Abstract
Natural killer (NK) cells, natural killer T (NKT) cells, and T lymphocytes were analyzed by using a flow cytometer in 225 human immunodeficiency virus (HIV)-positive individuals infected through the past sale of blood and plasma without receiving antiretroviral therapy in the People’s Republic of China. According to CD4 T-cell counts these HIV-infected adults were stratified into three groups: long-term slow progressors, HIV-infected subjects, and AIDS patients. NK cell counts in long-term slow progressors were higher compared to HIV infection and AIDS patients (P < 0.05) and lower compared to normal controls (P < 0.05), whereas NKT cell counts in slow progressors and the HIV infection group were not different from those of normal controls. NK cell counts in HIV-seropositive subjects were positively correlated with CD4 T-cell counts (P < 0.05), and NKT cell counts were positively correlated with CD4 T-cell and CD8 T-cell counts (P < 0.05). The CD8 T-cell counts were higher in slow progressors compared to those with HIV infection, AIDS patients, and normal controls. These results indicated that HIV infection causes alterations of NK cells and T cells in slow progressors, HIV-infected subjects, and AIDS patient groups, but no difference was found in NKT cell counts and percentages in slow progressors and the HIV-infected group compared to normal controls.
Among human immunodeficiency virus (HIV)-infected persons, a small percentage maintaining CD4 T-cell counts of >500 cells/μl for more than 10 years without receiving antiretroviral therapy are known as long-term slow progressors (SPs) (9, 10, 12, 13, 22). The factors involved in the long-term survival of these patients have been the subject of intense investigations since these factors may provide information important in the development of HIV-1 vaccines and treatments. However, since it is difficult to identify the time when these individuals were infected, studies for such long-term survivors are limited.
The People’s Republic of China is at significant risk for a generalized HIV/AIDS epidemic. There was a severe HIV epidemic among people who sold blood and plasma in the past in the rural areas. The duration of infection time for these people is very narrow (1993 to 1996) because the unsafe blood banks were set up around 1993 in the local areas, and the government closed these banks in 1996 to prevent the transmission of HIV.
There is also a small percentage of long-term SPs among people infected with HIV through past sales of blood and plasma who have lived with HIV for nearly 8 to 11 years but still show normal CD4 T-cell counts without having received highly active antiretroviral therapy (HAART) treatment. In contrast to the small percentage of long-term SPs, other people infected with HIV in the same way, at the same time period, and in the same local areas have decreased CD4 T cells, have developed AIDS, or have even died of AIDS. Why then do these HIV-seropositive people appear so different? Study of the immunological parameters of these long-term SPs comparing them with typical progressors, including HIV-infected subjects and AIDS patients, will be helpful for the prevention and control of HIV infection in the People’s Republic of China. Because there are few HIV-seropositive cases infected through past sales of blood and plasma in Western countries, most studies of HIV have focused on individuals infected with HIV through sexual contact or sharing needles; thus, studies of SPs infected via blood donation are limited.
After 20 years of intensive study on the acquired immune system for HIV infection, innate immune system has drawn much attention recently. Natural killer (NK) cells are an essential part of the immune system, representing 10 to 15% of circulating lymphocytes, and are important mediators of both natural and adaptive immunity. NK cells participate in immune surveillance against malignancies and virus infection (4, 8, 16, 20). NK cells are CD3-negative, large granular lymphocytes that express characteristic NK cell markers such as CD56 and CD16 (7, 18-19). A second set of NK cells—natural killer T (NKT) cells—are found within T-cell populations, and identification is based on expression of T-cell-receptor molecules and certain NK cell markers. NKT cells have been implicated in tumor rejection, control of microbial infection, and suppression of autoimmune diseases (3, 14, 21, 24). Understanding the responses of the innate immune system to HIV infection may be very important for HIV research.
