Abstract
A simple and sensitive high-performance liquid chromatographic method is described for the determination of colistimethate sodium in plasma and urine. The accuracy and reproducibility was within 10.1 and 11.2% with rat plasma and urine, respectively. Several commonly coadministered antibacterial agents do not interfere with the assay.
Serious infections caused by the multidrug-resistant Pseudomonas aeruginosa are a significant clinical problem (18) and result in high morbidity and mortality among cystic fibrosis patients and immunocompromised hosts (6, 17). Colistin, also known as polymyxin E, was the first antibiotic with notable in vitro activity against P. aeruginosa (1). Although the availability of less toxic antipseudomonal antibiotics relegated colistin to the status of a reserve agent, the subsequent development of multidrug resistance in P. aeruginosa has made colistin of interest once more, as it possesses the advantage of rapid bactericidal activity and only slowly leads to the development of resistance (5, 11). Colistin is a multicomponent polypeptide antibiotic composed mainly of colistin A (polymyxin E1) and colistin B (polymyxin E2) (12, 13). There are two different forms of colistin available commercially, colistin sulfate for oral and topical use and colistimethate sodium (CMS) for parenteral and aerosol therapy. CMS is produced by treating the primary amine groups of the α,γ-diaminobutyric acid residues in colistin with formaldehyde followed by sodium bisulfite.
Although in vitro antibacterial potency is reduced by sulfomethylation (2, 3, 10, 15, 16), toxicity is dramatically decreased and some undesirable side effects, such as painful irritation at subcutaneous or intramuscular injection sites, are avoided (2, 3). CMS has the potential to hydrolyze in aqueous solutions and form an extremely complex mixture of partially sulfomethylated derivatives as well as colistin. These hydrolysis products possess increased antibacterial activities (2, 3).
There are several high-performance liquid chromatography (HPLC) methods for the assay of colistin in biological samples (4, 7, 9), but none has been reported for CMS. Microbiological assay is currently the common analytical method for measuring CMS in biological fluids (8). The present report describes a simple and sensitive HPLC method for the assay of CMS in plasma and urine. It involves accelerated hydrolysis of CMS to colistin in biological fluids, extraction of the generated colistin, and derivatization prior to HPLC analysis according to a previously described method for the quantification of colistin (9).
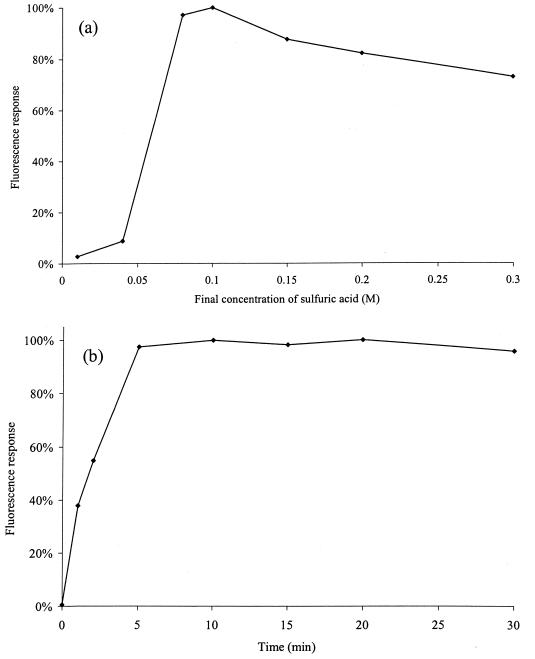
The optimal concentration of sulfuric acid for the hydrolysis of CMS (Sigma, St. Louis, Mo.) in rat plasma was determined by mixing 150-μl samples of drug-free rat plasma, freshly spiked with CMS (25 mg/liter of plasma), with 30 μl of netilmicin sulfate solution (5.0 mg/liter, internal standard; Schering-Plough, Kenilworth, N.J.) and 20 μl of sulfuric acid solution (0.1, 0.4, 0.8, 1.0, 1.5, 2.0, or 3.0 M). After 30 min at room temperature, the reactions were stopped by the addition of 40 μl of sodium hydroxide solution (at a concentration of 0.1, 0.4, 0.8, 1.0, 1.5, 2.0, or 3.0 M, respectively). To determine the optimal time for hydrolysis, samples (150 μl each) of drug-free rat plasma freshly spiked with CMS (25 mg/liter) were mixed with 30 μl of netilmicin sulfate solution (5.0 mg/liter) and 20 μl of sulfuric acid solution (1.0 M). The reactions were stopped at 1, 2, 5, 10, 15, 20, and 30 min by the addition of 40 μl of sodium hydroxide (1.0 M). The fluorescent responses arising from the sample preparation, derivatization, and HPLC analysis (see below) were determined. Analyses were performed in duplicate for each concentration or time point. Mean data for the responses from derivatized colistin under different conditions for hydrolysis of CMS are shown in Fig. 1. The optimum conditions for the hydrolysis of CMS in rat plasma involved a final sulfuric acid concentration of 0.08 to 0.1 M, with a reaction time of 10 min.
