Abstract
The ability to simultaneously concentrate diverse microbes is an important consideration for sample collection methods that are used for emergency response and environmental monitoring when drinking water may be contaminated with an array of unknown microbes. This study focused on developing a concentration method using ultrafilters and different combinations of a chemical dispersant (sodium polyphosphate [NaPP]) and surfactants. Tap water samples were seeded with bacteriophage MS2, Escherichia coli, Enterococcus faecalis, Cryptosporidium parvum, 4.5-μm microspheres, Salmonella enterica serovar Typhimurium, Bacillus globigii endospores, and echovirus 1. Ten-liter tap water samples were concentrated to ∼250 ml in 12 to 42 min, depending on the experimental condition. Initial experiments indicated that pretreating filters with fetal bovine serum or NaPP resulted in an increase in microbe recovery. The addition of NaPP to the tap water samples resulted in significantly higher microbe and microsphere recovery efficiencies. Backflushing of the ultrafilter was found to significantly improve recovery efficiencies. The effectiveness of backflushing was improved further with the addition of Tween 80 to the backflush solution. The ultrafiltration method developed in this study, incorporating the use of NaPP pretreatment and surfactant solution backflushing, was found to recover MS2, C. parvum, microspheres, and several bacterial species with mean recovery efficiencies of 70 to 93%. The mean recovery efficiency for echovirus 1 (49%) was the lowest of the microbes studied for this method. This research demonstrates that ultrafiltration can be effective for recovering diverse microbes simultaneously in tap water and that chemical dispersants and surfactants can be beneficial for improving microbial recovery using this technique.
In the event of microbiological contamination of drinking water, even low concentrations of organisms can put water consumers at risk. Due to factors such as sampling delay, microbial die-off, and dilution, it is often the case that human pathogens and fecal microflora are present in environmental waters at sufficiently low concentrations such as to preclude the use of simple “grab sampling” for water testing. Thus, it is desirable to have rapid and robust methods for concentrating large volumes of drinking water to detect and quantify pathogens and other microbes in these samples. For situations where drinking water may be contaminated but no clinical data are available to identify the pathogen of concern, it is critical that a sample concentration technique that can simultaneously concentrate viruses, bacteria, and parasites is used. The benefits of simultaneous recovery techniques are also increasingly being recognized for those applications where cost-effective monitoring of microbial water quality for diverse microbe types is desired.
Tangential flow ultrafiltration is a technique that can be used for the simultaneous concentration of diverse waterborne microbes in a single step (6, 13, 14). Ultrafilters having appropriate molecular weight cutoffs (MWCOs) concentrate microorganisms in the retentate while allowing water to pass through the pores. Hollow-fiber ultrafiltration is typically run in a tangential (or cross-flow) mode where the retentate is recirculated until the desired concentration factor is achieved. The scouring effect of the cross-flow recirculation decreases the tendency for microbes to adhere to filter surfaces and reduces the possibility of filter fouling (6, 13, 17, 21).
In the early 1980s, tangential-flow hollow-fiber ultrafiltration was investigated and found to be effective for recovering viruses in large-volume (20- to 100-liter) tap water samples (3). More recently, research by Morales-Morales et al. (13) reported greater than 50% recoveries for bacteriophages, Escherichia coli, and Cryptosporidium parvum oocysts seeded into 10-liter surface water samples. While the simultaneous ultrafiltration recovery results of Morales-Morales et al. were good, their technique relies on the use of a calf serum protocol to pretreat (“block”) the ultrafilter membranes prior to filtration. For certain applications (e.g., rapid response and field-based filtration), pretreatment with calf serum may not be appropriate or practical due to concerns related to theamount of time needed for the calf serum blocking procedure (e.g., overnight) and potential for microbial contamination growth in calf serum-blocked filters during storage and transport.
In the present study, we report the results of research investigating the effects of a sodium polyphosphate chemical dispersant that shows promise as a reagent for minimizing the adhesion of microbes to filter surfaces. Polyphosphates are highly negatively charged chemicals that have been used to decrease bacterial adhesion to soil (16), disperse Cryptosporidium oocysts and Giardia cysts prior to flow cytometry (9), and keep minerals in suspension during industrial processing (15). They work as dispersants by altering the surface charge of microbes, particles, and filter surfaces. When used as a bulk water additive, sodium polyphosphate (NaPP) compounds can significantly reduce (i.e., make more negative) the zeta potential of suspended microbes (16). Sodium polyphosphate compounds are commercially produced in various phosphate chain lengths, from two-unit compounds (sodium pyrophosphate) to six-unit compounds (hexametaphosphate) to 17-unit or greater polymers.
Whereas NaPP is of interest for increasing microbial recovery efficiencies by surface charge repulsion, surfactants (e.g., Tween 80) are of interest for minimizing hydrophobic interactions between microbes and ultrafilter surfaces (13). Surfactant molecules have hydrophilic and hydrophobic regions that orient at surfaces in a manner that typically modifies hydrophobic surfaces to become more hydrophilic. Tween 80 is a well-established nonionic surfactant for microbial laboratory techniques where it is desired to minimize microbial adhesion to surfaces or to desorb microbes that are adhered to surfaces (12, 13).