The level of natural immunity in HIV carriers in the People’s Republic of China who have been infected via past blood donation has not been examined. To our knowledge, a study of NKT cells in the SP group infected with HIV for more than 8 years and with normal CD4 T-cell counts has not been reported elsewhere. We investigated here the alterations of NK cells, NKT cells, and T lymphocytes in SPs and compared them to HIV-infected individuals and AIDS patients infected through the prior sale of blood and plasma in the People’s Republic of China.
MATERIALS AND METHODS
Study population.
Diagnosis of HIV-1 infection was made on the basis of a positive anti-HIV enzyme-linked immunosorbent assay (Vironostika; Organon Teknika, The Netherlands) and confirmed by a positive Western immunoblot (Gene Lab Diagnostics, Singapore). A total of 225 HAART-naive HIV carriers who were infected through past sales of blood and plasma in rural areas of the People’s Republic of China were asked to participate in the study. These individuals had been infected with HIV for 8 to 11 years. HIV-infected individuals were stratified according to CD4 T-cell counts. If the CD4 T-cell count was >500 cells/μl and the subject showed no HIV symptoms, we defined him or her as an HIV-positive long-term SP. If the CD4 T-cell count was <500 cells/μl but more than 200 cells/μl and the subject had no HIV symptoms, we defined him or her as an HIV-infected person. If the CD4 T-cell count was <200 cells/μl or the CD4 T-cell count gave indications of AIDS, we categorized the HIV-infected individual as an AIDS patient. According to these criteria, there were 64 SPs in the present study, with 41 males (64.1%) and 23 females (35.9%); the mean of age was 40.11 ± 7.6 years old, with a 10.2% positive rate of hepatitis B virus surface antigen and a 96.7% positive rate of anti-hepatitis C virus antibody, making up 28.4% of this HIV-seropositive study population. There were 122 HIV-infected persons, with 64 (52.5%) males and 58 (47.5%) females; the mean of age was 41.8 ± 8.34 years old, with a 5.9% positive rate of hepatitis B virus surface antigen and a 94.9% positive rate of anti-hepatitis C virus antibody, making up 54.2% of this HIV-seropositive study population. There were 39 AIDS patients, with 19 males (48.7%) and 20 females (51.3%); the mean of age was 42.42 ± 7.58 years old, with a 4.2% positive rate of hepatitis B virus surface antigen and a 98.2% positive rate of anti-hepatitis C virus antibody, making up 17.3% of this HIV-seropositive study population. Among the three groups, there were no significant differences based on age, sex, or coinfection of hepatitis B virus (HBV) or HCV statistically analyzed by one-way analysis of variance followed by use of the Student-Newman-Keuls test or the Fisher exact test. A total of 130 HIV-negative healthy persons were used as normal controls, with 68 males (52.3%) and 62 females (47.7%); the mean of age was 43.91 ± 14.74 years old, with normal blood cell counts, normal levels of hemoglobin, and normal liver functions and without any history of immune system disease. All subjects included here gave informed consent under the auspices of the appropriate research and ethics committees.
Blood collection.
Whole blood was collected into EDTA Vacutainer tubes and transported to our AIDS Research Center. The blood samples were analyzed by flow cytometer on the same day.
Determination of T-cell, NK cell, and NKT cell counts.
CD4 T-cell, CD8 T-cell, NK cell, and NKT cell counts were measured with a FACScalibur flow cytometer (Becton Dickinson). A single-platform, “lyse-no-wash” procedure was performed with Trucount tubes and with TriTEST CD4FITC/CD8 PE/CD3PerCP or CD3FITC/CD56/16PE/CD45PerCP reagents (Becton Dickinson). We pipetted 20 μl of TriTEST CD4FITC/CD8PE/CD3PerCP or CD3FITC/CD56/16PE/CD45PerCP reagent and 50 μl of anticoagulated whole blood into the bottom of the TruCOUNT Tubes and incubated them for 15 min in dark at room temperature (20 to 25°C). We added 450 μl of 1× FACS lysing solution to the tubes, incubated them for 15 min in the dark at room temperature, and analyzed the samples with a flow cytometer. CD4 T cells were defined as CD3+ CD4+ lymphocytes, CD8 T cells were defined as CD3+ CD8+ lymphocytes, NK cells were defined as CD3− CD56/16+ lymphocytes, and NKT cells were defined as CD3+ CD56/16+ lymphocytes.