FIG. 1.
Effects of the concentration of sulfuric acid (a) and time (b) on the hydrolysis of CMS to colistin in rat plasma. Response is expressed as a percentage of the maximum observed response.
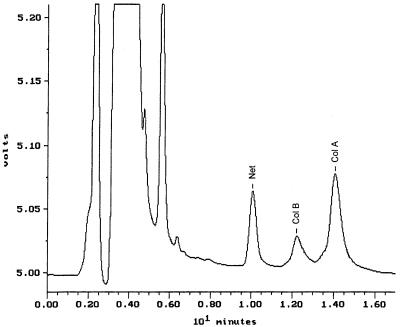
Sample pretreatment included mixing netilmicin sulfate solution (30 μl, 5 mg/liter) with 150 μl of plasma or 200 μl of urine. After the addition of 20 μl of sulfuric acid (1.0 M), CMS was hydrolyzed for 10 min and the reactions were stopped by the addition of 40 μl of sodium hydroxide (1.0 M). The entire mixture was pretreated and transferred to a cartridge (Sep-Pak C18, 100 mg; Waters, Milford, Mass.), and colistin was derivatized by procedures described for a previously reported HPLC method for colistin (9). An aliquot (100 μl) of the final solution was chromatographed on the HPLC column (steel, 5-μm-particle-size Ultrasphere C18, 250- by 4.6-mm inside diameter; Alltech, Dandenong, Australia) with a mobile phase of acetonitrile-tetrahydrofuran-water (50:30:20, vol/vol/vol) pumped at 1 ml/min. Modification of the previous mobile phase (9) gave a much shorter HPLC run time (18 min), which improved the speed and the economy of the assay. Fluorescence was measured at 315 nm following excitation at 260 nm, with the gain set at ×100. A typical chromatogram from plasma collected from a rat at 10 min after an intravenous bolus of CMS (15 mg/kg of body weight) is shown in Fig. 2. The derivatives were eluted in the order of netilmicin, colistin B, and colistin A. Analysis of drug-free rat plasma showed that there were no peaks at the corresponding retention times of the peaks of interest.
FIG. 2.
Chromatogram of a rat plasma sample collected 10 min after an intravenous bolus of 15 mg of CMS/kg. Net, Col B, Col A, peaks of netilmicin, colistin A, and colistin B, respectively.
