Abstract
To determine the effects of porcine circovirus type 2 (PCV2) maternal antibodies on and response to experimental PCV2 infection, 24 piglets were divided into four groups on the basis of the enzyme-linked immunosorbent assay titers of PCV2 maternal antibodies: group A (n = 6; sample/positive [S/P] ratio, <0.2), group B (n = 5; S/P ratio, >0.2 to <0.5), and groups C (n = 8) and D (n = 5) (S/P ratio, >0.5). Piglets in groups A, B, and C were inoculated with PCV2 at day 0 and challenged with PCV2 at day 42. Group D piglets were not exposed to PCV2 at day 0 but were challenged at day 42. Before challenge, seroconversion to PCV2 antibodies occurred in five of six group A piglets, and the antibody level rose above the cutoff level in one of five group B piglets. Viremia was detected in five of six, four of five, and two of eight pigs in groups A, B, and C, respectively. After challenge, PCV2 DNA was detectable from 7 to 21 days postchallenge in the sera from six of six, four of five, three of eight, and five of five pigs in groups A, B, C, and D, respectively. The results indicated that protection against PCV2 infection conferred by maternal antibodies is titer dependent: higher titers are generally protective, but low titers are not.
Porcine circovirus (PCV) was initially isolated as a persistent contaminant of the porcine kidney PK-15 cell line (25). PCV is a ubiquitous virus that does not cause any disease in piglets (3, 26). Recently, a new swine disease named postweaning multisystemic wasting syndrome (PMWS) (14) was linked to a variant strain of PCV (1). The PMWS-associated PCV was designated PCV type 2 (PCV2), whereas the nonpathogenic PK-15 cell-derived PCV was designated PCV1 (2, 7). PCV2, a member of the family Circoviridae (27), is a nonenveloped, single-stranded DNA virus of 1,768 bp (1, 2, 5, 7, 13). The ORF2 gene of PCV2 encodes the major capsid protein that contains neutralizing epitopes (5, 10, 12, 16), whereas the ORF1 gene encodes Rep proteins that are involved in virus replication (5, 13). The genetic determinant for PCV2 virulence is not known (5), although two amino acids in the capsid gene are involved in PCV2 attenuation (10).
PMWS mainly affects 5- to 16-week-old pigs (2, 14, 23). The characteristic clinical symptoms of PMWS include progressive weight loss, dyspnea, enlargement of lymph nodes, diarrhea, pallor, and jaundice (2, 23). The hallmark microscopic lesions in PCV2-infected pigs are lymphoid depletion and histiocytic replacement of lymphoid follicles (2, 23). Piglets coinfected with PCV2 and porcine parvovirus, PCV2 and porcine reproductive and respiratory syndrome virus, or PCV2 and Mycoplasma hyopneumoniae had more severe clinical disease and PCV2-associated lesions than piglets infected with PCV2 (4, 6, 15, 19, 20, 22). A vaccine against PCV2 is not yet available, although an experimental vaccine based on a chimeric virus of PCV1 and PCV2 is very promising (8, 9, 11).
Passively acquired antibodies generally confer protection against viral infections to newborn piglets (21, 24, 28). For example, maternal antibodies to group A porcine rotavirus play an important role in reducing the severity of clinical diseases following rotavirus infection, but the level of protection depended upon the titer of the circulating maternal antibodies in the newborns (21, 28). Similarly, higher titers of maternal antibodies to classical swine fever virus (CSFV) inhibited both cell-mediated and humoral immune responses to a CSFV vaccine, but lower titers of maternal antibodies (titer, <64) have no significant effect on CSFV vaccine (24). Under field conditions, PCV2 infection is widespread and most breeding age pigs are seropositive, and consequently, the majority of newborns have variable levels of PCV2 maternal antibodies (18). The window of time over which passive antibodies decay is wide and variable (18). The objective of this study was to assess the role of PCV2 maternal antibodies in preventing PCV2 infection in piglets.
