Abstract
Bacterial enoyl-acyl carrier protein (ACP) reductase (FabI) catalyzes the final step in each elongation cycle of bacterial fatty acid biosynthesis and is an attractive target for the development of new antibacterial agents. High-throughput screening of the Staphylococcus aureus FabI enzyme identified a novel, weak inhibitor with no detectable antibacterial activity against S. aureus. Iterative medicinal chemistry and X-ray crystal structure-based design led to the identification of compound 4 [(E)-N-methyl-N-(2-methyl-1H-indol-3-ylmethyl)-3-(7-oxo-5,6,7,8-tetrahydro-1,8-naphthyridin-3-yl)acrylamide], which is 350-fold more potent than the original lead compound obtained by high-throughput screening in the FabI inhibition assay. Compound 4 has exquisite antistaphylococci activity, achieving MICs at which 90% of isolates are inhibited more than 500 times lower than those of nine currently available antibiotics against a panel of multidrug-resistant strains of S. aureus and Staphylococcus epidermidis. Furthermore, compound 4 exhibits excellent in vivo efficacy in an S. aureus infection model in rats. Biochemical and genetic approaches have confirmed that the mode of antibacterial action of compound 4 and related compounds is via inhibition of FabI. Compound 4 also exhibits weak FabK inhibitory activity, which may explain its antibacterial activity against Streptococcus pneumoniae and Enterococcus faecalis, which depend on FabK and both FabK and FabI, respectively, for their enoyl-ACP reductase function. These results show that compound 4 is representative of a new, totally synthetic series of antibacterial agents that has the potential to provide novel alternatives for the treatment of S. aureus infections that are resistant to our present armory of antibiotics.
The emergence of antibacterial resistance is a growing worldwide problem and governs the need for new drugs directed at novel antibacterial targets (2, 14). However, over the last two decades, only one new class of antibiotics has been commercialized, and there is a concerning dearth of antibacterials with novel mechanisms of action in development. Bacterial fatty acid biosynthesis is an essential process that supplies precursors for the assembly of important cellular components, including phospholipids, lipoproteins, lipopolysaccharides, mycolic acids, and the cell envelope. In mammals, all enzymatic activities associated with acyl chain elongation are encoded by a single polypeptide, whereas in bacteria, the pathway is comprised of several discrete enzymes. This difference in organization makes the bacterial fatty acid biosynthetic enzymes potentially selective antibacterial targets. Prior to the availability of entire bacterial genomes, the majority of work on bacterial fatty acid biosynthesis focused on Escherichia coli. However, recent access to fully sequenced and assembled bacterial genomes has now enabled the identification of all the pathway enzymes in a variety of important clinical pathogens. This has permitted informed judgments to be made on the prospective spectrum of each of the component enzymes (12).
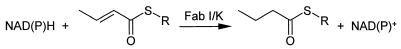
The last step in the fatty acid biosynthetic pathway is performed by enoyl-acyl carrier protein (ACP) reductase (FabI), which is responsible for reduction of the double bond in the enoyl-ACP derivative (Fig. 1) (5). In Staphylococcus aureus and E. coli this enzyme has been shown to be the antibacterial target of triclosan and diazaborines, thereby demonstrating the essentiality of FabI in these organisms (1, 3, 4, 9, 15). Prior to the genomics era, FabI was believed to be the only enoyl-ACP reductase in bacteria. However, access to and analysis of key bacterial genomes demonstrated that FabI is absent in some organisms, and an alternative enoyl-ACP reductase, FabK, is present in several important clinical pathogens (Fig. 1) (6). For example, FabK is the sole enoyl-ACP reductase in Streptococcus pneumoniae and both FabI and FabK have been found in pathogens such as Enterococcus faecalis and Pseudomonas aeruginosa (6). Consequently, FabI represents a selective antibacterial target for those pathogens such as S. aureus wherein FabI is the sole enoyl-ACP reductase. Alternatively, a compound that possesses inhibitory potency against both FabK and FabI would be expected to possess a far broader spectrum of antibacterial activity.
FIG. 1.
Reaction scheme for bacterial enoyl-ACP reductase (FabI).
In the present communication, we report on the discovery and characterization of a new class of FabI-directed antibacterials, representatives of which demonstrate clinical potential.
(This material was presented in part at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 16 to 19 December 2001 [D. J. Payne et al., 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1688, 2002; W. H. Miller et al., 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1689, 2001; M. A. Seefeld et al., 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1690, 2001].)