Calibration curves for CMS in rat plasma were prepared at concentrations of 0.33, 0.67, 2.7, 5.3, 6.7, 13.3, 26.7, and 53.3 mg/liter and in rat urine at concentrations of 0.25, 0.5, 2.0, 4.0, 5.0, 10, 20, and 40 mg/liter. Linear calibration curves were constructed from the relationship between the ratios of the summed peak areas of the derivatives of colistin A and B to that of netilmicin and concentrations of CMS. Reproducibility and accuracy were assessed by the intraday and interday assays with quality control samples containing 1.3 and 40.0 mg of CMS per liter of rat plasma or 1.0 and 30 mg/liter of rat urine. Data on the accuracy and reproducibility of the assay for plasma and urine are presented in Table 1. Regression coefficients for calibration curves for plasma and urine were greater than 0.995 and 0.983, respectively. The linear-regression equation for rat plasma had a mean slope ± standard deviation of 0.112 ± 0.010 and an intercept of −0.055 ± 0.038 (n = 3); corresponding values for rat urine were 0.178 ± 0.026 and −0.008 ± 0.079 (n = 3), respectively. The different volumes of plasma and urine account for the difference in mean slopes. Rat plasma containing CMS at a concentration of 26.7 mg/liter was assayed in triplicate as described above. Derivatives eluted from the cartridge and kept at an ambient temperature for 24 h gave a mean fluorescent response of 95% (n = 3) of the value for the freshly eluted derivatives. This demonstrated that pretreatment with sulfuric acid did not affect the stability of the colistin-9-fluorenylmethoxy carbonyl (FMOC) derivatives, which remained stable for at least 24 h at an ambient temperature. Duplicate samples of drug-free rat plasma containing colistin (2.0 mg/liter) and pretreated with sulfuric acid (1.0 M) for 30 min gave a mean fluorescence response which was 102% of the response from samples without pretreatment (n = 2). This indicated that colistin formed from hydrolysis of CMS was stable under these conditions and that its derivatization with FMOC-Cl was not affected by pretreatment with sulfuric acid. The validation confirmed the reliability of the HPLC method for measuring concentrations of CMS in rat plasma and urine. Furthermore, this method has been found to give similar accuracy and reproducibility when it is applied to human plasma (0.97 ± 0.03 and 29.2 ± 1.4 mg/liter for quality control samples containing 1.0 and 30 mg of CMS per liter, respectively; n = 6). The HPLC method has been used for pharmacokinetic studies of rats and humans, and the results will be reported separately.
TABLE 1.
Reproducibility and accuracy of assay of CMS in rat plasma and urine
| Time course (no. of samples) | Concn of sample (mg/liter) | Plasma
|
Urine
|
||
|---|---|---|---|---|---|
| Mean concn (mg/liter) | Relative SD (%) | Mean concn (mg/liter) | Relative SD (%) | ||
| Intraday (6) | 1.3 | 1.4 | 8.8 | ||
| 40 | 39 | 2.4 | |||
| 1.0 | 1.0 | 4.7 | |||
| 30 | 30 | 6.2 | |||
| Interday (3) | 1.3 | 1.3 | 10.1 | ||
| 40 | 40 | 4.1 | |||
| 1.0 | 1.0 | 11.2 | |||
| 30 | 29 | 7.8 | |||
In patients with cystic fibrosis, ceftazidime (Glaxo Wellcome, Boronia, Australia), meropenem (Zeneca, Cheshire, United Kingdom), aztreonam (Bristol-Myers Squibb, Noble Park, Australia), piperacillin (Lederle Laboratories, Baulkham Hills, Australia), ciprofloxacin (Bayer AG, Pymble, Australia), tobramycin (Eli Lilly, West Ryde, Australia), or ticarcillin (SmithKline Beecham, Ermington, Australia) may be coadministered with CMS to treat infections caused by P. aeruginosa and flucloxacillin (Rhone-Poulenc Roger, Australia) may be coadministered for infections caused by Staphylococcus aureus. Samples of rat plasma containing these antibiotics at a concentration of 8.0 mg/liter were assayed as described above for CMS. None of these compounds interfered with the chromatographic analysis of the derivatives of colistin and netilmicin.
