Abstract
Chemically defined media allow for a variety of metabolic studies that are not possible with undefined media. A defined medium, AM3, was created to expand the experimental opportunities for investigating the fermentative metabolism of succinate-producing Actinobacillus succinogenes. AM3 is a phosphate-buffered medium containing vitamins, minerals, NH4Cl as the main nitrogen source, and glutamate, cysteine, and methionine as required amino acids. A. succinogenes growth trends and end product distributions in AM3 and rich medium fermentations were compared. The effects of NaHCO3 concentration in AM3 on end product distribution, growth rate, and metabolic rates were also examined. The A. succinogenes growth rate was 1.3 to 1.4 times higher at an NaHCO3 concentration of 25 mM than at any other NaHCO3 concentration, likely because both energy-producing metabolic branches (i.e., the succinate-producing branch and the formate-, acetate-, and ethanol-producing branch) were functioning at relatively high rates in the presence of 25 mM bicarbonate. To improve the accuracy of the A. succinogenes metabolic map, the reasons for A. succinogenes glutamate auxotrophy were examined by enzyme assays and by testing the ability of glutamate precursors to support growth. Enzyme activities were detected for glutamate synthesis that required glutamine or α-ketoglutarate. The inability to synthesize α-ketoglutarate from glucose indicates that at least two tricarboxylic acid cycle-associated enzyme activities are absent in A. succinogenes.
Biobased chemical production is a growing multibillion dollar industry converting renewable resources into valuable products (20, 21). A $15 billion market could be based on succinate for producing bulk chemicals such as 1,4-butanediol (a precursor to “stronger-than-steel” plastics), ethylenediamine disuccinate (a biodegradable chelator), diethyl succinate (a green solvent for replacement of methylene chloride), and adipic acid (nylon precursor) (24). However, the cost of biobased succinate is not yet competitive with petrochemical-based alternatives such as maleic anhydride. The development of a cost-effective industrial succinate fermentation will rely on organisms able to produce high concentrations of succinate at high rates.
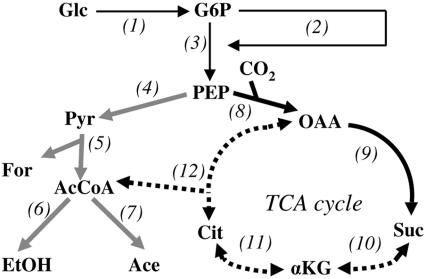
Actinobacillus succinogenes is a capnophilic, facultatively anaerobic, gram-negative bacterium that naturally produces high concentrations of succinate as a fermentation end product in addition to formate, acetate, and ethanol (4-6, 18). A. succinogenes converts glucose to phosphoenolpyruvate (PEP), at which point metabolism splits into the following two branches: (i) the formate-, acetate- and ethanol-producing C3 pathway, and (ii) the succinate-producing C4 pathway (Fig. 1). Metabolic engineering of A. succinogenes has begun, with the aim of achieving a homosuccinate fermentation. The most notable success has arisen from inactivation of pyruvate-formate lyase (PFL) by selecting mutants resistant to fluoroacetate (6, 13). A. succinogenes PFL mutants have increased succinate yields; however, significant amounts of pyruvate are also formed.
FIG. 1.
Simplified metabolic map of the central metabolism of A. succinogenes. Thin black arrows represent glucose uptake, pentose phosphate pathway, and Embden-Meyerhoff-Parnas pathway reactions. Gray arrows represent C3 pathway reactions. Thick black arrows represent C4 pathway reactions. Dashed arrows represent TCA-associated reactions that have not been tested. 1, hexokinase or PEP:glucose phosphotransferase; 2, pentose phosphate pathway; 3, Embden-Meyerhoff-Parnas pathway; 4, pyruvate kinase and PEP:glucose phosphotransferase; 5, pyruvate-formate lyase; 6, acetaldehyde dehydrogenase and alcohol dehydrogenase; 7, phosphotransacetylase and acetate kinase; 8, PEP carboxykinase; 9, malate dehydrogenase, fumarase, and fumarate reductase; 10, succinyl-CoA synthetase, αKG dehydrogenase, and αKG synthase; 11, isocitrate dehydrogenase and aconitase; 12, citrate lyase and citrate synthase. Metabolites: Glc, glucose; G6P, glucose-6-phosphate; Pyr, pyruvate; For, formate; AcCoA, acetyl-CoA; EtOH, ethanol; Ace, acetate; OAA, oxaloacetate; Suc, succinate; Cit, citrate.
Modern, efficient metabolic engineering strategies rely on a thorough understanding of the metabolism under study and of how the metabolism responds to environmental and genetic perturbations (2, 16). This understanding can be obtained by using 13C labeling experiments to measure intracellular metabolic fluxes. These experiments require a defined growth medium so that cell components (e.g., amino acids) are synthesized from a labeled substrate (e.g., [13C]glucose) and not from complex medium components, such as yeast extract. Furthermore, an accurate metabolic map is essential for metabolic flux analyses. A defined medium for growing wild-type A. succinogenes, AM3, is described. A common experiment for succinate-producing capnophiles is conducted using AM3 with different NaHCO3 concentrations, which provides new insights into A. succinogenes metabolism. Finally, we improve the A. succinogenes metabolic map in the poorly characterized region of its tricarboxylic acid (TCA) cycle by using experiments made possible by one of the amino acid auxotrophies of A. succinogenes and by the advent of AM3.