In this study we examined the use of hollow-fiber ultrafiltration to concentrate E. coli, Enterococcus faecalis, Salmonella enterica serovar Typhimurium, Bacillus globigii endospores, bacteriophage MS2, echovirus 1, C. parvum oocysts, and 4.5-μm polystyrene microspheres from 10 liters of tap water. These microbes were selected to cover the range of pathogenic microbe classes of specific interest to us in the context of response to water-related microbe contamination events. E.coli and E. faecalis were selected as gram-negative and gram-positive microbe models for pathogenic bacteria. B. globigii spores were selected as the surrogate for Bacillus anthracis spores, and bacteriophage MS2 was used as a surrogate for human enteric viral pathogens because of its small size, icosahedral shape, and assay simplicity. Microspheres (4.5 μm) were used as a surrogate for Cryptosporidium oocysts, based on previous research indicating that these microspheres were useful models for the physical removal of Cryptosporidium oocysts (4). The goals of this study were to (i) compare the effectiveness of NaPP to that of fetal bovine serum (FBS) for blocking ultrafilter cartridges prior to use, (ii) evaluate the effectiveness of NaPP as an additive to water samples for improving ultrafilter recovery efficiencies, and (iii) investigate ultrafilter backflushing (with or without surfactants) as a technique for improving microbe recovery efficiencies.
MATERIALS AND METHODS
Water samples.
Tap water samples were obtained the day of testing from the CDC laboratory faucet. Ten liters of water was dispensed into a graduated carboy for volume measurement and then transferred to a 10-liter cubitainer (Cole-Parmer). Water was dechlorinated by adding 5 ml of stock sodium thiosulfate (40 mg/ml) to achieve final concentration of 20 mg thiosulfate per liter feed water.
Microorganisms.
Escherichia coli (ATCC 11775), Enterococcus faecalis (ATCC 19433), Salmonella enterica serovar Typhimurium (ATCC 14028), and Bacillus globigii endospores (acquired from the U.S. Environmental Protection Agency, Cincinnati, OH) were seeded at levels of 106 CFU into dechlorinated tap water samples and assayed by membrane filtration before and after ultrafiltration according to Standard Methods for the Examination of Water and Wastewater (1). All necessary dilutions were made in phosphate-buffered saline (Dulbecco's modification) (0.01 M, pH 7.40) amended with 0.01% (wt/vol) Tween 80 (Fisher T164) and 0.001% (wt/vol) Antifoam A (Sigma catalog no. A-5758) (20) to disperse aggregates of the study organisms. E. coli was enumerated using mFC agar; E. faecalis, using mEnterococcus agar (1); S. enterica, using selenite-cysteine agar (a modification of standard method 9260B, with 1% agar added to selenite-cysteine broth) (18); and B. globigii endospores, by placing the membrane on AK sporulation agar no. 2 (Becton Dickinson catalog no. 210912). Viruses were also seeded into the 10-liter water samples at levels of 106 PFU. Bacteriophage MS2 (ATCC 15597-B1) was enumerated by plaque assay per U.S. Environmental Protection Agency method 1602 (19). Echovirus 1 (Farouk strain; ATCC VR-1038) was enumerated by plaque assay using BGMK cells and neutral red agar overlay. Echovirus used in the experiments was a stock produced by selection of a large plaque from a Farouk strain plaque assay followed by propagation of this isolate by standard methods. This stock of echovirus 1 replicated more rapidly than the initial stock from the ATCC and provided a routine plaque assay with results obtained in 2 days. Prior to assay, water concentrates were extracted with one-half volume of chloroform to remove bacteria. The aqueous extract was allowed to sit in an open petri dish for 5 min to allow any chloroform residues to volatilize. A 1/10 volume of 10× Eagle’s minimal essential medium was added to the water samples to provide conditions suitable for the plaque assay. C. parvum oocysts (Iowa Strain) were acquired from Waterborne, Inc., and from the CDC laboratory of Michael Arrowood. All oocysts were between 1 and 3 months old when used for experiments. C. parvum oocysts were seeded into water samples at a level of 106 and enumerated by a direct immunofluorescence assay technique using MeriFluor (Meridian Bioscience) labeling reagents according to U.S. Environmental Protection Agency method 1623 (20). Microspheres (4.5-μm Fluoresbrite YG polystyrene microspheres; Polysciences, Inc.) were seeded into water samples at levels of 3 × 104 and enumerated by filtering through 3-μm-pore-size polycarbonate track-etched membrane filters (GE Osmonics, Minnetonka, MN) and counting the spheres by fluorescence microscopy at a total magnification of ×100.
Ultrafilter setup.
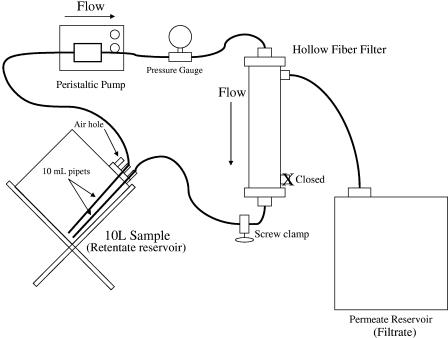
The filtration unit was set up as shown in Fig. 1. New high-performance, platinum-cured L/S 24 silicone tubing (Masterflex; Cole-Parmer Instrument Co.) was used for each filtration experiment. All tubing connections and clamps were autoclaved, and the brass fitting to the pressure gauge was sanitized with 3% hydrogen peroxide and 10% bleach solution (0.6% sodium hypochlorite) and then washed thoroughly with deionized water prior to use in the filtration setup. Nonseeded, negative control ultrafiltration runs were performed to ensure that the disinfection procedure was effective in preventing carryover contamination between experiments. No carryover contamination was observed between experiments. The hollow-fiber ultrafilters were Fresenius Hemoflow F80A polysulfone dialysis filters with a MWCO of 15,000 to 20,000, a surface area of 1.8 m2, and a fiber inner diameter of 200 μm (Fresenius Medical Care, Lexington, MA). New filters were used for each experiment, although the F80A dialyzers can be sanitized, stored, and reused according to the manufacturer's guidance. A Cole-Parmer model 7550-30 peristaltic pump was used for all experiments.