HIV viral load measurement.
HIV RNA was extracted from plasma samples stored at −70°C and amplified by a standardized reverse transcription-PCR assay according to the manufacturer's instructions (COBAS Amplicor; HIV-1 Monitor Test Version 1.5; Roche Diagnostics). The detection level in plasma was defined as 400 HIV-1 RNA copies/ml by our laboratory. An HIV viral load was transformed to log10 values for all statistical analyses.
Statistical analysis.
The analysis of variance were performed by analysis of variance and subsequent Student-Newman-Keuls test. The viral load was logarithmically transformed before analysis, and the geometric means and standard deviations (SD) were used to summarize the results for viral load. The means and SD were used for other data. Correlation between two quantitative variance was measured by using the Pearson correlation coefficient. P values of <0.05 were considered statistically significant. All analyses were carried out by using SPSS11.5 software.
RESULTS
NK and NKT cells in SPs, HIV-infected subjects, AIDS patients, and normal controls.
NK cells and NKT cells were measured in all subjects (Table 1). The absolute NK cell counts were significantly higher in SPs compared to the HIV-infected subjects (P < 0.05) and AIDS patients (P < 0.05) and lower compared to the normal controls (P < 0.05). The percentage of NK cells in SPs was lower than that of normal controls (P < 0.05), the same as that of the HIV-infected subjects (P > 0.05), and lower than that of AIDS patient group (P < 0.05). The percentage of NK cells in HIV infection group was much lower than that of normal controls and the AIDS group (P < 0.05).
TABLE 1.
NK and NKT cells in SPs, HIV-infected subjects, AIDS patients, and normal controlsa
| Group | n | Mean ± SD
|
|||
|---|---|---|---|---|---|
| NKb | NK% | NKTb | NKT% | ||
| Normal controls | 130 | 407 ± 223 | 18.92 ± 7.74 | 124 ± 92 | 5.83 ± 4.11 |
| SPs | 64 | 310 ± 159A | 12.44 ± 5.90A | 128 ± 85 | 5.12 ± 3.27 |
| HIV | 122 | 241 ± 160AB | 13.41 ± 7.41A | 100 ± 58 | 5.57 ± 2.9 |
| AIDS | 39 | 190 ± 143ABC | 16.84 ± 8.39BC | 76 ± 65AB | 7.23 ± 6.04ABC |
Superscripts: A, compared to the control group, P < 0.05; B, compared to the SP group, P < 0.05; C, compared to the HIV group, P < 0.05.
NK and NKT cell counts are given in cells per microliter.
The absolute numbers of NKT cells were significantly higher in SPs compared to AIDS patients (P < 0.05). No significant differences were found among SPs, HIV-infected subjects, and normal controls. The percentage of NKT cells in SPs was not different from HIV-infected subjects and normal controls but lower than that of the AIDS group (P < 0.05).
Correlation of NK cells and NKT cells with T lymphocytes and viral load in all HIV-seropositive subjects.