MATERIALS AND METHODS
Chemicals, bacteria, and culture conditions.
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. Escherichia coli K-12 (ATCC 10798) and A. succinogenes type strain 130Z (ATCC 55618) were obtained from the American Type Culture Collection. All liquid cultures were incubated at 37°C and shaken at 250 rpm. Cultures were inoculated with cell suspensions that were harvested in late log phase, washed twice in sterile saline, and resuspended in an appropriate volume of sterile saline to give a starting optical density at 660 nm (OD660) of 0.1 after inoculation.
Identification of a defined growth medium, AM3.
The defined medium was based on the phosphate buffer of the rich medium, medium A, commonly used to grow A. succinogenes (13, 14, 18). It contained the following, per liter: 15.5 g K2HPO4, 8.5 g NaH2PO4 · H2O, 1 g NaCl, 2 g NH4Cl, and 10 ml mineral mix. This basal solution was aliquoted into 28-ml anaerobic test tubes. The tubes were then sealed with rubber bungs and aluminum crimps, repeatedly flushed, and evacuated with N2. After autoclaving, each tube received filter-sterilized vitamin mix, kanamycin, amino acids, glucose, and NaHCO3 to achieve final respective concentrations of 2 ml/liter, 10 μg/ml, 0.08%, 50 mM, and 30 mM. Concentrations of basal solution and supplement stocks were adjusted to give total culture volumes of 10 ml. Soluble NaHCO3 was used instead of insoluble MgCO3 (5, 6, 18) to facilitate growth measurements by optical density. NaHCO3 stock solutions (1 M) were prepared under a 100% CO2 atmosphere, as described previously (19). An equal volume of sterile 100% CO2 was used to replace any volume of NaHCO3 taken from the stock solutions. The final medium pH was 6.9 to 7.1, depending on the amount of NaHCO3 added. The mineral mix was based on Lovley (11) and contained the following, per liter: 1.5 g nitrilotriacetic acid, 3 g MgSO4 · 7H2O, 0.5 g MnSO4 · H2O, 0.1 g FeSO4 · 7H2O, 0.1 g CaCl2 · 2H2O, 0.1 g CoCl2 · 6H2O, 13 mg ZnCl2, 10 mg CuSO4 · 5H2O, 10 mg AlK(SO4)2 · 12H2O, 10 mg H3BO3, 25 mg Na2MoO4, 25 mg NiCl2 · 6H2O, 25 mg Na2WO4 · 2H2O, and 10 mg NaSeO3. The vitamin mix was based on Wolin et al. (22) and contained the following, per liter: 10 mg biotin, 10 mg folic acid, 50 mg pyridoxine HCl, 25 mg thiamine HCl, 25 mg riboflavin, 25 mg nicotinic acid, 25 mg pantothenic acid, 0.5 mg cyanocobalamin, 25 mg p-aminobenzoic acid, and 25 mg thioctic acid.
The inoculum was A. succinogenes 130Z grown from frozen glycerol stocks in 10 ml of BBL Trypticase soy broth (Becton Dickinson, Sparks, MD) containing 50 mM glucose, 30 mM NaHCO3, and 10 μg/ml kanamycin in 15-ml screw-cap glass tubes with air as headspace. The defined medium was inoculated with 0.5 ml of washed cell suspension. The original defined medium supporting growth contained 12 amino acids (i.e., glutamate, aspartate, cysteine, tyrosine, phenylalanine, serine, alanine, isoleucine, valine, arginine, leucine, and methionine) that were chosen based on literature for Haemophilus influenzae defined media (7, 8). Cells grown in this medium were washed and used to inoculate various defined media containing 11 amino acids, each medium missing one of the initial 12 amino acids. This procedure was repeated with fewer and fewer amino acids until the amino acids required for growth were identified. The defined medium with the fewest amino acids still supporting growth was called AM3.
Growth of A. succinogenes on AM3 solid agar.
AM3 agar was prepared as described for the liquid medium, with the addition of 1.5% Bacto agar (Becton Dickinson) and with or without 10 g/liter MgCO3 prior to autoclaving. NaHCO3 was added to some preparations after autoclaving to a final concentration of 30 mM. A. succinogenes was grown in liquid AM3 and washed. After aerobic inoculation, the plates were incubated at 37°C in an anaerobic jar with a CO2 headspace.
Determination of growth trends and fermentation balances in AM3 and medium A.