MATERIALS AND METHODS
Bacterial strains.
The bacterial strains included in the antimicrobial activity assays were obtained from the GlaxoSmithKline culture collection, Upper Providence, Pa. Included were standard laboratory and reference strains as well as isolates from various clinical sources. Efflux knockouts, LS-2 (acrA knockout) and LS-3 (acrB knockout) (13), derived from Haemophilus influenzae RD-51907, were obtained from H. Nikaido (University of California, Berkeley).
Enzyme inhibition assays.
Enzyme inhibition assays were carried out in half-area, 96-well microtiter plates. Compounds were evaluated in 150-μl (FabI) or 100-μl (FabK) assay mixtures containing components specific for each enzyme (see below). Inhibitor concentrations were typically varied over the range of 0.01 to 10 μM. The consumption of NAD(P)H was monitored for 20 min at 30°C by following the change in absorbance at 340 nm (Fig. 1). Fifty percent inhibitory concentrations (IC50s) were estimated from a fit of the initial velocities to a standard, four-parameter logistic model (equation 1) by using GraFit 4.0 (Erithacus Software, Staines, United Kingdom) and were typically reported as the mean ± standard deviation of duplicate determinations.
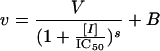 |
where v is the measured enzymatic rate, [I] is the concentration of inhibitor, V is the fitted uninhibited rate minus the background, B is the background, IC50 is the concentration of inhibitor at 0.5V, and s is a slope factor describing the steepness of the curve. The model assumes that v falls with increasing [I].
S. aureus FabI assays contained 100 mM sodium ADA [ADA is N-(2-acetamido)-2-iminodiacetic acid; pH 6.5], 4% glycerol, 25 μM crotonoyl-ACP, 50 μM NADPH, and an appropriate dilution of S. aureus FabI (approximately 20 nM). Initial velocities were estimated from the slope of a linear fit of the progress curves. H. influenzae FabI assays contained MDTG buffer [100 mM 2-(N-morpholino)ethanesulfonic acid (MES), 51 mM diethanolamine, 51 mM triethanolamine (pH 6.5), 4% glycerol], 25 μM crotonoyl-ACP, 50 μM NADH, and 9 nM H. influenzae FabI. Initial velocities were estimated from an exponential fit of the nonlinear progress curves and are equal to the slope of the tangent at time zero (0 min). Assays for S. pneumoniae FabK contained MDTG buffer (pH 7.5), 100 mM NH4Cl, 25 μM crotonoyl-ACP, 50 μM NADH, and 1.5 nM S. pneumoniae FabK. Initial velocities were determined as described above for S. aureus FabI.
Antimicrobial activity assay.
Whole-cell antimicrobial activity was determined by broth microdilution. Test compounds were dissolved in dimethyl sulfoxide (DMSO) and diluted 1:10 in water to produce a 256-μg/ml stock solution. By using a 96-well microtiter plate, a Microlab AT Plus 2 dilution instrument (Hamilton Co., Reno, Nev.) serially diluted 50 μl of the stock solution into cation-adjusted Mueller Hinton broth (Becton Dickinson, Cockeysville, Md.). After the compounds were diluted, a 50-μl aliquot of the test isolate (∼106 CFU/ml) was added to each well of the microtiter plate. Inoculated plates were incubated at 35°C in ambient air for 18 to 24 h. The MIC was determined as the lowest concentration of compound that inhibited visible growth.
Mode-of-antibacterial-action studies.
Generation and validation of strains of H. influenzae and S. aureus overexpressing FabI have been described previously (15; S. B. Winram, S. M. Green, M. S. Head, R. A. Daines, J. T. Lonsdale, and D. D. Jaworski, Abstr. 101st Gen. Meet. Am. Soc. Microbiol., abstr. A-157, 2001). An increase in the MIC for the strain overexpressing FabI relative to that for the wild type is indicative of FabI being the mode of antibacterial action. The modes of action of the compounds were also examined biochemically. The effects of the FabI inhibitors on the incorporation of [14C]isoleucine, [14C]thymidine, [14C]uridine, [14C]N-acetylglucosamine, and [14C]acetate in S. aureus were examined by methodologies described and validated previously (14). Selective inhibition of acetate incorporation indicates that the mode of antibacterial action of the compound is through inhibition of fatty acid biosynthesis.
Selectivity and cytotoxicity.
