated. The low abundance detected was not due to poor efficiency of PCR, because the DNA quality of all the field samples and PCR efficiency of standard DNA (from cultured P. shumwayae) added to random field DNA samples were verified (not shown). In comparison, however, P. shumwayae appeared to be more abundant than P. piscicida in some of the geographic locations and time periods covered in this study, except in Long Island Sound, where P. shumwayae was not detected (Lin et al., unpublished data).
Although P. shumwayae appeared to occur more frequently at warmer temperatures and intermediate salinities in the Neuse River, as did P. piscicida (5), the relationship between P. shumwayae abundance and environmental factors remains elusive. The low abundance of P. shumwayae cannot be explained by limitation of prey, because incubation of field-collected water samples with Rhodomonas sp. added did not increase P. shumwayae abundance in most cases. In the Neuse River, the higher P. shumwayae abundances were detected at a medium range of chlorophyll concentrations, and the few data points from other locations also fell in that range (Fig. 5C). Apparently, neither total phytoplankton abundance nor a presumably favored prey alga seems to regulate P. shumwayae abundance. Some top-down (predation) control mechanism may be important, because Pfiesteria spp. are known to be grazed by other microzooplankton (23). The results from the present study strongly suggest the need for more focused field studies to assess the relative importance of the bottom-up and top-down control of P. shumwayae dynamics. The protocol developed in this study will facilitate such studies.
Acknowledgments
We thank Wayne Litaker and Patricia Tester's research team at National Ocean Services for Pfiesteria shumwayae culture and field samples, Robert Andersen from Bigelow Laboratory for Pfiesteria piscicida and Pfiesteria-like cultures, and Donald M. Anderson from WHOI for Alexandrium tamarense culture. We are also grateful to David Goshorn from the Department of Natural Resources of Maryland for samples from Chesapeake Bay tributaries, to Matthew Lyons from the Water Quality Monitoring Group of the Connecticut Department of Environmental Protection for samples from Long Island Sound, to Peter Sattler from the New York Interstate Environmental Commission for samples from the East River (A1 and A2), to Xuchen Wang from the University of Massachusetts at Boston for samples from Boston Harbor, to Dave Ullman and Yubo Hou for samples from Narragansett Bay, to Daniel Veras for samples from Chile, to Po Wang and Tiezhu Mi for samples from Jiaozhou Bay, and to Yahui Gao for samples from Xiamen Harbor, China.
This study was supported by ECOHAB funding (grants NA86OP0491 and NA16OP2797).
Footnotes
ECOHAB publication number 131.
REFERENCES
- 1.Akihito, I. A., T. Kobayashi, K. Ikeo, T. Imanishi, H. Ono, Y. Umehara, C. Hamamatsu, K. Sugiyama, Y. Ikeda, Y. Sakemoto, A. Fumihito, S. Ohno, and T. Gojobori. 2000. Evolutionary aspects of gobioid fishes based upon a phylogenetic analysis of mitochondrial cytochrome b genes. Gene 259:5-15. [DOI] [PubMed] [Google Scholar]
- 2.Avise, J. C. 1986. Mitochondrial DNA and the evolutionary genetics of higher animals. Philos. Trans. R. Soc. London Biol. 312:235-242. [DOI] [PubMed] [Google Scholar]
- 3.Baldauf, S. L., A. J. Roger, I. Wenk-Sifert, and W. F. Doolittle. 2000. A kingdom-level phylogeny of eukaryotes based on combined protein data. Science 290:972-974. [DOI] [PubMed] [Google Scholar]
- 4.Berry, J. P., K. S. Reece, K. S. Rein, D. G. Baden, L. W. Haas, W. L. Ribeiro, J. D. Shields, R. V. Snyder, W. K. Vogelbein, and R. E. Gawley. 2002. Are Pfiesteria species toxicogenic? Evidence against production of ichthyotoxins by Pfiesteria shumwayae. Proc. Natl. Acad. Sci. USA 99:10970-10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burkholder, J. M., and H. B. Glasgow, Jr. 1997. Pfiesteria piscicida and other Pfiesteria-like dinoflagellates: behavior, impacts, and environmental controls. Limnol. Oceanogr. 42:1052-1055. [Google Scholar]
- 6.Callejas, C., and M. D. Ochando. 2000. Recent radiation of Iberian barbel fish (Teleostei, Cyprinidae) inferred from cytochrome b genes. J. Hered. 91:283-288. [DOI] [PubMed] [Google Scholar]
- 7.Conroy, C. J., and J. A. Cook. 1999. MtDNA evidence for repeated pulses of speciation within arvicoline and murid rodents. J. Mammal. Evol. 6:221-245. [Google Scholar]
- 8.Conway, D. J., C. Fanello, J. M. Lloyd, B. M. A. Al-Joubori, A. H. Baloch, S. D. Somanath, C. Roper, A. M. J. Oduola, B. Mulder, M. M. Povoa, B. Singh, and A. W. Thomas. 2000. Origin of Plasmodium falciparum malaria is traced by mitochondrial DNA. Mol. Biochem. Parasitol. 111:163-171. [DOI] [PubMed] [Google Scholar]