Anoxic medium A and AM3 (11-ml final volume in 28-ml test tubes) were inoculated with 0.25 ml of washed cells grown in identical media. Medium A differs from AM3 by having 5 g/liter of yeast extract in place of the vitamins, minerals, amino acids, NaCl, and NH4Cl in AM3. Both media contained 150 mM NaHCO3. Growth was monitored throughout log phase by measuring OD660 with a Spectronic 20 (Bausch and Lomb, Rochester, NY) spectrophotometer, which does not require culture sampling. Growth rates were determined from four to six measurements. Samples (<1 ml) were collected at the beginning of incubation, once during log phase (0.6 to 1.0 OD660), and at the end of log phase. The optical densities of these samples were determined using a DU 650 spectrophotometer (Beckman, Fullerton, CA). These OD660 values (more precise than those obtained with the Spectronic 20) were used to calculate the final OD and the carbon and electron balances. Glucose and metabolic end products in the sample supernatants were separated by high-performance liquid chromatography (Waters, Milford, MA) on a 300- by 7.8-mm Aminex HPX-87H column (Bio-Rad, Hercules, CA) at 23°C with 4 mM H2SO4 as the eluent, at a flow rate of 0.6 ml/min. Glucose and ethanol were quantified using a Waters 410 differential refractometer, and organic acids were quantified using a Waters 2487 UV detector at 210 nm.
Determination of fermentation balances, growth rates, and product formation rates in AM3 with different NaHCO3 concentrations.
Anoxic AM3 was prepared as described above but with NaHCO3 concentrations ranging from 5 to 150 mM. The inoculum was 0.25 ml of washed culture grown in AM3 medium of identical NaHCO3 concentrations. Sample collection and determination of cell densities, growth rates, and end product concentrations were performed as described above. CO2 was detected by transferring 1 ml of culture headspace and 0.3 ml of liquid cultures to separate bung-sealed 13-ml serum vials. The liquid sample was acidified with 50 μl of 3.2 N H2SO4. Vial headspace contents were sampled using a pressure syringe and injected into a series 750 gas chromatograph (GOW-MAC, Bethlehem, PA) equipped with a Carbosphere column, methanizer, and flame ionization detector. Specific rates were calculated as described previously for batch cultures (15, 16). For example, to calculate a specific product formation rate, the equation rp = YXP μ, was used, where rp is the specific product formation rate, YXP is the amount of product produced per gram of biomass, and μ is the growth rate.
Preparation of crude cell extracts and enzyme assays.
A. succinogenes 130Z was grown in 450 ml medium A containing 33 mM glucose and 15 mM NaHCO3 in 1-liter spherical flasks with an N2 headspace. Cultures were harvested in log phase by centrifugation, washed once with 200 ml 0.1 M Tris-HCl (pH 7.7), and resuspended in 20 ml 0.1 M Tris-HCl (pH 7.7). Cells were lysed by two passages through a French press at 1,200 to 1,400 lb/in2 under an N2 headspace. Cell extracts were stored at −20°C before assays. The cell extract protein concentration (i.e., 1.8 mg protein/ml) was quantified by the bicinchoninic acid assay (Pierce, Rockford, IL) with bovine serum albumin as the standard (12).
Enzyme activities were assayed by measuring the oxidation or reduction of NADP(H) using a Cary 300 spectrophotometer (Varian, Palo Alto, CA). An extinction coefficient of 6.23 cm−1 mM−1 at 340 nm was used for NADPH (18). Reagents were dissolved in 0.1 M Tris-HCl (pH 8.0), and reactions were carried out in triplicate in 1-ml volumes at 37°C. The reaction mixture for the glutamate dehydrogenase assay contained 40 mM NH4Cl, 5 mM α-ketoglutarate (αKG), 0.3 mM NADPH, 1 mM CaCl2, and 25 μl cell extract. The reaction was started by the addition of αKG. Glutamate synthase activity was tested in the presence of 5 mM glutamine, 5 mM αKG, 0.3 mM NADPH, 1 mM CaCl2, and 25 μl cell extract. The reaction was started by the addition of glutamine. Isocitrate dehydrogenase was assayed using anoxic reagents in rubber-stoppered cuvettes that were evacuated and flushed with N2 as described previously (23). The reaction mixture contained 0.1 M NaCl, 5 mM MgCl2, 1 mM dithiothreitol, 0.3 mM NADP+, 50 μl cell extract, and 5 mM isocitrate (17). Cell extracts (15.2 mg protein/ml) from E. coli K-12 aerobically grown in LB with 25 mM glucose were used as a positive control. The reaction was started with the addition of isocitrate. No enzyme activity was detected in any assay when NAD(H) was used in place of NADP(H).
Test of potential glutamate precursors to support growth.
Anoxic defined medium was prepared as described for AM3, but glutamate and/or NH4Cl was omitted where appropriate. Filter-sterilized stock solutions of potential glutamate precursors (i.e., αKG, glutamine, aspartate, isocitrate, and citrate) were added to the autoclaved medium to 15 mM final concentration. The inoculum was 0.25 ml of A. succinogenes grown in AM3 and washed. The culture volume was 12 ml. The turbidity was monitored using a Spectronic 20 until stationary phase was reached or for 5 days. If growth occurred, cells were washed as described above and used to inoculate an identical medium to ensure that growth was not due to nutrient carryover.
RESULTS AND DISCUSSION
Creation of the defined growth medium, AM3.