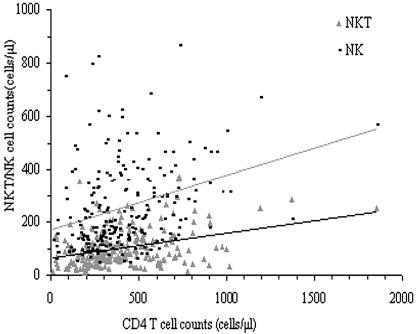
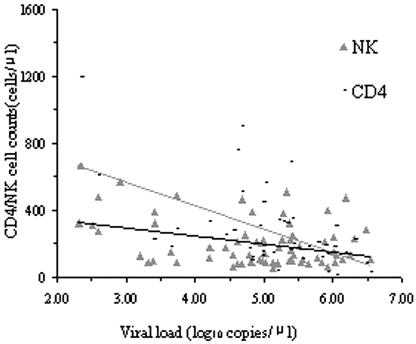
The absolute NK cell counts were positively correlated with CD4 T-cell counts (r = 0.394, P < 0.01) and not correlated with CD8 T-cell counts. The absolute NKT cell counts were positively correlated with CD4 T-cell and CD8 T-cell counts (r = 0.225 P < 0.01; r = 0.269, P < 0.01) (Fig. 1). The HIV viral loads were negatively correlated with NK cell counts, CD4 T-cell counts, CD4 T-cell percentage, and CD4/CD8 T-lymphocyte ratio (r = −0.362 [P < 0.01], r = −0.563 [P < 0.01], r = −0.658 [P < 0.01], and r = −0.632 [P < 0.01], respectively) (Fig. 2).
FIG. 1.
Correlation of CD4 T cells to NK and NKT cells in HIV-seropositive subjects. The absolute counts of NK cells, NKT cells, and CD4 T cells were measured with a flow cytometer. The absolute NK cell counts and NKT cell counts were positively correlated with CD4 T-cell counts (r = 0.394, P < 0.01; r = 0.225, P < 0.01).
FIG. 2.
Correlations of viral load to CD4 T cells and NK cells in all HIV-seropositive subjects. The HIV viral loads, NK cell counts, and CD4 T-cell counts were measured. The HIV viral loads was negatively correlated with NK cell counts and CD4 T-cell counts (r = −0.362 [P < 0.01] and r = −0.563 [P < 0.01], respectively).
T lymphocytes in SPs, HIV-infected subjects, AIDS patients, and normal controls.
CD3 T and CD8 T lymphocytes were measured in all subjects (Table 2). The results showed that absolute CD3 T-cell counts were significantly higher (P < 0.05) in SPs compared to normal controls, HIV infection, and AIDS patients (P < 0.05). The CD3 T-cell counts were significantly lower (P < 0.05) in the HIV-infected subjects and the AIDS patient group than in normal controls (P < 0.05).
TABLE 2.
T lymphocytes in SPs, HIV infected subjects, AIDS patients, and normal controlsa
| Group | n | Mean ± SD
|
||||
|---|---|---|---|---|---|---|
| CD3b | CD4% | CD4b | CD8% | CD8b | ||
| Normal controls | 130 | 1,610 ± 487 | 53.75 ± 10.07 | 852 ± 251 | 40.55 ± 9.59 | 660 ± 290 |
| SPs | 64 | 1,911 ± 542A | 38.94 ± 11.12A | 712 ± 224A | 57.8 ± 11.74A | 1,139 ± 499A |
| HIV | 122 | 1,397 ± 507AB | 26.07 ± 8.73AB | 334 ± 83AB | 71.45 ± 9.57AB | 1,032 ± 484A |
| AIDS | 39 | 845 ± 342ABC | 16.38 ± 8.27ABC | 127 ± 57ABC | 81.79 ± 8.69ABC | 704 ± 320BC |
Superscripts: A, compared to the control group, P < 0.05; B, compared to the SP group, P < 0.05; C, compared to the HIV group, P < 0.05.
CD3, CD4, and CD8 cell counts are given in cells per microliter.
The absolute CD8 T-cell count was significantly higher in SPs compared to normal controls and AIDS patients (P < 0.05) and not different from HIV-infected subjects (P > 0.05). The CD8 T-cell count in HIV infection was higher than that of AIDS patients (P < 0.05). The percentage of CD8 T cells in SPs was remarkably higher than in normal controls (P < 0.05) but much lower than that of HIV-infected subjects and the AIDS patient group (P < 0.05). The percentage of CD8 T cells in HIV-infected subjects and AIDS patients was higher than that of normal controls (P < 0.05).
The CD4/CD8 T-lymphocyte ratio in SPs (0.75 ± 0.39) was significantly higher than in HIV-infected subjects (0.39 ± 0.20) and AIDS patients (0.21 ± 0.14) (P < 0.05), whereas it was lower than in normal controls (1.45 ± 0.68) (P < 0.05) (data not shown).