The compounds were evaluated against human fatty acid synthase as described previously (16). For cytotoxicity testing, the compounds were tested against proliferating human A549 lung cells (American Type Culture Collection) and grown in RPMI 1640 medium supplemented with 10% fetal bovine serum. The cells were incubated in 75-cm2 tissue culture flasks at 37°C in a 5% CO2 atmosphere until a confluent monolayer was achieved. Lung cells were trypsinized from the culture flasks and washed in fresh culture medium. The cells were enumerated with a hemocytometer and diluted in culture medium to yield an inoculum containing 4 × 105 cells/ml. FabI inhibitors were prepared as a double dilution series in Nunc 96-well flat-bottom plates with RPMI 1640 medium-10% fetal bovine serum. Fifty microliters of inoculum (2 × 104 cells/well) was added to each of the wells of the microtiter plates. The plates were incubated at 37°C in 5% CO2 for 96 h. Following drug exposure, cell viability was determined with the tetrazolium dye WST-1 (Roche Molecular Biochemicals), in accordance with the recommendations of the manufacturer. The 50% toxic concentrations of the FabI inhibitors were the lowest concentrations of compounds which caused a ≥50% decrease in cell viability compared with the viability of the drug-free control containing cells and culture medium only.
In vitro plasma protein binding.
Equilibrium dialysis was carried out with Spectra/Por 4 dialysis membrane discs (molecular mass cutoff, 12,000 to 14,000 Da). The membranes were soaked in 0.1 M phosphate-buffered saline (100 mM Na2HPO4, 150 mM NaCl [pH 7.4]) overnight and blotted on tissue prior to cell assembly. Dialysates were prepared in plasma to achieve a concentration of 2,000 ng/ml (final DMSO concentration, 0.5%); similar solutions were prepared in buffer to test for nonspecific binding. Triplicate 50-μl aliquots were collected to verify the initial concentration. Dialysis was carried out at 37°C in a water bath for 6 h at approximately 15 rpm. Following incubation, spiked plasma and buffer from each of the cells were collected, and the volumes withdrawn were determined gravimetrically to account for the volume shift. Three 50-μl aliquots of each spiked plasma sample and control buffer sample were stored at −30°C until analysis. The total amount of analyte recovered was determined by measurement of the initial concentration in the plasma sample and the analyte concentrations on both sides of the membrane. The percentage of each compound bound to plasma proteins following equilibrium dialysis was calculated by the following equation: percent bound = 100 × (Cplasma − Cbuffer) × [(Vplasma/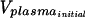 )/(Cplasma − Cbuffer)] × (Vplasma/
)/(Cplasma − Cbuffer)] × (Vplasma/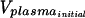 ) + Cbuffer, where C is concentration and V is volume. Quantitative analysis of plasma and buffer samples was performed by the high-pressure liquid chromatography-mass spectrometry-mass spectrometry (HPLC-MS-MS) method. The lower limit of quantitation was 10.0 ng/ml.
) + Cbuffer, where C is concentration and V is volume. Quantitative analysis of plasma and buffer samples was performed by the high-pressure liquid chromatography-mass spectrometry-mass spectrometry (HPLC-MS-MS) method. The lower limit of quantitation was 10.0 ng/ml.
In vivo pharmacokinetics.
Male Sprague-Dawley rats (Charles River, Raleigh, N.C.) were housed according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (11). Use of all animals was preapproved by the internal Institutional Animal Care and Use Committee. All dosages were administered as solutions in either isotonic saline (intravenous [i.v.] doses) or analytical-grade water (oral doses) and contained up to 1.0% DMSO and 10% (vol/vol) polyethylene glycol 400 to aid in dissolution of the test compound. All studies were conducted in a crossover fashion, with the same animals receiving the i.v. and oral dosages at least 2 days apart. Concentration-time profiles after both i.v. and oral administrations were obtained; data were analyzed by noncompartmental techniques (WinNolin, version 2.1; Pharsight, Palo Alto, Calif.).
Analytical procedure.
Quantitative analyses of plasma and buffer samples for all test compounds were performed by the HPLC-MS-MS method. Each test compound was isolated from 50 μl of sample by protein precipitation with acetonitrile and was quantified with a Sciex API 365 instrument with a turbo-ion spray interface. In each experiment, the lower limit of quantitation for each analyte was 10.0 ng/ml.
In vivo efficacy studies.