A. succinogenes grew slowly (0.06 h−1) when first transferred from Trypticase soy broth to defined medium containing the initial 12 amino acids, with final OD660 values ranging from 0.7 to 1.1. After several transfers in defined medium, growth rates increased to 0.14 h−1. This improvement could be due to a slow response in gene regulation to suit the new growth conditions or to genetic drift. After amino acids were removed from the defined medium one at a time, the amino acid requirements of A. succinogenes were determined to be cysteine, glutamate, and methionine. To improve the A. succinogenes growth rate and final OD in defined medium, concentrations of amino acids, NH4Cl, vitamin mix, and mineral mix were varied, and their effects on growth rate and final OD were determined. Mineral mix, vitamin mix, and amino acids were required for anaerobic growth on glucose. Increasing the vitamin concentration from 2 ml/liter to 10 ml/liter doubled the growth rate and tripled the final OD. A. succinogenes grew without NH4Cl when glutamate, cysteine, and methionine were present, but the growth rate (0.03 ± 0.00 h−1 [mean ± standard deviation]) and final OD660 (0.44 ± 0.07) were low. The improved medium, called AM3, contained the following, per liter: 15.5 g K2HPO4, 8.5 g Na2HPO4 · H2O, 1 g NaCl, 2 g NH4Cl, 0.15 g l-glutamate, 0.08 g l-cysteine-HCl, 0.08 g l-methionine, 10 ml mineral mix, 10 ml vitamin mix, 30 mmol NaHCO3, and 50 mmol glucose.
A. succinogenes also grew on solid AM3 agar. One-millimeter-diameter colonies developed after 2 to 4 days of incubation under CO2 gas phase at 37°C. Colonies developed with and without MgCO3 or NaHCO3.
Growth trends and fermentation balances in AM3 and medium A.
In a defined medium, bacteria are forced to synthesize a number of cellular building blocks that would otherwise be available from rich medium components. For this reason, growth rates were lower in AM3 (0.24 ± 0.01 h−1) than in medium A (0.43 ± 0.01 h−1). The final OD660 in AM3 (2.82 ± 0.05) was slightly lower than that in medium A (3.03 ± 0.14). Since most of the succinate is produced during log phase, fermentation balances were based on log-phase samples. While carbon and electron recoveries for cultures grown in AM3 were near 100%, recoveries for cultures grown in medium A exceeded 100% (Table 1), likely because carbon and electron recoveries take into account only the glucose consumed. The yeast extract carbon in medium A is ∼50% that of the supplied glucose, according to BD Diagnostic Systems and Doyle et al. (1). Thus, there is ample carbon in yeast extract to explain a 117% carbon recovery in medium A. Yeast extract may also have contributed to the higher formate and acetate yields and to the lower succinate product ratio [i.e., succinate/(ethanol plus acetate)] in medium A than in AM3. With no undefined carbon sources to track in AM3, the comparison of fermentation balances in AM3 and medium A illustrates how a chemically defined medium facilitates metabolic studies.
TABLE 1.
Log-phase fermentation balances of A. succinogenes in AM3 and medium Aa
| Medium | Product formed (mmol/100 mmol glucose consumed)
|
Carbon recoveryc (%) | Electron recoveryd (%) | Succinate product ratioe | ||||
|---|---|---|---|---|---|---|---|---|
| Succinate | Formate | Acetate | Ethanol | Biomassb | ||||
| Defined (AM3) | 70 ± 1 | 61 ± 3 | 64 ± 2 | 9 ± 2 | 166 ± 5 | 97 ± 2 | 106 ± 2 | 0.97 ± 0.02 |
| Rich (medium A) | 70 ± 1 | 99 ± 6 | 80 ± 3 | 16 ± 4 | 199 ± 5 | 117 ± 1 | 117 ± 0 | 0.73 ± 0.02 |
Data are means ± standard deviations of results from triplicate cultures.
Biomass was determined using assumed values of 567 mg dry cell weight/ml per OD660 and a cell composition of CH2O0.5N0.2 (24.967 g/mol) (18).
Carbon in product divided by carbon in glucose consumed. An assumption was made that 1 mol of CO2 was fixed per mol of succinate produced (18). Therefore, C3H6O2 was used as the chemical composition of succinate derived from glucose consumed.
Electron recoveries are based on available hydrogen (3).
Ratio of succinate to acetate plus ethanol. It was assumed that all formate is formed from PFL and is accounted for in the sum of acetate and ethanol. Production of less formate than the sum of acetate and ethanol produced may be due to pyruvate dehydrogenase activity; however, this has not yet been proven for A. succinogenes.
Effect of AM3 NaHCO3 concentration on fermentation balances, growth rates, and metabolic rates.