DISCUSSION
Innate immunity may play a role in preventing HIV infection or progression to AIDS (11, 23). It has been determined that a few HIV-infected people with very low CD4 T-cell counts (<50/μl) who have prolonged asymptomatic periods had significantly higher NK cell toxicity and NK cell numbers than AIDS patients with symptoms and were not different from HIV-negative controls with regard to NK cell cytotoxicity and NK cell numbers despite their lower CD4 T-cell numbers and higher viral loads (11). On the other hand, impairment of NK cell number and function by HIV infection was also reported (1-2, 5, 15), although different races may show different outcomes in HIV infection. The impairment of NK cell number, percentage, and function in North American HIV-seropositive subjects was demonstrated compared to the HIV-seronegative group, but there was no significant difference between the Thai HIV-seronegative and HIV-seropositive groups (6).
The present study shows that NK cells in HIV-infected subjects and AIDS patients were significantly lower compared to normal controls and that the numbers of NK cells were significantly lower in AIDS patients compared to HIV-infected subjects. We also show that HIV infection dysregulated the NK cells with disease progression in HIV-seropositive individuals infected via past sales of blood and plasma in China. The result is consistent with findings in North American HIV-seropositive subjects (6) and another published study (17) with North American subjects, but quite different from the findings in a Thai HIV-seropositive group (6). The reasons for this difference may be related to different sample sizes, the infecting HIV subtype, or the infection time. In our study, we had a total of 225 HIV-seropositive subjects infected with HIV through past sales of blood and plasma for 8 to 11 years and who were predominantly infected with HIV subtype B′. Although there were 68 Thai HIV-positive subjects in that report (6) and the participants were mostly infected with HIV subtype E, the infection time was not specified.
The percentage of NK cells in our study decreased in the HIV-infected group compared to the normal control group, whereas in the AIDS group it increased and was not different from that of the normal control group. A previous study indicated that the NK cell percentage in Thai HIV-seropositive subjects was not different from HIV-seronegative subjects, but the NK cell percentage in North American HIV-seropositive subjects decreased compared to HIV-seronegative subjects (6). The main reason for this difference is that the disease stages of the subjects were not considered in that study; subjects studied at different stages may lead to different results.
Studies of innate immunity in subjects infected HIV for more than 8 years with normal CD4+ T-cell counts and without HAART therapy are rare, especially with respect to the alteration of NKT cells. The present study investigated changes of NK, NKT, and T cells in Chinese SPs. The results showed that NK cell counts were lower in SPs compared to normal controls and higher compared to HIV infection and AIDS patients; they were positively correlated with CD4 T-cell counts and negatively correlated with HIV viral loads. These findings may indicate that HIV infection leads to the impairment of CD4 T cells and causes NK cell counts to decline subsequently. Although NKT cell counts in SPs were not different with normal controls and there was a trend toward increased NKT cell counts in SPs, this may associated with delayed disease progression; further studies are needed to verify this. The CD8 T cells in SPs and HIV-infected individuals increased significantly compared to normal controls. The absolute CD8 T-cell counts were positively correlated with NKT cell counts, and we believe both NKT and CD8 T cells may exert anti-HIV effects. In the AIDS stage, although the absolute NKT and CD8 number decreased, the body trying to keep anti-HIV effects causes the percentages of NKT and CD8 T cells to significantly increase.
Although the present study is based on cross-sectional investigation, the subjects were infected around the same time period but with the different disease progression. We may presume from the data that the HIV infection that caused CD4 T cells to decrease also caused the NK cells to decrease; NKT cell counts kept normal for a period of time and then declined, whereas CD8 T-cell counts increased first and then decreased to the normal range in the AIDS stage.
Our investigation showed that long-term SPs represented 28.4% of the HIV-positive past blood donors, a number higher than that reported previously (5%) (10). The reason for this is that we probably just cross-sectionally investigated live subjects who had lived for 8 to 11 years after the HIV infection occurred, and thus those who died from AIDS were not counted.