Groin abscess infection in specific-pathogen-free male Sprague-Dawley rats (Charles River) was caused by S. aureus WCUH29, with each animal receiving 6.4 log10 CFU. FabI inhibitors were administered by either intraperitoneal injection or oral gavage at a dose of 50 mg/kg of body weight at 1, 4, 7, and 24 h postinfection in a volume of 1 ml/rat. Amoxicillin-potassium clavulanate (350/50 mg/kg), vancomycin, ciprofloxacin, and erythromycin were included as positive antibiotic controls. Each therapy group was comprised of five animals. Approximately 24 h after cessation of therapy the groin abscess was excised and homogenized in 1 ml of phosphate-buffered saline for the enumeration of viable bacterial numbers. The samples were inoculated (20 μl) in triplicate onto blood agar plates by a modified Miles-Misra technique (17), and the colonies were counted following overnight incubation at 37°C. The outcome measure was the number of bacteria in the abscess at the end of therapy. Results are presented as group means with standard deviations. Statistical analysis was performed by Student's t test, with P values of ≤0.05 considered significant.
RESULTS
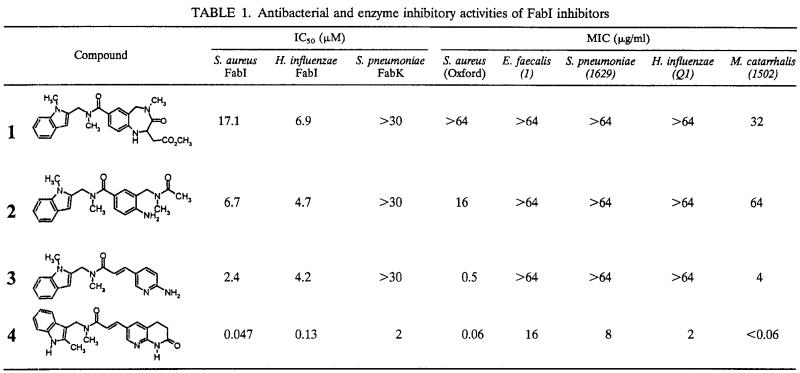
Structure-activity relationships. High-throughput screening of 305,189 compounds from the GlaxoSmithKline compound collection identified the benzodiazepine derivative (compound 1) as an inhibitor of S. aureus FabI when it was used at micromolar concentrations (Table 1). Preliminary lead compound optimization studies, described previously (10; Seefeld et al., 41st ICAAC; Miller et al., 41st ICAAC), led to the identification of compound 2, which exhibits increased potency against S. aureus FabI. Further optimization by X-ray crystal structure-based design led to the identification of an aminopyridine derivative, compound 3, that was an inhibitor of S. aureus FabI at low micromolar concentrations.
TABLE 1.
Antibacterial and enzyme inhibitory activities of FabI inhibitors
Optimization of compound 3 led to the identification of the lactam derivative, compound 4, which achieved a >100-fold increase in potency against the S. aureus FabI. Compound 4 also exhibited inhibitory activity against FabK at low micromolar concentrations. Compounds 1 to 4 had no detectable activities against the human fatty acid biosynthetic enzyme (IC50, >100 μM), and compounds 2 to 4 exhibited no significant cytotoxicities (50% toxic concentration, >250 μg/ml) against the A549 lung cells.
Antibacterial evaluation.
The hit compound from high-throughput screening (compound 1) had no detectable antibacterial activity against S. aureus (MIC, >64 μg/ml), yet optimization of this lead compound afforded compounds with significant antibacterial activities. The MIC of compound 2 for S. aureus was 16 μg/ml, and the MIC of the subsequent derivative, compound 3, for S. aureus was 0.5 μg/ml (Table 1). The analog optimized further, compound 4, possessed exquisite activity against S. aureus and good activities against other key gram-positive and gram-negative organisms. When evaluated with a panel of S. aureus (n = 22) and Staphylococcus epidermidis (n = 9) clinical isolates, the MICs at which 90% of isolates are inhibited (MIC90s) of compound 4 were 0.03 and 0.06 μg/ml, respectively, which are significantly lower than those of any of the commercial antibiotics tested (Table 2). Compound 4 was also shown to be >30-fold more potent against efflux pump deletion mutants of H. influenzae than against the wild-type strain, suggesting that the compound is a substrate for efflux pumps in this species (Table 3). Further testing of compound 4 against a spectrum of gram-negative organisms demonstrated that the MICs of the compound were 1 to 16 μg/ml for H. influenzae, Proteus mirabilis, and E. coli. MICs of 32 to 64 μg/ml were detected for Klebsiella pneumoniae and Enterobacter spp., but no antibacterial activity was detected against Serratia marcescens, Stenotrophomonas maltophilia, or P. aeruginosa (Table 4). The antibacterial activity of compound 4 in the presence of the efflux pump inhibitor Phe-Arg beta-naphthylamide dihydrochloride (8) was increased 4- to 100-fold against H. influenzae, P. mirabilis, E. coli, K. pneumoniae, and Enterobacter spp., but the efflux inhibitor did not potentiate the antibacterial activity against S. marcescens, S. maltophilia, or P. aeruginosa (data not shown).