- 9.Glasgow, H. B., J. M. Burkholder, S. L. Morton, and J. Springer. 2001. A second species of ichthyotoxic Pfiesteria (Dinamoebales, Dinophyceae). Phycologia 40:234-245. [Google Scholar]
- 10.Guillard, R. R., and J. H. Ryther. 1962. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve). Gran. Can. J. Microbiol. 18:229-239. [DOI] [PubMed] [Google Scholar]
- 11.Jakobsen, K. S., T. Tengs, A. Vatne, H. A. Bowers, D. W. Oldach, J. M. Burkholder, H. B. Glasgow, P. A. Rublee, and D. Klaveness. 2002. Discovery of the toxic dinoflagellate Pfiesteria in northern European waters. Proc. R. Soc. London B 269:211-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura, M. 1980. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 16:111-120. [DOI] [PubMed] [Google Scholar]
- 13.Lewitus, A. J., K. C. Hayes, B. M. Willis, J. M. Burkholder, H. B. Glasgow Jr; A. F. Holland, P. P. Maier, P. A. Rublee, R. Magnien. 2002. Lewitus, A. J., K. C. Hayes, B. M. Willis, J. M. Burkholder, H. B. Glasgow Jr., A. F. Holland, P. P. Maier, P. A. Rublee, and R. Magnien. 2002. Low abundance of the dinoflagellates, Pfiesteria piscicida, P. shumwayae, and Cryptoperidiniopsis spp., in South Carolina tidal creeks and open estuaries. Estuaries 25:586-597. [Google Scholar]
- 14.Lin, S., H. Zhang, D. Spencer, J. Norman, and M. Gray. 2002. Widespread and extensive editing of mitochondrial mRNAs in dinoflagellates. J. Mol. Biol. 320:727-739. [DOI] [PubMed] [Google Scholar]
- 15.Litaker, R. W., M. W. Vandersea, S. R. Kibler, K. S. Reece, N. A. Stokes, K. A. Steidinger, D. F. Millie, B. J. Bendis, R. J. Pigg, and P. A. Tester. 2003. Identification of Pfiesteria piscicida (Dinophyceae) and Pfiesteria-like organisms using internal transcribed spacer-specific PCR assays. J. Phycol. 39:754-761. [Google Scholar]
- 16.Oldach, D., C. F. Delwiche, K. S. Jakobsen, T. Tengs, E. G. Brown, J. W. Kempton, E. F. Schaefer, H. A. Bowers, H. B. Glasgow, Jr., J. M. Burkholder, K. A. Steidinger, and P. A. Rublee. 2000. Heteroduplex mobility assay-guided sequence discovery: elucidation of the small subunit (18S) rRNA sequences of Pfiesteria piscicida and related dinoflagellates from complex algal culture and environmental sample DNA pools. Proc. Natl. Acad. Sci. USA 97:4303-4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pearson, O. P., and M. F. Smith. 1999. Genetic similarity between Akodon olivaceus and Akodon xanthorhinus (Rodentia: Muridae) in Argentina. J. Zool. 247:43-52. [Google Scholar]
- 18.Rhodes, L. L., J. M. Burkholder, H. B. Glasgow, P. A. Rublee, C. Allen, and J. E. Adamson. 2002. Pfiesteria shumwayae (Pfiesteriaceae) in New Zealand. N. Zealand J. Mar. Freshwat. Res. 36:621-630. [Google Scholar]
- 19.Rublee, P. A., J. W. Kempton, E. F. Schaefer, C. Allen, J. Harris, D. W. Oldach, H. Bowers, T. Tengs, J. M. Burkholder, and H. B. Glasgow. 2001. Use of molecular probes to assess geographic distribution of Pfiesteria species. Environ. Health Perspect. 109(Suppl.):765-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saccone, C., C. Gissi, C. Lanave, A. Larizza, G. Pesole, and A. Reyes. 2000. Evolution of the mitochondrial genetic system: an overview. Gene 261: 153-159. [DOI] [PubMed] [Google Scholar]
- 21.Saito, K., T. Drgon, J. A. F. Robledo, D. N. Krupatkina, and G. R. Vasta. 2002. Characterization of the rRNA locus of Pfiesteria piscicida and development of standard and quantitative PCR-based detection assays targeted to the nontranscribed spacer. Appl. Environ. Microbiol. 68:5394-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 23.Stoecker, D. K., K. Stevens, and D. E. Gustafson. 2000. Grazing on Pfiesteria piscicida by microzooplankton. Aquat. Microbiol. Ecol. 22:261-270. [Google Scholar]
- 24.Utermohl, H. 1958. Zur Vervollkommnung der quantitativen Phytoplankton-Methodik. Mitt. Int. Ver. Theor. Angew. Limnol. 9:1-38. [Google Scholar]
- 25.Zhang, H., and S. Lin. 2002. Detection and quantification of Pfiesteria piscicida using mitochondrial cytochrome b gene sequence. Appl. Environ. Microbiol. 68:989-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang, H., and S. Lin. 2003. Complex gene structure of the form II Rubisco in the dinoflagellate Prorocentrum minimum (dinophyceae). J. Phycol. 39:1160-1171. [Google Scholar]
- 27.Zhang. H., D. Bhattacharya, and S. Lin. 2005. Phylogeny of dinoflagellates based on mitochondrial cytochrome b and nuclear small subunit rDNA sequence comparisons. J. Phycol. 41:411-420. [Google Scholar]
- 28.Zhang, H., N. Mikawa, Y. Yamada, N. Horie, A. Okamura, T. Utoh, S. Tanaka, and T. Motonobu. 1999. Detection of foreign eels in the natural waters of Japan by PCR. Fish. Sci. 65:684-686. [Google Scholar]