The HIV infection times for these blood donors in China is easier to identify than infection times from sexual contact or intravenous drug use. The SPs of these past blood donors are important subjects to be studied for natural anti-HIV mechanisms and may provide an opportunity to investigate both viral and host factors that influence the rate of disease progression.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation Committee of China (NSFC30471548) and the Key Technologies Research and Development Program of the Tenth Five-Year Plan from Ministry of Science and Technology of China (2004BA719A12).
REFERENCES
- 1.Ahmad, A., and R. Ahmad. 2003. HIV's evasion of host's NK cell response and novel ways of its countering and boosting anti-HIV immunity. Curr. HIV Res. 1:295-307. [DOI] [PubMed] [Google Scholar]
- 2.Azzoni, L., E. Papasavvas, J. Chehimi, J. R. Kostman, K. Mounzer, J. Ondercin, B. Perussia, and L. J. Montaner. 2002. Sustained impairment of IFN-gamma secretion in suppressed HIV-infected patients despite mature NK cell recovery: evidence for a defective reconstitution of innate immunity. J. Immunol. 168:5764-5770. [DOI] [PubMed] [Google Scholar]
- 3.Biron, C., and L. Brossay. 2001. NK cells and NKT cells in innate defense against viral infections. Curr. Opin. Immunol. 13:458-464. [DOI] [PubMed] [Google Scholar]
- 4.Biron, C. A. 1997. Activation and function of natural killer cell responses during viral infections. Curr. Opin. Immunol. 9:24-34. [DOI] [PubMed] [Google Scholar]
- 5.De Maria, A., M. Fogli, P. Costa, G. Murdaca, F. Puppo, D. Mavilio, A. Moretta, and L. Moretta. 2003. The impaired NK cell cytolytic function in viremic HIV-1 infection is associated with a reduced surface expression of natural cytotoxicity receptors (NKp46, NKp30, and NKp44). Eur. J. Immunol. 33:2410-2418. [DOI] [PubMed] [Google Scholar]
- 6.de Souza, M. S., C. Karnasuta, A. E. Brown, L. E. Markowitz, S. Nitayaphan, R. P. Garner, J. G. McNeil, D. L. Birx, and J. H. Cox. 2000. A comparative study of the impact of HIV infection on natural killer cell number and function in Thais and North Americans. AIDS Res. Hum. Retrovir. 16: 1061-1066. [DOI] [PubMed] [Google Scholar]
- 7.Diefenbach, A., and D. Raulet. 2001. Strategies for target cell recognition by natural killer cells. Immunol. Rev. 181:170-184. [DOI] [PubMed] [Google Scholar]
- 8.Dines, I., V. M. Rumjanek, and P. M. Persechini. 2004. What is going on with natural killer cells in HIV infection? Int. Arch. Allergy Immunol. 133: 330-339. [DOI] [PubMed] [Google Scholar]
- 9.Gillespie, G. M., R. Kaul, T. Dong, H. B. Yang, T. Rostron, J. J. Bwayo, P. Kiama, T. Peto, F. A. Plummer, A. J. McMichael, and S. L. Rowland-Jones. 2002. Cross-reactive cytotoxic T lymphocytes against a HIV-1 p24 epitope in slow progressors with B*57. AIDS 16:961-972. [DOI] [PubMed] [Google Scholar]
- 10.Hogan, C. M., and S. M. Hammer. 2001. Host determinants in HIV infection and disease. Part 1. Cellular and humoral immune responses. Ann. Intern. Med. 134:761-776. [DOI] [PubMed] [Google Scholar]
- 11.Ironson, G., E. Balbin, G. Solomon, J. Fahey, N. Klimas, N. Schneiderman, and M. A. Fletcher. 2001. Relative preservation of natural killer cell cytotoxicity and number in healthy AIDS patients with low CD4 cell counts. AIDS 15:2065-2073. [DOI] [PubMed] [Google Scholar]