TABLE 2.
MIC90 and MIC50s of compound 4 and commercially available antibiotics for 22 multidrug-resistant clinical isolates of S. aureus
| Compound | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
|
S. aureus (n = 22)
|
S. epidermidis (n = 9)
|
|||||
| 90% | 50% | Range | 90% | 50% | Range | |
| Amoxicillin | >16 | >16 | 0.06->16 | >16 | 4 | 0.06->16 |
| Oxacillin | >16 | 0.5 | 0.125->16 | 16 | 0.5 | 0.125-16 |
| Gentamicin | >16 | 1 | 0.25->16 | 4 | 0.125 | 0.06-4 |
| Erythromycin | >16 | 1 | 0.125->16 | >16 | 0.25 | 0.06->16 |
| Ciprofloxacin | >16 | 0.25 | 0.06->16 | >16 | 0.125 | 0.125->16 |
| Tetracycline | >16 | 2 | 0.125->16 | >16 | 0.25 | 0.125->16 |
| Chloramphenicol | 16 | 8 | 2->16 | 8 | 4 | 2-8 |
| Meropenem | >16 | 0.125 | 0.06->16 | 16 | 0.5 | 0.06-16 |
| Cefuroxime | >16 | 4 | 1->16 | >16 | 0.5 | 0.25->16 |
| Compound 4 | 0.06 | 0.03 | 0.008-0.06 | 0.06 | 0.03 | 0.03-0.06 |
TABLE 3.
MICs of Fab I compounds for the H. influenzae efflux knockouts
| Compound | MIC (μg/ml)
|
||
|---|---|---|---|
| RD-51907 (parent) | LS-2 (acrA knockout) | LS-3 (acrB knockout) | |
| Erythromycin | 4 | 0.125 | 0.125 |
| Amoxicillin | 0.125 | 0.25 | 0.125 |
| Compound 4 | 2 | ≤0.06 | ≤0.06 |
TABLE 4.
MICs of compound 4 for gram-negative organisms
| Organism | MIC (μg/ml)
|
||
|---|---|---|---|
| Ciprofloxacin | Amoxicillin | Compound 4 | |
| H. influenzae H128 | 0.008 | >16 | 2 |
| H. influenzae ATCC 49247 | 0.008 | 4 | 1 |
| H. influenzae Neqas 4012 | 0.008 | 8 | 4 |
| H. influenzae Speeding | 0.008 | 1 | 2 |
| M. catarrhalis Ravasio | 0.03 | 4 | 0.125 |
| M. catarrhalis CL2 | 0.03 | 8 | ≤0.06 |
| E. coli UTI17 | 0.008 | 4 | 8 |
| E. coli 41548 | 0.008 | >16 | 8 |
| E. coli ATCC 25922 | NRa | 8 | 8 |
| K. pneumoniae E70 | 0.03 | >16 | 32 |
| K. pneumoniae ATCC13883 | 0.06 | >16 | 64 |
| K. pneumoniae KP1 | 0.03 | >16 | 32 |
| P. aeruginosa 4 | 0.125 | >16 | >64 |
| P. aeruginosa K799wt | 1 | >16 | >64 |
| P. aeruginosa Z61 | 0.5 | 0.5 | >64 |
| Enterobacter aerogenes SA19 | 0.016 | >16 | 64 |
| Enterobacter cloacae N1 | 0.016 | >16 | >64 |
| P. mirabilis C889 | 0.016 | >16 | 16 |
| P. mirabilis W229 | 0.03 | >16 | 16 |
| S. marcescens Ba58 | 0.125 | >16 | >64 |
| S. marcescens US20 | 0.06 | >16 | >64 |
| S. maltophilia RC1 | 2 | >16 | >64 |
| S. maltophilia NCTC 10257 | 0.5 | >16 | >64 |
NR, no result.
Mode-of-antibacterial-action studies.