To select piglets with various levels of PCV2 maternal antibodies for the study, a total of 106 specific-pathogen-free piglets free of M. hyopneumoniae and porcine reproductive and respiratory syndrome virus were acquired from a commercial source at 12 days of age. All piglets were tested for the presence of maternal antibody by a PCV2-specific enzyme-linked immunosorbent assay (ELISA) prior to grouping (17). Of the 106 piglets tested, 24 piglets were selected for this experiment solely on the basis of the titers of PCV2 maternal antibodies. Piglets were separated into four different groups and rooms on the basis of the titers of maternal antibodies detected 2 days prior to experimental exposure to PCV2. Six piglets with maternal antibodies at a sample/positive (S/P) ratio of <0.2 were placed in group A, and five piglets with maternal antibodies at an S/P ratio between 0.2 and 0.5 were placed in group B. Of the 13 pigs with maternal antibodies at an S/P ratio of >0.5, 8 were assigned to group C and 5 to group D (Table 1). The ELISA S/P ratio cutoff is determined to be 0.2 (17); therefore, piglets at an S/P ratio <0.2 are considered negative for maternal antibodies. Piglets with an S/P ratio of >0.2 to <0.5 are considered to have a low level of detectable maternal antibodies, whereas piglets with an S/P ratio of >0.5 have a high level of maternal antibodies (18).
TABLE 1.
Serum viral DNA loads (genomic copy per ml serum) in pigs throughout the study as detected by quantitative PCR
| Groupa or parameter | Pig IDb | Serum viral DNA load atc:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 dpi | 7 dpi | 14 dpi | 21 dpi | 28 dpi | 35 dpi | 42 dpi (0 dpc) | 49 dpi (7 dpc) | 56 dpi (14 dpc) | 63 dpi (21 dpc) | ||
| Group A | 10 | − | − | − | 2.6 × 107 | 1.4 × 107 | 1.2 × 106 | 3.2 × 106 | 3.4 × 106 | 1.2 × 106 | 4.7 × 106 |
| 13 | − | − | − | − | − | − | − | 2.5 × 105 | 1.3 × 106 | 2.0 × 105 | |
| 36 | − | − | 3.0 × 106 | 5.5 × 108 | 3.7 × 108 | 3.1 × 107 | 2.56 × 108 | 1.8 × 109 | 1.1 × 107 | 1.5 × 107 | |
| 39 | − | − | − | 6.3 × 108 | 2.4 × 107 | 6.2 × 106 | − | 1.4 × 106 | 1.2 × 105 | 8.7 × 105 | |
| 47 | − | − | 1.3 × 105 | 5.0 × 105 | 1.9 × 106 | 5.3 × 104 | 1.7 × 105 | 2.0 × 105 | 3.0 × 105 | 2.9 × 105 | |
| 53 | − | − | − | 2.8 × 108 | 6.3 × 106 | 1.0 × 109 | 7.8 × 105 | 3.0 × 105 | 2.4 × 107 | 5.6 × 104 | |
| No. of pigs with viremiad | 0/6 | 0/6 | 2/6 | 5/6 | 5/6 | 5/6 | 4/6 | 6/6 | 6/6 | 6/6 | |
| Group B | 21 | − | − | − | − | 1.0 × 107 | 3.5 × 107 | 2.3 × 106 | 5.8 × 107 | 3.5 × 108 | 6.0 × 105 |
| 26 | − | − | − | 4.7 × 106 | 7.5 × 104 | 8.7 × 104 | 1.7 × 106 | 5.4 × 105 | 1.9 × 105 | 8.6 × 104 | |
| 29 | − | − | − | 4.6 × 106 | 5.8 × 106 | 2.5 × 105 | 1.3 × 106 | 1.4 × 106 | 2.2 × 106 | 2.6 × 106 | |
| 43 | − | − | − | − | 3.3 × 104 | 5.5 × 104 | 8.4 × 105 | 9.6 × 104 | 3.2 × 104 | 1.5 × 106 | |
| 71 | − | − | − | − | − | − | − | − | − | − | |
| No. of pigs with viremia | 0/5 | 0/5 | 0/5 | 2/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | 4/5 | |
| Group C | 9 | − | − | − | − | − | − | − | − | − | − |
| 15 | − | − | − | 1.9 × 104 | 2.3 × 105 | 5.6 × 104 | 3.6 × 104 | 3.6 × 104 | 9.8 × 104 | − | |