FIG. 1.
Schematic of 10-liter ultrafiltration experimental setup.
Ultrafilter blocking.
Ultrafilters were blocked using two basic types of reagents: NaPP or calf serum reagents (either fetal bovine serum [Invitrogen catalog no. 26400-036] or calf serum [Invitrogen catalog no. 16170-078]). Dilutions of calf serum reagents were made using 0.2-μm filter-sterilized deionized water. For overnight blocking using FBS or calf serum, the ultrafilter cartridge was filled with 5% FBS (or calf serum) and rocked on a shaker platform for a period of at least 16 h. For short-protocol (1-h) blocking, 5% FBS (or calf serum) was recirculated through the ultrafilter cartridge for 1 h before the system was rinsed and the 10-liter microbe recovery experiment was performed. For experiments performed using NaPP-blocked ultrafilters, the filters were pretreated with a polymeric (n = 17) NaPP compound (Sigma-Aldrich catalog no. 305553). NaPP blocking was performed immediately prior to experiments by passing 1 liter of NaPP solution (0.1% to 0.001% NaPP in deionized water, depending on the experimental condition) through the ultrafilter cartridge at a recirculation rate of 1,700 ml/min with no back pressure and the permeate port of the ultrafilter cartridge open. It typically took 15 min to complete the blocking procedure.
Filtration reagents.
Dechlorinated tap water was seeded with approximately 106 cells of each microbe used for the different experimental conditions studied. In addition to being investigated as a blocking reagent, NaPP was also added to water samples to investigate the effectiveness of the chemical as a dispersant in solution. NaPP concentrations of 0.1 to 0.001% were used for these water amendment experiments. Tween 80 was also investigated as a water sample amendment and as a backflushing reagent. For experiments where Tween 80 was added to water samples, either 1 ml of Tween 80 was added to 10 liters of water (to give 0.01%, vol/vol) or 2 ml of a 10% solution of Tween 80 was added to 10 liters of water (to give 0.002%, vol/vol). When Tween 80 was added to water samples, 50 μl of Antifoam A was also added to the samples to minimize foaming during the ultrafiltration procedure.
Ultrafilter backflushing.
Various backflushing conditions were investigated as the method development process progressed. Initially, backflush solutions containing only NaPP were investigated. For these experiments, the solution used to backflush the ultrafilter contained the same concentration of NaPP that was added to the water sample. Backflushing using Tween surfactants was investigated using solutions containing 0.5 to 1.0% Tween 80 or a combination of 0.01% Tween 80 and 0.01% Tween 20. Two experiments were performed when 0.5% Tween 80 was used, and five experiments were performed when 1% Tween 80 was used. For experiments where Tween surfactants were used as backflushing reagents, the backflushing solution also contained 0.001% Antifoam A and 0.01% NaPP. UV absorbance measurements (at a wavelength of 200 to 254 nm) were performed to evaluate the relative breakthrough of various concentrations of Tween 80 solutions (0.001% to 0.5%) through the ultrafilter.
Filtration parameters.
The peristaltic pump was set to pump water from the retentate reservoir at 1,700 ml/min. Generally, the system was operated at 7 to 8 lb/in2 to achieve a permeate rate of approximately 800 ml/min (and a corresponding cross-flow rate within the hollow-fiber ultrafilter of 900 ml/min). Filtration was performed until 100 to 150 ml of concentrated sample remained in the retentate reservoir. At this point the intake tubing was withdrawn from the retentate reservoir and the peristaltic pump used to force as much of the retentate as possible into the retentate reservoir. For experiments where backflushing was not performed, pressurized air was used to recover residual retentate remaining in the ultrafilter system (achieving ∼10 to 15% increased volumetric recovery). For experiments where backflushing was performed, the peristaltic pump was used to pump the backflush solution into the ultrafilter cartridge through the open permeate port at a rate of 150 ml/min until approximately 100 ml of liquid was collected.
Statistical analysis.
Recovery efficiency for each microbe and microbial surrogate was calculated by first calculating the total number of each microbe/surrogate in the preconcentrated 10-liter tap water sample and final ultrafilter concentrate sample based on the measured concentrations of each microbe/surrogate in these samples. Once the total number of each microbe/surrogate was calculated for an experiment, the recovery efficiency was calculated by dividing the total number of the microbe/surrogate in the final ultrafilter concentrate sample by the total number in the preconcentrated 10-liter tap water sample (with the fraction multiplied by 100 to report the recovery percentage). The significance of experimental variables for each microbe or surrogate was determined using two-tailed t tests for independent samples. To investigate the effect of an experimental condition on recovery of multiple microbial parameters, the two-factor analysis of variance (ANOVA) test was used. The significance level (α) was set at 0.05. All recovery efficiencies were included as reported in calculations of descriptive statistics regardless of whether the value exceeded 100%. For statistical comparisons utilizing ANOVA, however, data values greater than 100% were truncated to 99.9% and transformed to logits in order to normalize the distribution of the outcome variable.