In mechanism-of-action studies, the MICs of compounds 2, 3, and 4 for an S. aureus FabI overexpressor were elevated, and all three compounds selectively inhibited acetate incorporation in S. aureus (Table 5; Fig. 2). These results demonstrate that the antibacterial modes of action of these compounds in S. aureus are via inhibition of FabI. In addition, the MIC of compound 4 for an H. influenzae construct that overexpresses FabI was increased compared to that for the wild-type strain. However, like the triclosan control, compound 4 did not give rise to selective inhibition of acetate incorporation in S. pneumoniae (Fig. 2).
TABLE 5.
Demonstration of FabI mechanism of action against strains of H. influenzae and S. aureus overexpressing FabI
| Compound | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| S. aureus WTa | S. aureus FabI overexpressor | H. influenzae WT | H. influenzae FabI overexpressor | |
| 2 | 8 | >32 | NTb | NT |
| 3 | 2 | >32 | NT | NT |
| 4 | <0.06 | 2 | 8 | >128 |
WT, wild type.
NT, not tested.
FIG. 2.
Effects of FabI inhibitors on macromolecular processes in S. aureus and S. pneumoniae. (A) Compound 4 (20 μg/ml) against S. pneumoniae; (B) compound 3 (2 μg/ml) against S. aureus; (C) triclosan (6 μg/ml) against S. pneumoniae; (D) triclosan (20 μg/ml) against S. aureus. NAG, N-acetylglucosamine.
In vivo efficacy and pharmacokinetics.
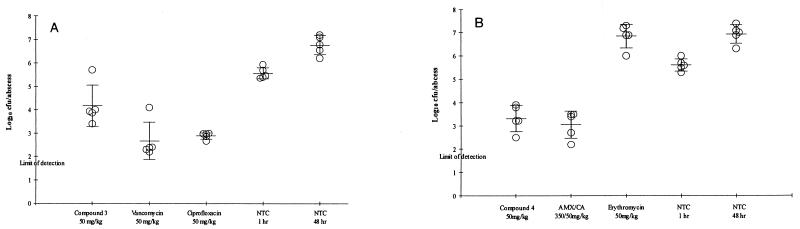
Compounds 3 and 4 exhibited low to moderate levels of clearance from plasma in the rat: 26.4 ± 6.9 and 5.1 ± 0.5 ml/min/kg, respectively. Both compounds demonstrated high levels of protein binding and good oral bioavailabilities when dosed as solutions (Table 6). In in vivo studies, compound 3 at 50 mg/kg was efficacious in the S. aureus (MIC, 0.5 μg/ml) groin abscess infection model following either intraperitoneal dosing (reduction in bacterial counts, 2.5 log10 CFU/abscess compared with the counts for untreated animals [P < 0.01]) or oral dosing (reduction in bacterial counts, 1.5 log10 CFU/abscess [P < 0.05]) (data not shown). Despite the high level of plasma protein binding, the concentration of the predicted free fraction of compound 4 following oral administration (50 mg/kg) remained above the MIC longer than that of compound 3 did following oral or intraperitoneal administration. This increase in time above the MIC of the free fraction of compound 4 (MIC, 0.03 μg/ml) correlated well with better activity than compound 3, achieving a 3.5-log reduction in bacterial counts compared with those for the untreated animals (P < 0.01). This reduction in bacterial counts was comparable (P > 0.05) to that achieved with amoxicillin-clavulanate (350/50 mg; 3.8-log reduction [P < 0.01]) which was administered to approximate a clinically relevant human dose in the rat (875/125 mg) (Fig. 3).
TABLE 6.
Pharmacokinetic parameters of FabI inhibitors in ratsa
| Compound | MRT (h) | CL (ml/min/kg) | VSS (liters/kg) | Oral bio- availability (%) | Plasma protein binding (%) | Cmax (μg/ml) | Time above MIC (h)b |
|---|---|---|---|---|---|---|---|
| 3 | 1.0 ± 0.3 | 26.4 ± 6.9 | 1.5 ± 0.3 | 100, 83.4c | 96.0 ± 1.2 | 90d | 10d |
| 4 | 0.7 ± 0.1 | 5.1 ± 0.5 | 0.2 ± 0.01 | 40.3 ± 2.2 | 97.4 ± 0.3 | 10.8e | 14e |
MRT, mean residence time; CL, plasma clearance; VSS, steady-state volume of distribution; Cmax, maximum concentration in plasma.
Unbound fraction.
n = 2.
Dose, 50 mg/kg, intraperitoneal.