| 30 | − | − | − | − | 2.7 × 105 | 1.7 × 104 | 3.3 × 105 | 3.3 × 105 | 3.3 × 105 | 7.5 × 104 | |
| 34 | − | − | − | − | − | − | − | − | − | − | |
| 50 | − | − | − | − | − | − | − | − | − | − | |
| 54 | − | − | − | − | − | − | − | − | − | − | |
| 61 | − | − | − | − | − | − | − | 9.2 × 105 | 4.3 × 105 | 1.5 × 105 | |
| 68 | − | − | − | − | − | − | − | − | − | − | |
| No. of pigs with viremia | 0/8 | 0/8 | 0/8 | 1/8 | 2/8 | 2/8 | 2/8 | 3/8 | 3/8 | 2/8 | |
| Group D | 4 | − | − | − | − | − | − | − | − | − | 1.1 × 106 |
| 12 | − | − | − | − | − | − | − | 3.9 × 105 | − | ||
| 81 | − | − | − | − | − | − | − | − | 2.5 × 105 | 2.1 × 104 | |
| 82 | − | − | − | − | − | − | − | − | 7.5 × 106 | 3.9 × 105 | |
| 106 | − | − | − | − | − | − | − | − | 1.2 × 105 | − | |
| No. of pigs with viremia | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 0/5 | 4/5 | 4/5 | |
Group A, negative maternal antibody (S/P ratio of <0.2); group B, low maternal antibody (S/P ratio of >0.2 to <0.5); groups C and D, high maternal antibody (S/Pratio of >0.5).
Pig ID, pig identification number.
Serum viral DNA load (number of genomic copies per milliliter of serum) in pigs at the indicated day postinoculation (dpi) or day postchallenge (dpc). −, negative by quantitative PCR.
Number of pigs with viremia/total number of pigs in the group.
An infectious PCV2 virus stock was generated by transfecting PK-15 cells with a PCV2 infectious DNA clone (8-11), and the infectivity titer of the PCV2 virus stock was subsequently determined as previously described (8-11) and used for the animal experiment. Piglets from groups A, B, and C (Table 1) were all exposed to PCV2 at day 0: each received 3 ml (1 × 103.55 50% tissue culture infective doses) of PCV2 by slow instillation into the nasal cavity. Piglets in group D were not exposed to PCV2 at day 0 (Table 1). Piglets in groups A, B, C, and D were all challenged with 3 ml (1 × 104.7 50% tissue culture infective doses) of a homologous PCV2 at 42 days postinoculation (dpi). To maximize the exposure of pigs to PCV2 challenge, approximately 1 ml of inoculum was given intramuscularly, and 2 ml was given intranasally. All piglets were bled prior to inoculation and weekly thereafter, and necropsied at 21 days postchallenge (dpc).
Viremia and PCV2 DNA load in sera were determined by a modified quantitative PCR, and the standardization of the assay has previously been described (9-11). A standard dilution series with a known amount of plasmid containing a single copy of the PCV2 genome was run simultaneously with samples in each reaction (9, 11). After amplification, a melt curve analysis was performed to assure the correct product was formed. Quantification of viral genomic copies per milliliter (GC/ml) of serum was then carried out essentially as previously described (10, 11). The sera were also tested for anti-PCV2 antibodies by ELISA as previously described (17). S/P ratios of PCV2 antibody and PCV2 DNA loads were compared and evaluated by a simple t test, and analysis of variance and regression analysis were performed using the TTEST and GLM procedures of SAS (version 9.1; SAS Institute, Inc., Cary, NC).