Succinate production by A. succinogenes requires CO2, presumably as a substrate for PEP carboxykinase (Fig. 1) (9, 18). We previously showed that A. succinogenes produces more succinate and less alternative end products when the MgCO3 concentration was increased in medium A (18). To confirm that this trend holds for AM3, we compared end product distributions in AM3 with different NaHCO3 concentrations. Indeed, increasing the NaHCO3 concentration in AM3 increased the succinate product ratio (Table 2). In addition to confirming this trend, we also wanted to determine an optimal NaHCO3 concentration for succinate production in AM3. In our previous study, the maximum MgCO3 concentration tested was equimolar to that of the supplied glucose (i.e., 55 mM) (18). Here we tested NaHCO3 concentrations up to 3 times the molar concentration of supplied glucose. As seen in Table 2, the succinate production ratio plateaued at NaHCO3 concentrations above 75 mM. Although not quantitatively precise, CO2 was detected in all cultures at the time of log-phase sampling (data not shown). Therefore, the reported succinate yields are not due to complete CO2 consumption. While CO2 was not completely consumed, the end product distributions suggest that NaHCO3, at 5 and 25 mM, may be limiting succinate production.
TABLE 2.
Effect of NaHCO3 concentration on end product distribution and growth rate in AM3a
| NaHCO3 concn (mM) | Product formed (mmol/100 mmol glucose consumed)
|
Carbon recovery (%) | Electron recovery (%) | Succinate product ratio | Growth rate (h−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Succinate | Formate | Acetate | Ethanol | Biomass | |||||
| 5 | 33 ± 1 | 117 ± 1 | 72 ± 1 | 51 ± 7 | 146 ± 6 | 101 ± 1 | 109 ± 2 | 0.27 ± 0.02 | 0.24 ± 0.01 |
| 25 | 52 ± 2 | 91 ± 1 | 68 ± 1 | 28 ± 3 | 169 ± 4 | 101 ± 2 | 110 ± 2 | 0.54 ± 0.03 | 0.32 ± 0.01 |
| 75 | 67 ± 1 | 58 ± 5 | 61 ± 3 | 14 ± 1 | 199 ± 10 | 102 ± 4 | 113 ± 4 | 0.90 ± 0.03 | 0.25 ± 0.00 |
| 125 | 71 ± 1 | 60 ± 2 | 64 ± 1 | 10 ± 3 | 176 ± 1 | 100 ± 2 | 110 ± 2 | 0.96 ± 0.05 | 0.23 ± 0.01 |
| 150 | 70 ± 0 | 61 ± 3 | 64 ± 2 | 9 ± 2 | 166 ± 5 | 97 ± 2 | 106 ± 2 | 0.97 ± 0.02 | 0.24 ± 0.01 |
Data are means ± standard deviations of results from triplicate cultures. Biomass, carbon recovery, electron recovery, and succinate product ratio were calculated as described in footnotes b to e in Table 1.
As shown in Table 2, the average growth rate was statistically higher at 25 mM NaHCO3 than at any other NaHCO3 concentration (two-tailed t test, equal variance, P = 0.001). This high growth rate can be explained by the metabolic rates shown in Table 3. At 5 mM NaHCO3, C3 flux (estimated from specific acetate and ethanol formation rates) is high, while C4 flux (i.e., the specific succinate formation rate) is low. At 25 mM NaHCO3, C3 flux remains high, with a maximum acetate formation rate, and C4 flux reaches a maximum. At 75 mM NaHCO3 and above, C4 flux remains high, while C3 flux decreases by about half. ATP can be derived from PEP conversion to succinate via the C4 pathway (1.67 mol ATP) and from ethanol (1 mol ATP) or acetate (2 mol ATP) productions via the C3 pathway. With high C3 and C4 fluxes at 25 mM NaHCO3, more ATP is generated for biosynthesis, explaining the high growth rate under these conditions. The maximum succinate formation rate at 25 mM NaHCO3 also indicates that NaHCO3 is not limiting succinate production, unlike what the end product distributions in Table 2 suggest.
TABLE 3.
Effect of NaHCO3 concentration on specific metabolic rates and estimated fluxes
| NaHCO3 concn (mM) | Specific rate (mmol · g biomass−1 · h−1)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Glucose consumption | Succinate formation or C4 flux | Formate formation | Acetate formation | Ethanol formation | Estimated C3 fluxa | Estimated net ATP formationb | Estimated glycolytic fluxc | |
| 5 | 6.6 ± 0.0 | 2.2 ± 0.1 | 7.8 ± 0.1 | 4.8 ± 0.0 | 3.4 ± 0.4 | 8.2 ± 0.5 | 16.6 ± 0.4 | 10.4 ± 0.4 |
| 25 | 7.7 ± 0.0 | 4.0 ± 0.2 | 7.0 ± 0.1 | 5.3 ± 0.1 | 2.2 ± 0.2 | 7.4 ± 0.2 | 19.4 ± 0.5 | 11.4 ± 0.3 |
| 75 | 5.0 ± 0.2 | 3.4 ± 0.1 | 2.9 ± 0.1 | 3.1 ± 0.0 | 0.7 ± 0.1 | 3.8 ± 0.1 | 12.5 ± 0.2 | 7.2 ± 0.1 |
| 125 | 5.3 ± 0.2 | 3.7 ± 0.1 | 3.2 ± 0.1 | 3.4 ± 0.1 | 0.5 ± 0.1 | 3.9 ± 0.2 | 13.5 ± 0.3 | 7.6 ± 0.2 |
| 150 | 5.7 ± 0.3 | 4.0 ± 0.1 | 3.5 ± 0.1 | 3.6 ± 0.1 | 0.5 ± 0.1 | 4.2 ± 0.2 | 14.5 ± 0.4 | 8.2 ± 0.4 |
Estimated C3 flux is specific acetate formation rate plus specific ethanol formation rate.