Dose, 50 mg/kg, oral.
FIG. 3.
(A) Efficacy of compound 3 administered intraperitoneally against a groin abscess infection in rats caused by S. aureus WCUH29; (B) efficacy of compound 4 administered orally against a groin abscess infection in rats caused by S. aureus WCUH29. AMX/CA, amoxicillin-clavulanate; NTC, nontreated control.
DISCUSSION
As a result of the distribution of FabI and FabK in clinical pathogens, a FabI-directed antibacterial should possess antibacterial activity against those pathogens in which FabI is the sole enoyl-ACP reductase (e.g., S. aureus, H. influenzae, Moraxella catarrhalis, and E. coli) but not against S. pneumoniae, enterococci, or P. aeruginosa, which utilize either FabK or both FabI and FabK. However, inhibitors with dual FabI and FabK inhibitory activities are expected to have antibacterial activities against FabK-utilizing organisms, and the results of the present study support this expectation.
Compound 2 had improved potency against S. aureus FabI compared to the potency of the original screening hit compound, which may account for the increase in antibacterial activity against S. aureus. Antibacterial activity against this pathogen improved further with the increase in inhibitory potency against FabI achieved with compound 3. Neither compound 2 nor 3 demonstrated any detectable activity against FabK, and thus, their lack of activity against E. faecalis and S. pneumoniae is to be expected.
Compound 4 has significantly improved inhibitory potency against FabI. This resulted in impressive potency against a panel of multidrug-resistant strains of S. aureus and S. epidermidis and an increase in potency against other FabI-utilizing organisms such as H. influenzae, E. coli, and M. catarrhalis. However, this compound also possesses some inhibitory potency against FabK, which may explain the antibacterial activity observed against S. pneumoniae and E. faecalis. Although the modes of antibacterial action of compounds 2 to 4 could all be clearly demonstrated to be via FabI in S. aureus and H. influenzae (where appropriate), we were unable to obtain data to illustrate that the mode of antibacterial activity of compound 4 in S. pneumoniae was via FabK, and this evaluation is ongoing.
Compound 4 also possessed activity against a broader range of gram-negative organisms. The studies with the H. influenzae efflux pump deletion mutants clearly demonstrate that this compound is a substrate for efflux pumps, and this is further illustrated in several other gram-negative organisms when the antibacterial activity of compound 4 was determined in the presence of Phe-Arg beta-naphthylamide dihydrochloride, an efflux pump inhibitor of gram-negative organisms. However, even in the presence of the efflux inhibitor, the MIC of compound 4 was >64 μg/ml for S. marcescens, S. maltophilia, and P. aeruginosa. Although the impermeability of these organisms was likely to be a contributing factor, the lack of activity against P. aeruginosa may also be a result of the potential presence of a plethora of enoyl-ACP reductases in this organism (7).
Compounds 3 and 4 demonstrate the potential clinical utilities of representatives of this novel series, with the efficacy of compound 4 equaling that of amoxicillin-clavulanate in the S. aureus groin abscess infection model. Interestingly, the high levels of protein binding observed with these compounds did not affect their in vivo efficacies, suggesting that sufficient unbound compound was available during the infection process.
This work describes the chemical optimization of the activity of a hit compound obtained from high-throughput screening against S. aureus FabI. The original screening hit compound possessed weak inhibitory activity against FabI and no significant antibacterial activity. Iterative medicinal chemistry and structure-based design improved the inhibitory potency of the original lead compound by >350-fold, and exquisite activity against multidrug-resistant S. aureus has been built into the series. The potential utility of this series is exemplified by compound 4, the MIC90s of which were >500-fold lower that those of each of the commercially available antibiotics tested. Furthermore, since these compounds exhibit activities against both FabK and FabI, the potential also exists to optimize and develop these compounds as broader-spectrum antibacterial agents.
In conclusion, we have described the discovery of a novel series of highly potent FabI-directed antibiotics. These novel FabI- and FabK-directed antibacterials have clear potentials as new therapeutic options for tackling infections caused by multidrug-resistant pathogens and also serve to highlight the potential of the bacterial fatty acid biosynthetic enzymes as a source of novel antibacterial targets for the 21st century.