RESULTS
FBS and NaPP ultrafiltration conditions.
Experiments were initially performed to investigate the effect on MS2 recovery efficiency when FBS was used to block ultrafilters and 0.1% NaPP was added to water samples. Subsequent recovery tests were performed with S. enterica serovar Typhimurium. Backflushing was not used in these tests, and the final ultrafilter retentate volumes were 150 ml on average. Results of the MS2 and Salmonella tests suggested that FBS blocking improved the recovery efficiency of the ultrafilter procedure, although the differences in the baseline (no blocking) and FBS blocking data were not significant (P = 0.27) due to small sample size, lack of power, and high relative standard deviations for some of the data (Table 1). When 0.1% NaPP was added to the water samples, in addition to blocking the ultrafilter overnight with FBS, the mean MS2 and Salmonella recovery efficiencies were 108% and 49%, respectively. When FBS blocking was performed for 1 h instead of overnight, the mean MS2 recovery was 71% (when 0.1% NaPP was also used), which was significantly lower than the 108% mean recovery measured when FBS blocking was performed overnight (P = 0.007). MS2 breakthrough of the ultrafilter during these tests was <0.05% of the seed level. The addition of 0.1% NaPP was associated with significantly higher recovery efficiencies of MS2 and Salmonella when FBS-blocked filters were used (P ≤ 0.0001).
TABLE 1.
Ultrafiltration recovery efficiencies for various FBS blocking and sodium polyphosphate sample amendment conditions
| Blocking condition | na | Water sample amendment | Mean % recovery ± SD
|
|
|---|---|---|---|---|
| MS2 | Salmonella | |||
| No blocking | 4, 3 | None | 44 ± 30 | 10 ± 1 |
| 5% FBS, overnight | 7, 4 | None | 51 ± 19 | 31 ± 30 |
| 7, 8 | 0.1% NaPP | 108 ± 16 | 49 ± 23 | |
| 5% FBS, 1 h | 3, 0 | 0.1% NaPP | 71 ± 11 | ND |
| 3, 0 | None | 50 ± 14 | ND | |
Number of experiments for MS2 and Salmonella, respectively.
Effectiveness of NaPP for filter blocking and backflushing.
Based on the experimental results shown in Table 1, additional experiments were performed to investigate the effect of NaPP at various concentrations when added to water samples, as well as when used to backflush ultrafilters. Calf serum was also used (as a less expensive alternative to FBS) in this follow-on set of experiments for comparison with NaPP as a blocking reagent. The ultrafilter procedure using overnight calf serum blocking and addition of 0.1% NaPP to the 10-liter water sample (condition A) resulted in mean retentate recovery efficiencies of 50% or higher for all study microbes (Table 2). The use of 0.1% NaPP as a filter blocking reagent and water amendment (condition B) resulted in relatively high mean retentate recoveries of MS2 and E. coli (71 and 74%, respectively) but lower recoveries of E. faecalis (12%) and microspheres (32%). However, the lower recoveries of E. faecalis and microspheres were significantly increased to 76 and 93%, respectively, by backflushing using 0.1% NaPP (Table 2). No statistically different recoveries were found when blockings of the ultrafilters with calf serum and 0.1% NaPP were compared (P = 0.36). For all the conditions shown in Table 2, final concentrate volumes were approximately 250 ml, with the additional backflush liquid (∼100 ml) added to the retentate volume (∼150 ml).
TABLE 2.
Recovery efficiencies for microbes and microspheres under various ultrafiltration conditions
| Conditiona | Filter/sampleb | Mean % recoveryc± SD
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS2
|
E. coli
|
E. faecalis
|
Salmonella
|
B. globigii spores
|
Microspheres
|
||||||||
| Retentate | Total | Retentate | Total | Retentate | Total | Retentate | Total | Retentate | Total | Retentate | Total | ||
| A | 5% Calf serum/0.1% NaPP | 84 ± 13 | 89 ± 15 | 70 ± 13 | 72 ± 13 | 71 ± 10 | 93 ± 3 | 62 | 79 | 52 | 63 | 103 | 110 |
| B | 0.1% NaPP for both | 71 ± 25 | 82 ± 25 | 74 ± 8 | 98 ± 8 | 12 ± 4 | 76 ± 9 | NDd | ND | ND | ND | 32 ± 15 | 93 ± 14 |
| C | 0.01% NaPP for both | 86 ± 20 | 96 ± 21 | 72 ± 15 | 93 ± 15 | 24 ± 5 | 87 ± 11 | ND | 65 ± 9 | ND | 46 ± 15 | 15 ± 10 | 59 ± 13 |
| D | 0.01% NaPP/0.01% Tween 80 | 105 ± 23 | 106 ± 23 | 121 | 123 | 126 | 133 | 80 | 84 | 106 | 107 | 116 | 117 |
| E | 0.01% NaPP/0.002% Tween 80 | 70 | 73 | 129 | 134 | 87 | 106 | ND | ND | ND | ND | 100 | 103 |
For condition A, n = 3 to 5 for MS2, E. coli, and E. faecalis and n = 2 for all others; for condition B, n = 3 or 4 for all parameters; for condition C, n = 4 for all; for condition D, n = 4 for MS2 and n = 2 for all others; and for condition E, n = 2 for all.