Estimated net ATP formation equals estimated ATP formation minus estimated ATP consumption flux in central metabolism. The assumptions of ATP consumption and formation by central metabolic pathways are as follows: (i) glucose uptake consumes 1 ATP either by ATP-utilizing hexokinase or by PEP:glucose phosphotransferase system, preventing ATP production by pyruvate kinase; (ii) 1 ATP is consumed by phosphofructokinase; (iii) pyruvate kinase, acetate kinase, and PEP carboxykinase each produce 1 ATP; and (iv) fumarate reductase produces 0.67 ATP per reaction (10).
Estimated glycolytic flux equals specific succinate production rate plus estimated C3 flux.
It is also worth noting that the high specific product formation rates at 25 mM NaHCO3 are allowed by a high glucose consumption rate (Table 3). This high glucose uptake rate (and expected corresponding high glycolytic flux) should be able to support a succinate formation rate higher than the observed 4 mmol · g biomass−1 · h−1. Not enough is known at this stage to identify the regulatory mechanism or mechanisms behind this observation. Among the possible explanations is that high NaHCO3 concentrations inhibit the C3 pathway and that the C4 pathway cannot process substrate faster than 4 mmol · g biomass−1 · h−1. This bottleneck would in turn inhibit glucose uptake and glycolysis. Another possibility is that NaHCO3 (or CO2) inhibits glucose uptake and/or glycolysis. Any of these mechanisms would have important implications for the metabolic engineering of A. succinogenes succinate production. Future metabolic flux analyses, in which genetic or environmental perturbations affect individual pathway branches, will focus on identifying what limits the succinate production rate in A. succinogenes.
A. succinogenes is missing at least two TCA cycle-associated enzyme activities.
A. succinogenes was found to be auxotrophic for cysteine, methionine, and glutamate. Glutamate auxotrophy was initially surprising since A. succinogenes cell extracts have aspartate:glutamate transaminase activity (18). Figure 2 shows possible enzyme activities leading to glutamate, not all of which are known to be present in A. succinogenes. Several glutamate precursors (i.e., αKG, isocitrate, citrate, and succinate) are TCA cycle intermediates. It is still unclear whether A. succinogenes has a complete TCA cycle (Fig. 1). Because a complete TCA cycle would mean at least two pathways for succinate production and/or consumption, we used the glutamate auxotrophy of A. succinogenes to our advantage to study a poorly characterized region of the A. succinogenes central metabolic map. Table 4 shows that αKG can replace glutamate in the growth medium when NH4Cl is present, indicating in vivo glutamate dehydrogenase activity. Aspartate plus αKG also supported growth, while aspartate alone did not. These results suggest that aspartate:glutamate transaminase is functional in vivo. Alternatively, aspartase activity could convert aspartate to fumarate and NH4+, and then NH4+ used with αKG by glutamate dehydrogenase could produce glutamate. Growth on glutamine indicates the presence of a glutamine deaminating activity (e.g., glutamine synthetase or carbamoyl phosphate synthetase). In vitro enzyme activity assays suggested that glutamate dehydrogenase (1,100 ± 180 nmol · min−1 · mg protein−1) and glutamate synthase (30 ± 10 nmol · min−1 · mg protein−1) are also functional in A. succinogenes. Taken together, these results suggest that all the enzyme activities (i.e., those numbered 3, 6, 7, and 8) below αKG in Fig. 2 are present in A. succinogenes.
FIG. 2.