The method described in this paper measures the summed concentrations of all sulfomethyl derivatives of colistin, including colistin, that are present in samples. When combined with the previously reported method for measuring colistin in plasma (9), it has the advantage of being able to determine both the concentrations of colistin alone and the concentrations of full and partial sulfomethyl derivatives of colistin in samples. The lack of response from plasma freshly spiked with CMS (10 mg/liter) to the HPLC method for colistin (9) proved that no FMOC derivatives from CMS were eluted at the same retention times as those of the FMOC derivatives of colistin. Furthermore, this showed that, following collection of plasma from humans or animals to whom CMS has been administered, sample pretreatment by the method for colistin (9) does not cause any conversion of CMS to colistin.
It should be noted that the concentration of each substituted form of CMS cannot be determined by the method. In addition, in biological samples collected following administration of CMS, the concentrations calculated from the calibration curves are apparent values with reference to those for fresh standard preparations of CMS. The actual mass of CMS in biological samples collected following CMS administration may be less than the apparent value due to a random loss of sulfomethyl groups (each with a molecular weight of 94) during the hydrolysis of CMS in vivo. Therefore, measured values for concentrations of CMS are most appropriately considered as apparent concentrations of CMS, reflecting fully and partially sulfomethylated colistin.
Previous reports have shown that the activity of colistin against Escherichia coli, P. aeruginosa, Enterobacter aerogenes, and Klebsiella pneumoniae ranged from 3- to 10-fold the activity of CMS (10, 15). A rapid increase in antibacterial activity was observed after CMS was stored at 37°C in 0.1 M phosphate buffer (pH 7.0) (3). Work in our laboratory has also shown hydrolysis of CMS to colistin during storage in isotonic phosphate buffer (pH 7.4) and in Mueller-Hinton broth at 37°C. Therefore, with microbiological assays requiring overnight incubation, the antimicrobial activity of the sample increases with time. Our preliminary findings from using the present HPLC method to measure concentrations of fully and partially sulfomethylated colistin (CMS) in the plasma of humans after a dose of CMS and to measure concentrations of colistin alone (9) suggest that the ratio of CMS to colistin changes with time. Hence, any comparison of the concentrations of CMS measured by HPLC and by microbiological methods is rendered meaningless. Furthermore, microbiological assays with samples containing any other antibiotics active against the test strains are likely to be unreliable. A recent report employed derivatization with dansyl chloride and HPLC to measure the concentrations of “colistin” in human plasma and urine after an intravenous dose of CMS (14). However, it was not clear which form, colistin, CMS, or both, was quantified. Additionally, the limit of quantification was relatively high (5 mg/liter with 1 ml of plasma) and the method required greater than 170 min for sample pretreatment.
In summary, a simple HPLC method for the assay of CMS in plasma and urine has been developed and validated. To our knowledge, this is the first report of an HPLC method for the analysis of CMS in biological fluids. The use of the present method to measure apparent concentrations of CMS and of a separate HPLC method to measure the more microbiologically active colistin (9) is substantially better than using the only other HPLC method reported to date (14) or similarly less specific microbiological methods. For those performing pharmacokinetic studies with CMS, the combined use of two HPLC methods will be of far greater value than previous assays and will allow a more detailed description of the pharmacokinetics of CMS than has hitherto been possible.