Filter, blocking condition for the ultrafilter; sample, sample amendment condition.
Retentate efficiencies reflect microbe recovery in ultrafilter concentrates without backflushing; total efficiencies reflect additional microbe recovery achieved by backflushing the ultrafilters (total = retentate + backflushing). Backflushing for conditions A and B was performed using 0.1% NaPP. For conditions C, D, and E, 0.01% NaPP was used.
ND, not determined.
In addition to investigation of ultrafiltration using calf serum and/or 0.1% NaPP, experiments were also performed to determine if lower concentrations of NaPP could be as effective as 0.1% NaPP for blocking and sample amendment (Table 2). When using 0.01% NaPP to block ultrafilters, as well as adding NaPP to water samples to a concentration of 0.01% (condition C), retentate and total recoveries for MS2, E. coli, E. faecalis, and microspheres were not significantly different than when using 0.1% NaPP, by two-factor ANOVA (P = 0.58 and 0.98, respectively). With backflushing, mean total recoveries by the condition C ultrafiltration procedure were 59% or higher for all microbial parameters tested, with the exception of B. globigii endospores, for which the mean recovery was 46% (Table 2). Two replicate experiments were also performed using 0.001% NaPP, but the mean total E. coli recovery for these experiments was 48%, so it was decided that higher concentrations of NaPP were preferable for the ultrafiltration protocol (data not shown).
Use of Tween 80 as a water sample amendment.
Based on data for conditions B and C in Table 2, additional experiments with NaPP were performed using 0.01% NaPP for blocking, sample amendment, and backflushing. However, the data for E. faecalis, B. globigii spores, and microspheres suggested that the use of a surfactant (in addition to NaPP) could be effective for minimizing hydrophobic interactions between these particles and filtration system surfaces (and thereby increasing recovery efficiencies). Four replicate experiments were performed with the addition of 0.01% Tween 80 to the 10-liter water samples (condition D), and two replicate experiments were performed with the addition of 0.002% Tween 80 to the water samples. When 0.01% Tween 80 was used as a sample amendment, mean retentate recoveries for all study microbes were 80% or higher (Table 2). Backflushing using a 0.01% NaPP solution was found to improve recoveries only slightly, from 1 to 7%. Mean recoveries using 0.002% Tween 80 were similar to, but slightly lower than, those when 0.01% Tween 80 was used. As shown in Table 2, many of the recovery efficiencies were over 100% when Tween 80 was used as a sample amendment. These data suggest that the seed stocks contained microbial aggregates that were not completely monodispersed by the use of vortexing and a diluent containing Tween 80.
While mean recovery efficiencies were generally higher when Tween 80 was added to water samples (versus conditions A, B, and C), the Tween 80 addition was observed to cause significant fouling of the ultrafilters. Without the addition of Tween 80, permeate rates (for conditions A, B, and C) were approximately 810 ml/min at a system pressure of 7.2 lb/in2 (data not shown). The total filtration time for these experiments (conditions A, B, and C) was approximately 13 min (range, 12 to 14 min). With 0.01% Tween 80 in the water samples, average permeate rates were 330 ml/min, even with higher average system pressures of approximately 9.7 lb/in2. Fouling was also observed when 0.002% Tween 80 was added to water samples. When 0.002% Tween 80 was used, average permeate rates were 580 ml/min at an average system pressure of 9.3 lb/in2. Total filtration times for the 0.01% Tween 80 sample amendment experiments were 32 min on average (range, 27 to 42 min), whereas the filtration times when 0.002% Tween 80 was used were 15 to 24 min. The overarching objective of this method development work was to develop a technique that could be used to process larger volumes of water (100 liters and higher). Thus, due to the fouling observed with Tween 80 sample amendments, it was decided that addition of Tween 80 to water samples was not practical under the conditions being studied. However, Tween 80 was studied further as a reagent for backflushing the ultrafilters.
Effect of Tween surfactants for backflushing.
Four basic ultrafiltration and backflushing conditions were investigated using the suite of pathogens and pathogen surrogates shown in Table 3. Total average filtration times for the four conditions were the same (approximately 13 min). The baseline experiments were performed without the use of NaPP or Tween for blocking, sample amendment, or backflushing (i.e., no treatment of the filters or water samples was performed, and backflushing was performed with tap water). When 0.01% NaPP was used for these procedures, mean microbial recoveries (including backflushing) were approximately 60% or higher, with the exception of B. globigii endospores, for which the mean recovery was 45%. The least-square mean microbial recovery for the baseline experiments (62%) was lower than that for the 0.01% NaPP-only experiments (68%). However, the recovery efficiencies measured for the 0.01% NaPP ultrafiltration experiments were not significantly higher for the suite of study microbes than those under the baseline condition (P = 0.26). When the backflush solution contained 0.01% Tween 80 and 0.01% Tween 20, mean recovery efficiencies for the microbial parameters were generally higher than when only 0.01% NaPP was used. The presence of 0.01% Tween 80 and 0.01% Tween 20 in the backflush solution was associated with significantly higher recoveries of the microbes in the suite shown in Table 3 than were obtained under the 0.01% NaPP-only condition (P = 0.009).