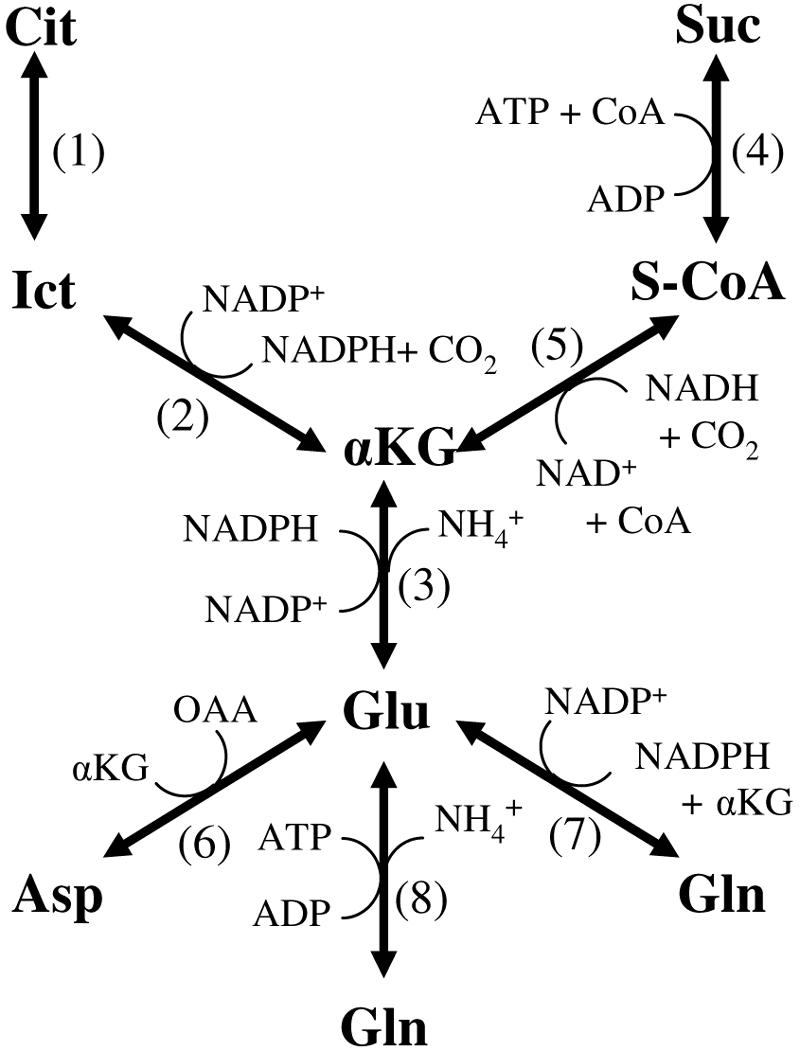
Possible enzyme activities leading to glutamate synthesis. 1, aconitase; 2, isocitrate dehydrogenase; 3, glutamate dehydrogenase; 4, succinyl-CoA synthetase; 5, αKG dehydrogenase; 6, aspartate:glutamate transaminase; 7, glutamate synthase; 8, glutamine synthetase. Metabolites include isocitrate (Ict) and succinyl-CoA (S-CoA).
TABLE 4.
Ability of glutamate precursors to support growth of A. succinogenes in AM3
| Glutamate precursor | Growtha |
|---|---|
| NH4+ | − |
| NH4+ + Glu | + |
| NH4+ + αKG | + |
| Gln | + |
| Asp | − |
| Asp + αKG | + |
Growth (+) is defined by an OD660 of >1.5 within 24 h. No growth (−) is defined by no OD660 increase within 5 days after inoculation. Tests were performed at least in duplicate.
These results also point to a single reason for A. succinogenes's glutamate auxotrophy: A. succinogenes cannot synthesize αKG from glucose. This inability means that enzymes are absent or inactive in two pathways: (i) between succinate and αKG in the reverse TCA cycle (especially since A. succinogenes produces ample succinate), and (ii) in the TCA cycle from acetyl-coenzyme A (acetyl-CoA) and oxaloacetate to citrate to αKG (Fig. 1). This conclusion is supported in part by the fact that no in vitro isocitrate dehydrogenase activity could be detected in either anaerobically or aerobically grown A. succinogenes cell extracts, while it was detected in E. coli cell extracts as a positive control [70 ± 10 nmol NADP(H) min−1 mg protein−1]. Growth experiments with citrate or isocitrate were not informative. A. succinogenes did not grow when citrate or isocitrate was supplied with NH4Cl or aspartate (data not shown) for at least two reasons: (i) it is not known whether citrate and isocitrate are taken up by A. succinogenes cells, and (ii) citrate prevented A. succinogenes growth at concentrations above 3 mM in the presence of glutamine or glutamate (data not shown). This inhibition was countered by adding extra minerals (data not shown), suggesting that citrate binds essential minerals (e.g., iron) and prevents mineral acquisition.
A. succinogenes is a promising catalyst for biobased production of succinate and possibly other chemicals (e.g., malate, fumarate, 5-aminolevulinate, αKG, and glutamate). We have described a chemically defined medium for growing A. succinogenes and for studying its metabolism. NaHCO3 concentrations between 5 and 75 mM had pronounced effects on fermentation end product distributions, but higher concentrations of NaHCO3 did not. A. succinogenes had an optimal growth rate at 25 mM NaHCO3, where both energy-producing pathways displayed their highest fluxes. αKG could be used in place of glutamate to support growth, indicating that at least two TCA cycle-associated enzyme activities are absent. The defined medium made testing growth on glutamate precursors possible. The discovery that A. succinogenes lacks a full TCA cycle is key information for the construction of an accurate A. succinogenes metabolic map that will be essential in future metabolic flux analyses and practical metabolic engineering designs for A. succinogenes-based chemical production.
Acknowledgments
This work was supported by National Science Foundation grant BES-0224596. James McKinlay was supported by a Michigan State University Quantitative Biology and Modeling Initiative fellowship.
We thank John Breznak, Yair Shachar-Hill, Harini Krishnamurthy, and Maris Laivenieks for helpful discussions.
REFERENCES
- 1.Doyle, A., M. N. Weintraub, and J. P. Schimel. 2004. Persulfate digestion and simultaneous colorimetric analysis of carbon and nitrogen in soil extracts. Soil Sci. Soc. Am. J. 68:669-676. [Google Scholar]
- 2.Fell, D. 1997. Understanding the control of metabolism. Portland Press, London, United Kingdom.