- 12.Jansen, C. A., E. Piriou, C. Bronke, J. Vingerhoedb, and S. Kostense. 2004. Characterization of virus-specific CD8+ effector T cells in the course of HIV-1 infection: longitudinal analyses in slow and rapid progressors. 113:299-309. [DOI] [PubMed]
- 13.Keoshkerian, E., L. J. Ashton, D. G. Smith, J. B. Ziegler, J. M. Kaldor, D. A. Cooper, G. J. Stewart, and R. A. Ffrench. 2003. Effector HIV-specific cytotoxic T-lymphocyte activity in long-term nonprogressors: associations with viral replication and progression. J. Med. Virol. 71:483-491. [DOI] [PubMed] [Google Scholar]
- 14.Kim, C., B. Johnston, and E. Butcher. 2002. Trafficking machinery of NKT cells: shared and differential chemokine receptor expression among Vα24+Vβ11+NKT cell subsets with distinct cytokine-producing capacity. Blood 100:11-16. [DOI] [PubMed] [Google Scholar]
- 15.Kottilil, S., T. W. Chun, S. Moir, S. Liu, M. McLaughlin, C. W. Hallahan, F. Maldarelli, L. Corey, and A. S. Fauci. 2003. Innate immunity in human immunodeficiency virus infection: effect of viremia on natural killer cell function. J. Infect. Dis. 187:1038-1045. [DOI] [PubMed] [Google Scholar]
- 16.Levy, J. A. 2001. The importance of the innate immune system in controlling HIV infection and disease. Trends Immunol. 22:312-316. [DOI] [PubMed] [Google Scholar]
- 17.Margolick, J. B., E. R. Scott, N. Odaka, and A. J. Saah. 1991. Flow cytometric analysis of gamma delta T cells and natural killer cells in HIV-1 infection. Clin. Immunol. Immunopathol. 58:126-138. [DOI] [PubMed] [Google Scholar]
- 18.Miller, J. 2001. Biology of natural killer cells in cancer, infection, and pregnancy. Exp. Hematol. 29:1157-1168. [DOI] [PubMed] [Google Scholar]
- 19.Moretta, L., M. C. Mingari, D. Pende, C. Bottino, R. Biassoni, and A. Moretta. 1996. The molecular basis of natural killer (NK) cell recognition and function. J. Clin. Immunol. 16:243-253. [DOI] [PubMed] [Google Scholar]
- 20.Murphy, W. J., C. Y. Koh, A. Raziuddin, M. Bennett, and D. L. Longo. 2001. Immunobiology of natural killer cells and bone marrow transplantation: merging of basic and preclinical studies. Immunol. Rev. 181:279-289. [DOI] [PubMed] [Google Scholar]
- 21.Oya, H., T. Kawamura, T. Shimizu, M. Bannai, H. Kawamura, M. Minagawa, H. Watanabe, K. Hatakeyama, and T. Abo. 2000. The differential effect of stress on natural killer T (NKT) and NK cell function. Clin. Exp. Immunol. 121: 384-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rhodes, D. I., L. Ashton, A. Solomon, A. Carr, D. Cooper, J. Kaldor, and N. Deacon. 2000.Characterization of three nef-defective human immunodeficiency virus type 1 strains associated with long-term nonprogression. J. Virol. 74:10581-10588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scott-Algara, D., L. X. Truong, P. Versmisse, A. David, T. T. Luong, N. V. Nguyen, I. Theodorou, F. Barre-Sinoussi, and G. Pancino. 2003. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J. Immunol. 171:5663-5667. [DOI] [PubMed] [Google Scholar]
- 24.Ullum, H., A. Cozzi Lepri, H. Aladdin, T. Katzenstein, J. Victor, A. N. Phillips, J. Gerstoft, P. Skinhoj, and B. Klarlund Pedersen. 1999. Natural immunity and HIV disease progression. AIDS 13:557-563. [DOI] [PubMed] [Google Scholar]