TABLE 3.
Simultaneous ultrafiltration recovery efficiencies using NaPP and different backflushing conditions
| Conditionsa | n | Mean % recovery ± SD
|
||||||
|---|---|---|---|---|---|---|---|---|
| MS2 | Echovirus | Salmonella | E. faecalis | B. globigii spores | C. parvum | Microspheres | ||
| No amendment/tap water | 3-4 | 34 ± 28 | 69 ± 15 | 67 ± 2 | 80 ± 24 | 33 ± 7 | 45 ± 36 | 81 ± 8 |
| 0.01% NaPP/0.01% NaPP | 3-6 | 59 ± 10 | 62 ± 12 | 63 ± 8 | 65 ± 18 | 45 ± 17 | 98 ± 17 | 79 ± 15 |
| 0.01% NaPP/0.01% Tween 80 + 0.01% Tween 20 | 3-4 | 65 ± 35 | 97 ± 58 | 74 ± 15 | 79 ± 20 | 70 ± 19 | 97 ± 20 | 98 ± 11 |
| 0.01% NaPP/0.5-1% Tween 80 | 5-8 | 91 ± 33 | 49 ± 47 | 70 ± 13 | 83 ± 13 | 84 ± 47 | 83 ± 17 | 102 ± 23 |
Either 0.01% NaPP was added to water samples and used for blocking filters, or no amendment of the sample or filter blocking was performed. The other reagents shown were used for backflushing.
For statistical purposes, ultrafiltration experiments performed with 0.5% Tween 80 (n = 2) and 1% Tween 80 (n = 5) in backflush solutions were combined for comparison with experiments in which the 0.01% Tween backflush solution was used. When these higher concentrations of Tween 80 (0.5 to 1%) were used in the backflush solution (and no Tween 20 was present), mean recoveries of most of the microbes, including B. globigii endospores, were 70% or higher (Table 3). The mean echovirus 1 recovery when using 0.5 to 1% Tween 80 backflushing was 49%, but the high variability in the echovirus 1 recovery for the five replicate experiments makes it difficult to fully assess the effectiveness of this ultrafilter condition for echovirus 1 recovery versus other conditions studied. The presence of 0.5 to 1% Tween 80 in the backflush solution was not associated with significantly higher microbial recoveries than the condition where 0.01% Tween 80 plus 0.01% Tween 20 was used for backflushing (P = 0.86), but it was associated with significantly higher recoveries than under the baseline condition (P = 0.015). UV absorbance measurements of the breakthrough of Tween 80 surfactant solutions through the ultrafilter indicated that five to six times as much Tween 80 was able to pass through the ultrafilter when a 0.5% Tween 80 solution was used for backflushing than when 0.01% Tween 80 was used (data not shown).
Although not the focus of this study, real-time (TaqMan) PCR and reverse transcription-PCR were performed using a R.A.P.I.D. real-time thermal cycler (Idaho Technology, Inc., Salt Lake City, UT) to determine if the NaPP and Tween 80 reagents were associated with PCR inhibition. No PCR inhibition was observed when approximately 1,000 infectious units of adenovirus 40 and echovirus 1 were seeded into 0.01% NaPP, 0.1% NaPP, and 0.01% Tween 80 solutions and the real-time PCR fluorescence curves (and crossing thresholds) were compared to real-time PCR results for the same virus seedings in nuclease-free reagent water (data not shown).
DISCUSSION
This study demonstrates that viruses, bacteria, and parasites can be simultaneously concentrated and efficiently recovered from tap water by using ultrafiltration. This study was performed using relatively high microbial seeding levels (106 PFU, CFU, or oocysts) in order to enable us to directly assay pre- and postultrafiltration samples by using methods requiring small analytical volumes (e.g., Cryptosporidium direct immunofluorescence assay microscopy and echovirus tissue culture). After the present study, replicate experiments were performed to test ultrafiltration recovery efficiencies for low seed levels. These experiments used the last condition as reported in Table 3 (0.01% NaPP-0.5% Tween 80 backflushing) and 1,000 of each organism (or particle) reported in Table 3 in 100 liters of water. Microbe and microsphere recovery efficiencies were calculated (data not shown) and found to be similar to the recoveries reported here (even with the necessary incorporation of secondary concentration procedures). The recovery data reported here reflect the efficiencies of the ultrafiltration procedures without incorporating inherent inefficiencies of other secondary concentration procedures (e.g., centrifugation or immunomagnetic separation). Similar to the present study, other researchers have demonstrated the application of hollow-fiber ultrafiltration for simultaneous recovery of diverse microbe types. The work of Morales-Morales et al. (13) showed that an ultrafilter having a MWCO of 50,000 was capable of recovering greater than 50% of E. coli cells, bacteriophages (T1 and PP7), and C. parvum oocysts when the ultrafilter was blocked with calf serum, Tween 80 (0.1%, vol/vol) was used as a sample amendment, and the filter was eluted with 0.05 M glycine. Morales-Morales et al. did not report permeate rate data, so it is not clear whether the use of Tween 80 as a sample amendment in their study was associated with membrane fouling and reduced permeate rates during ultrafiltration (as was observed in the present study). The present study is novel in that we investigated a class of chemical dispersants, sodium polyphosphate, for minimizing microbial adhesion to the ultrafilter system surfaces. In addition, we report the use of backflushing to recover microbes that adhere to the ultrafilter fibers and are not recovered during collection of the ultrafilter retentate. Backflushing using glycine and beef extract has been previously reported for recovering viruses from hollow-fiber ultrafilters (2). However, backflushing of ultrafilters with surfactants and/or dispersants has not been explored previously for simultaneously recovering viruses, bacteria, and parasites.