- 3.Gottschalk, G. 1986. Bacterial metabolism, 2nd ed. Springer-Verlag, New York, N.Y.
- 4.Guettler, M. V., D. Rumler, and M. K. Jain. 1999. Actinobacillus succinogenes sp. nov., a novel succinic-acid-producing strain from the bovine rumen. Int. J. Syst. Bacteriol. 49:207-216. [DOI] [PubMed] [Google Scholar]
- 5.Guettler, M. V., M. K. Jain, and B. K. Soni. April. 1996. Process for making succinic acid, microorganisms for use in the process and methods of obtaining the microorganisms. U.S. patent 5,504,004.
- 6.Guettler, M. V., M. K. Jain, and D. Rumler. November. 1996. Method for making succinic acid, bacterial variants for use in the process, and methods for obtaining variants. U.S. patent 5,573,931.
- 7.Herroitt, R. M., E. M. Meyer, and M. Vogt. 1970. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J. Bacteriol. 101:517-524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herroitt, R. M., E. Y. Meyer, M. Vogt, and M. Modan. 1970. Defined medium for growth of Haemophilus influenzae. J. Bacteriol. 101:513-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim, P., M. Laivenieks, C. Vieille, and J. G. Zeikus. 2004. Effect of overexpression of Actinobacillus succinogenes phosphoenolpyruvate carboxykinase on succinate production in Escherichia coli. Appl. Environ. Microbiol. 70:1238-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kroger, A., S. Biel, J. Simon, R. Gross, G. Unden, and C. R. D. Lancaster. 2002. Fumarate respiration of Wolinella succinogenes: enzymology, energetics and coupling mechanism. Biochim. Biophys. Acta 1553:23-28. [DOI] [PubMed] [Google Scholar]
- 11.Lovely, D. 2000. Dissimilatory Fe(III)- and Mn(IV)-reducing prokaryotes. .In Martin Dworkin (ed.), The prokaryotes. Springer-Verlag [Online.] http://141.150.157.117:8080/prokPUB/index.htm. Accessed December 2003.
- 12.McKinlay, J. B., and J. G. Zeikus. 2004. Extracellular iron reduction is mediated in part by neutral red and hydrogenase in Escherichia coli. Appl. Environ. Microbiol. 70:3467-3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park, D. H., M. Laivenieks, M. V. Guettler, M. K. Jain, and J. G. Zeikus. 1999. Microbial utilization of electrically reduced neutral red as the sole electron donor for growth and metabolite production. Appl. Environ. Microbiol. 65:2912-2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park, D. H., and J. G. Zeikus. 1999. Utilization of electrically reduced neutral red by Actinobacillus succinogenes: physiological function of neutral red in membrane-driven fumarate reduction and energy conservation. J. Bacteriol. 181:2403-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sauer, U., D. R. Lasko, J. Fiaux, M. Hochuli, R. Glaser, T. Szyperski, K. Würthrich, and J. E. Bailey. 1999. Metabolic flux ratio analysis of genetic and environmental modulations of Escherichia coli central carbon metabolism. J. Bacteriol. 181:6679-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephanopoulos, G. N., A. A. Aristidou, and J. Nielsen. 1998. Metabolic engineering: principles and methodologies. Academic Press, London, United Kingdom.
- 17.Thorsness, P. E., and D. E. Koshland, Jr. 1987. Inactivation of isocitrate dehydrogenase by phosphorylation is mediated by the negative charge of the phosphate. J. Biol. Chem. 262:10422-10425. [PubMed] [Google Scholar]
- 18.van der Werf, M. J., M. V. Guettler, M. K. Jain, and J. G. Zeikus. 1997. Environmental and physiological factors affecting the succinate product ratio during carbohydrate fermentation by Actinobacillus sp. 130Z. Arch. Microbiol. 167:332-342. [DOI] [PubMed] [Google Scholar]
- 19.Widdel, F., and F. Bak. 1992. Gram-negative mesophilic sulfate-reducing bacteria, p. 3358-3378. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K. H. Schleifer (ed.), The prokaryotes, 2nd ed., vol. 4. Springer-Verlag, New York, N.Y. [Google Scholar]
- 20.Wilke, D. 1999. Chemicals from biotechnology: molecular plant genetics will challenge the chemical and fermentation industry. Appl. Microbiol. Biotechnol. 52:135-145. [DOI] [PubMed] [Google Scholar]
- 21.Wilke, D. 1995. What should and what can biotechnology contribute to chemical bulk production? FEMS Microbiol. Rev. 16:89-100. [Google Scholar]
- 22.Wolin, E., M. Wolin, and R. Wolfe. 1963. Formation of methane by bacterial extracts. J. Biol. Chem. 238:2882-2886. [PubMed] [Google Scholar]
- 23.Zeikus, J. G., G. Fuchs, W. Kenealy, and R. K. Thauer. 1977. Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J. Bacteriol. 132:604-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeikus, J. G., M. K. Jain, and P. Elankovan. 1999. Biotechnology of succinic acid production and markets for derived industrial products. Appl. Microbiol. Biotechnol. 51:545-552. [Google Scholar]