REFERENCES
- 1.Baldock, C., J. B. Rafferty, S. E. Sedelnikova, P. J. Baker, A. R. Stuitje, A. R. Slabas, T. R. Hawkes, and D. W. Rice. 1996. A mechanism of drug action revealed by structural studies of enoyl reductase. Science 274:2107-2110. [DOI] [PubMed] [Google Scholar]
- 2.Bax, R., N. Mullan, and J. Verhoef. 2000. The millennium bugs—the need for and development of new antibacterials. Int. J. Antimicrob. Agents 16:51-59. [DOI] [PubMed] [Google Scholar]
- 3.Bergler, H., S. Fuchsbichler, G. Högenauer, and F. Turnowsky. 1996. The enoyl-[acyl-carrier-protein] reductase (FabI) of Escherichia coli, which catalyzes a key regulatory step in fatty acid biosynthesis, accepts NADH and NADPH as cofactors and is inhibited by palmitoyl-CoA. Eur. J. Biochem. 242:689-694. [DOI] [PubMed] [Google Scholar]
- 4.Heath, R. J., Y.-T. Yu, M. A. Shapiro, E. Olson, and C. O. Rock. 1998. Broad spectrum antimicrobial biocides target the FabI component of fatty acid synthesis. J. Biol. Chem. 273:30316-30320. [DOI] [PubMed] [Google Scholar]
- 5.Heath, R. J., and C. O. Rock. 1995. Enoyl-acyl carrier protein reductase (fabI) plays a determinant role in completing cycles of fatty acid elongation in Escherichia coli. J. Biol. Chem. 270:26538-26542. [DOI] [PubMed] [Google Scholar]
- 6.Heath, R. J., and C. O. Rock. 2000. A triclosan-resistant bacterial enzyme. Nature 406:145. [DOI] [PubMed] [Google Scholar]
- 7.Hoang, T. T., and H. P. Schweizer. 1999. Characterization of Pseudomonas aeruginosa enoyl-acyl carrier protein reductase (FabI): a target for the antimicrobial triclosan and its role in acylated homoserine lactone synthesis. J. Bacteriol. 181:5489-5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lomovskaya, O., M. S. Warren, A. Lee, J. Galazzo, R. Fronko, M. Lee, J. Blais, D. Cho, S. Chamberland, T. Renau, R. Leger, S. Hecker, W. Watkins, K. Hoshino, H. Ishida, and V. J. Lee. 2001. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother. 45:105-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McMurry, L. M., M. Oethinger, and S. B. Levy. 1998. Triclosan targets lipid synthesis. Nature 394:531-532. [DOI] [PubMed] [Google Scholar]
- 10.Miller, W. H., M. A. Seefeld, K. A. Newlander, I. N. Uzinskas, W. J. Burgess, D. A. Heerding, C. C. K. Yuan, M. S. Head, D. J. Payne, S. F. Rittenhouse, T. D., Moore, S. C. Pearson, V. Berry, W. E. DeWolf, P. M. Keller, B. J. Polizzi, X. Qiu, C. A. Janson, and W. F. Huffman. 2002. Discovery of aminopyridine-based inhibitors of bacterial enoyl-ACP reductase (FabI). J. Med. Chem. 45:246-3256. [DOI] [PubMed] [Google Scholar]
- 11.National Academy Press. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 12.Payne, D. J., P. V. Warren, D. J. Holmes, Y. Ji, and J. T. Lonsdale. 2001. Bacterial fatty acid biosynthesis: a genomics driven target for antibacterial drug discovery. Drug Discovery Today 6:537-544. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez, L., W. Pan, M. Vinas, and H. Nikaido. 1997. The acrAB homolog of Haemophilus influenzae codes for a functional efflux pump. J. Bacteriol. 21:6855-6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Setti, E. L., L. Quattrocchio, and R. G. Micetich. 1997. Current approaches to overcome bacterial resistance. Drugs Future 22:271-284. [Google Scholar]
- 15.Slater-Radosti, C., G. Van Aller, R. Greenwood, R. O. Nicholas, P. Keller, W. E. DeWolf, F. Fan, D. J. Payne, and D. D. Jaworski. 2001. Biochemical and genetic characterisation of the action of triclosan on Staphylococcus aureus. J. Antimicrob. Chemother. 48:1-6. [DOI] [PubMed] [Google Scholar]
- 16.SmithKline Beecham Corporation (D. J. Bergsma, C. Chapman, M. E. DePiera, C. E. Ellis, J. T. Lonsdale, and J. L. Mooney). July2001. Human FAS. U.S. patent 6,294,364.
- 17.Woodnutt, G., and V. Berry. 1999. Efficacy of high-dose amoxicillin-clavulanate against experimental respiratory tract infections caused by strains of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 43:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]