The results of this study show that concentrations of 0.01% to 0.1% of a long-chain sodium polyphosphate can improve microbial recovery efficiencies during ultrafiltration when used for blocking and/or sample amendment. These results were anticipated based on the chemicophysical properties of long-chain polyphosphates (e.g., high negative charge and steric effects) and previous studies reporting the effectiveness of polyphosphates for dispersing inorganic colloids (15) and Cryptosporidium oocysts and Giardia cysts (9, 11). Polyphosphates are used as food additives and have been shown to inhibit microbial growth (especially for gram-positive bacteria) under certain conditions in some studies (7). The inhibitory effect on bacterial growth has been hypothesized to be due to the cation-chelating effect of the macromolecules, which can lead to destabilization of a cell's outer membrane and leakage of cations from cells under certain conditions. However, research suggests that the concentration of the long-chain NaPP polymer used in the present study (0.01%) is not likely to cause significant physiological effects on waterborne microbes (8, 10). This dispersing agent was tested in the present study with gram-negative bacteria (E. coli and Salmonella) and gram-positive bacteria (E. faecalis and B. globigii endospores). No bactericidal effects were observed in these experiments.
In addition to NaPP, Tween 80 was investigated as a possible sample amendment for decreasing microbial adhesion to ultrafilter system surfaces and thereby increasing microbial recovery efficiencies. While the results of these tests (Table 2) indicated that the use of Tween 80 as a sample amendment was associated with highly efficient microbial recoveries, it was also observed that Tween 80 fouled the ultrafilter fibers and resulted in permeate rate reductions of approximately 30 to 60%. An increase in fouling was observed when the Tween 80 concentration in samples was increased from 0.002% to 0.01%. Correspondingly, the average filtration time for these Tween 80 experiments was 20 to 32 min (for 0.002% and 0.01% Tween 80, respectively) instead of the typical filtration rate of 13 min per 10-liter sample when no Tween 80 was added. As the ultimate goal of this research effort is to develop an ultrafiltration method for concentrating hundreds of liters of water, the permeation rate reductions associated with sample amendment with Tween 80 appear to make its use impractical for concentrating large-volume water samples by using the ultrafilters investigated in the present study.
FBS and calf serum were included in this study because these media have been shown by other researchers to be effective blocking agents for ultrafilters to minimize microbial adhesion and increase recoveries (13, 21). However, we had concerns that the overnight FBS/calf serum blocking protocols used in these studies required too much time for effective use in rapid response to water contamination events. Based on data from the MS2 recovery experiments using FBS blocking and NaPP sample amendment in the present study, it appeared that the overnight filter blocking protocol was more effective than the 1-hour protocol. However, data from FBS blocking without NaPP did not show this difference in the performance of the blocking protocols, so it is possible that further testing of the 1-hour protocol could show it to be as effective as overnight blocking. When experiments using NaPP to block ultrafilters resulted in similar microbial recovery (with backflushing) as when overnight FBS/calf serum blocking was used, we decided to continue using NaPP to block the ultrafilters used in subsequent experiments. The NaPP blocking procedure requires approximately 15 min to complete, as does the backflushing procedure. Backflushing was determined to be a critical component of the ultrafiltration protocol for significantly increasing microbial recoveries. Backflushing of the ultrafilters with surfactant (Tween 20 and/or Tween 80) was found to significantly increase the microbial recovery efficiency of the ultrafiltration protocol. With the exception of the highly variable echovirus data shown in Table 3, use of a backflush solution containing 0.5 to 1% Tween 80, 0.01% NaPP, and 0.001% Antifoam A resulted in overall microbial recovery efficiencies of 70% and higher for the simultaneous recovery of viruses, bacteria, and parasites. In addition, no inhibition of PCR or reverse transcription-PCR was observed for 0.01% NaPP and 0.01% Tween 80 solutions seeded with ∼1,000 infectious units of adenovirus 40 and echovirus 1 and tested using TaqMan assays (5). This ultrafiltration method (using 0.01% NaPP for filter blocking and sample amendment and a 0.5% Tween 80 surfactant solution containing 0.01% NaPP and 0.001% Antifoam A for backflushing) is the focus of continued study in our laboratory for simultaneous recovery and rapid molecular detection of diverse microbes in larger volumes of drinking water and surface water.
Acknowledgments
We thank Kevin Oshima and Geof Smith (New Mexico State University) and Ricardo DeLeon (Metropolitan Water District of Southern California) for their assistance with this project, Mike Arrowood (CDC) for providing C. parvum oocysts, and Gene Rice (U.S. Environmental Protection Agency) for providing B. globigii endospores.
The use of trade names and names of commercial sources is for identification only and does not imply endorsement by the Centers for Disease Control and Prevention or the U.S. Department of Health and Human Services.