REFERENCES
- 1.Banerjee, D., and D. Stableforth. 2000. The treatment of respiratory pseudomonas infection in cystic fibrosis: what drug and which way? Drugs 60:1053-1064. [DOI] [PubMed] [Google Scholar]
- 2.Barnett, M., S. R. Bushby, and S. Wilkinson. 1964. Sodium sulphomethyl derivatives of polymyxins. Br. J. Pharmacol. 23:552-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beveridge, E. G., and A. J. Martin. 1967. Sodium sulphomethyl derivatives of polymyxins. Br. J. Pharmacol. Chemother. 29:125-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Decolin, D., P. Leroy, A. Nicolas, and P. Archimbault. 1997. Hyphenated liquid chromatographic method for the determination of colistin residues in bovine tissues. J. Chromatogr. Sci. 35:557-564. [DOI] [PubMed] [Google Scholar]
- 5.Evans, M. E., D. J. Feola, and R. P. Rapp. 1999. Polymyxin B sulfate and colistin: old antibiotics for emerging multiresistant gram-negative bacteria. Ann. Pharmacother. 33:960-967. [DOI] [PubMed] [Google Scholar]
- 6.Giamarellou, H., and A. Antoniadou. 2001. Antipseudomonal antibiotics. Med. Clin. N. Am. 85:19-42. [DOI] [PubMed] [Google Scholar]
- 7.Le Brun, P. P. H., A. I. de Graaf, and A. Vinks. 2000. High-performance liquid chromatographic method for the determination of colistin in serum. Ther. Drug Monit. 22:589-593. [DOI] [PubMed] [Google Scholar]
- 8.Leroy, P., D. Decolin, S. Nicolas, P. Archimbault, and A. Nicolas. 1989. Residue determination of two co-administered antibacterial agents—cephalexin and colistin—in calf tissues using high-performance liquid chromatography and microbiological methods. J. Pharm. Biomed. Anal. 7:1837-1846. [DOI] [PubMed] [Google Scholar]
- 9.Li, J., R. W. Milne, R. L. Nation, J. D. Turnidge, K. Coulthard, and D. W. Johnson. 2001. A simple method for the assay of colistin in human plasma, using pre-column derivatization with 9-fluorenylmethyl chloroformate in solid-phase extraction cartridges and reversed-phase high-performance liquid chromatography. J. Chromatogr. B 761:167-175. [DOI] [PubMed] [Google Scholar]
- 10.Li, J., J. Turnidge, R. Milne, R. L. Nation, and K. Coulthard. 2001. In vitro pharmacodynamic properties of colistin and colistin methanesulfonate against Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Antimicrob. Agents Chemother. 45:781-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Littlewood, J. M., C. Koch, P. A. Lambert, N. Hoiby, J. S. Elborn, S. P. Conway, R. Dinwiddie, and F. Duncan-Skingle. 2000. A ten year review of colomycin. Respir. Med. 94:632-640. [DOI] [PubMed] [Google Scholar]
- 12.Orwa, J. A., C. Govaerts, R. Busson, E. Roets, A. Van Schepdael, and J. Hoogmartens. 2001. Isolation and structural characterization of colistin components. J. Antibiot. 54:595-599. [DOI] [PubMed] [Google Scholar]
- 13.Orwa, J. A., A. Van Gerven, E. Roets, and J. Hoogmartens. 2000. Development and validation of a liquid chromatography method for analysis of colistin sulphate. Chromatographia 51:433-436. [Google Scholar]
- 14.Reed, M. D., R. C. Stern, M. A. O'Riordan, and J. L. Blumer. 2001. The pharmacokinetics of colistin in patients with cystic fibrosis. J. Clin. Pharmacol. 41:645-654. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz, B. S., M. R. Warren, F. A. Barkley, and L. Landis. 1960. Microbiological and pharmacological studies of colistin sulphate and sodium colistin methanesulfonate. Antibiot. Annu. 7:41-60. [PubMed] [Google Scholar]
- 16.Stansly, P. G., R. G. Shepherd, and H. J. White. 1947. Polymyxin: a new chemotherapeutic agent. Bull. Johns Hopkins Hosp. 81:43-54. [PubMed] [Google Scholar]
- 17.Tatterson, L. E., J. F. Poschet, A. Firoved, J. Skidmore, and V. Deretic. 2001. CFTR and pseudomonas infections in cystic fibrosis. Front. Biosci. 6: D890-D897. [DOI] [PubMed] [Google Scholar]
- 18.Waterer, G. W., and R. G. Wunderink. 2001. Increasing threat of Gram-negative bacteria. Crit. Care Med. 29:N75-N81. [DOI] [PubMed] [Google Scholar]