REFERENCES
- 1.American Public Health Association. 1998. Standard methods for the examination of water and wastewater, 20th ed. American Public Health Association, New York, N.Y.
- 2.Belfort, G., Y. Rotem-Borensztajn, and E. Katzenelson. 1978. Virus concentration by hollow fiber membranes: where to now? Prog. Water Technol. 10:357-364. [Google Scholar]
- 3.Dziewulski, D. M., and G. Belfort. 1983. Virus concentration from water using high-rate tangential-flow hollow fiber ultrafiltration. Water Sci. Technol. 15:75-89. [Google Scholar]
- 4.Emelko, M. B., and P. M. Huck. 2004. Microspheres as surrogates for Cryptosporidium filtration. J. Am. Water Works Assoc. 96:94-105. [Google Scholar]
- 5.Jothikumar, N., T. L. Cromeans, V. R. Hill, X. Lu, M. D. Sobsey, and D. D. Erdman. 2005. Quantitative real-time PCR assays for detection of human adenoviruses and identification of serotypes 40 and 41. Appl. Environ. Microbiol. 71:3131-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kfir, R., C. Hilner, M. d. Preez, and B. Bateman. 1995. Studies evaluating the applicability of utilising the same concentration techniques for the detection of protozoan parasites and viruses in water. Water Sci. Technol. 31:417-423. [Google Scholar]
- 7.Knabel, S. J., H. W. Walker, and P. A. Hartman. 1991. Inhibition of Aspergillus flavus and selected gram-positive bacteria by chelation of essential metal cations by polyphosphates. J. Food Prot. 54:360-365. [DOI] [PubMed] [Google Scholar]
- 8.Lee, R. M., P. A. Hartman, D. G. Olson, and F. D. Williams. 1994. Bactericidal and bacteriolytic effects of selected food-grade phosphates, using Staphylococcus aureus as a model system. J. Food Prot. 57:276-283. [DOI] [PubMed] [Google Scholar]
- 9.Lepesteur, M., S. Blasdall, and N. J. Ashbolt. 2003. Particle dispersion for further Cryptosporidium and Giardia detection by flow cytometry. Lett. Appl. Microbiol. 37:218-229. [DOI] [PubMed] [Google Scholar]
- 10.Maier, S. K., S. Scherer, and M. J. Loessner. 1999. Long-chain polyphosphate causes cell lysis and inhibits Bacillus cereus septum formation, which is dependent on divalent cations. Appl. Environ. Microbiol. 65:3942-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCuin, R. M., T. M. Hargy, J. E. Amburgey, and J. L. Clancy. 2001. Improving methods for isolation of Cryptosporidium oocysts and Giardia cysts from source and finished waters. AWWA Water Quality Technology Conference, Nashville, Tenn.
- 12.Mendez, J., A. Audicana, A. Isern, J. Llaneza, B. Moreno, M. L. Tarancon, J. Jofre, and F. Lucena. 2004. Standardised evaluation of the performance of a simple membrane filtration-elution method to concentrate bacteriophages from drinking water. J. Virol. Methods 117:19-25. [DOI] [PubMed] [Google Scholar]
- 13.Morales-Morales, H. A., G. Vidal, J. Olszewski, C. M. Rock, D. Dasgupta, K. H. Oshima, and G. B. Smith. 2003. Optimization of a reusable hollow-fiber ultrafilter for simultaneous concentration of enteric bacteria, protozoa, and viruses from water. Appl. Environ. Microbiol. 69:4098-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oshima, K. H. 2001. Efficient and predictable recovery of viruses and Cryptosporidium parvum oocysts from water by ultrafiltration systems. Technical Completion Report 01-4-23949. New Mexico Water Research Resources Institute, New Mexico State University, Las Cruces.
- 15.Papo, A., L. Piani, and R. Ricceri. 2002. Sodium tripolyphosphate and polyphosphate as dispersing agents for kaolin suspensions: rheological characterization. Colloids Surfaces A 201:219-230. [Google Scholar]
- 16.Sharma, M. M., Y. I. Chang, and T. F. Yen. 1985. Reversible and irreversible surface charge modification of bacteria for facilitating transport througnh porous media. Colloids Surfaces 16:193-206. [Google Scholar]
- 17.Simmons, O. D., III, M. D. Sobsey, C. D. Heaney, F. W. Schaefer III, and D. S. Francy. 2001. Concentration and detection of cryptosporidium oocysts in surface water samples by method 1622 using ultrafiltration and capsule filtration. Appl. Environ. Microbiol. 67:1123-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith, G. B. Personal communication.
- 19.U.S. Environmental Protection Agency. 2001. Method 1602: male-specific (F+) and somatic coliphage in water by single agar layer (SAL). Procedure EPA 821-R-01-029. U.S. Environmental Protection Agency, Washington, D.C.
- 20.U.S. Environmental Protection Agency. 2001. Method 1623: Cryptosporidium and Giardia in water by filtration/IMS/FA. EPA-821-R-01-025. U.S. Environmental Protection Agency, Washington, D.C.
- 21.Winona, L. J., A. W. Ommani, J. Olszewski, J. B. Nuzzo, and K. H. Oshima. 2001. Efficient and predictable recovery of viruses from water by small scale ultrafiltration systems. Can. J. Microbiol. 47:1033-1041. [PubMed] [Google Scholar]