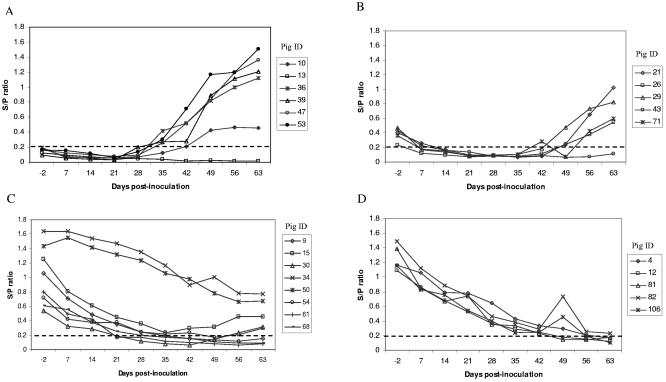
After exposure to PCV2, seroconversion started at 35 dpi in four of six piglets in group A. By 42 dpi, five of six piglets had seroconverted (Fig. 1). The onset of viremia in two of the six group A piglets occurred at 14 dpi, and they had a viral DNA load ranging from 105 to 106 GC/ml serum (Table 1). All but one piglet in group A had viremia by 42 dpi at the time points tested. The low levels of maternal antibodies in group B piglets waned by 14 dpi. At 21 dpi, the five group B piglets had become seronegative (S/P ratio, <0.2) and remained so through 35 dpi. At 42 dpi, one pig in group B (pig 71) seroconverted and the other four pigs were still seronegative (Fig. 1). Viremia was first detected in two of five group B piglets at 21 dpi, and by 42 dpi, four piglets had viremia. The high level of maternal antibodies in group C pigs gradually waned from 7 to 42 dpi, and there was no rise of antibody titer between 7 and 42 dpi in any piglets (Fig. 1). Viremia was detected in one of eight piglets (pig 15) at 21 dpi (Table 1), and by 42 dpi, only two piglets in group C had viremia, with a viral DNA load ranging from 104 to 105 GC/ml serum. Statistical analysis showed that the day of peak viremia in infected piglets was not related to the initial maternal antibody level (P = 0.50). Peak viremia level decreased with increasing maternal antibody levels present at −2 dpi (P = 0.025). The mean antibody level at −2 dpi in the piglets that became infected with PCV2 was lower (S/P ratio, 0.37; standard deviation, 0.328) than in piglets that did not become infected (S/P ratio, 0.84; standard deviation, 0.515) (P = 0.044).
FIG. 1.
Kinetics of PCV2 antibody in response to infection and PCV2 maternal antibody decay for each group of piglets throughout the experiment. Piglets in groups A, B, and C were exposed to PCV2 at day 0 (day postinoculation). Piglets in group D were not exposed to PCV2 at day 0 but were challenged with a homologous PCV2 at day 42, along with all piglets in the other groups. The criteria used to assign piglets to groups on the basis of maternal antibody levels prior to inoculation with PCV2 were as follows: group A, n = 6, negative maternal antibody (S/P ratio, <0.2); group B, n = 5, low level of maternal antibodies (S/P ratio, >0.2 to <0.5); groups C (n = 8) and D (n = 5), high level of maternal antibodies (S/P ratio, >0.5). Pig identification (ID) numbers are given. The broken lines show an S/P ratio of 0.2.
These results suggested that the presence of low levels of PCV2 maternal antibodies does not protect piglets from experimental PCV2 infection and that high levels of PCV2 maternal antibodies generally confer protection against PCV2 infection, but not total protection. Statistical analysis showed that peak viremia levels in piglets were reduced in those piglets with higher antibody levels at the time of inoculation (P = 0.025). When piglets in group D were challenged at day 42, all five piglets became infected. These results could explain why many neonatal piglets born to PCV2-positive sows are still susceptible to PCV2 infection in swine farms under field conditions.
To determine the length of protection that PCV2 maternal antibodies can confer to the piglets and to assess the outcome of prior PCV2 exposure on reinfection of piglets by PCV2, we challenged all piglets with PCV2 at 42 dpi. At the time of challenge, five of six pigs in group A were seropositive in response to the initial PCV2 exposure at dpi 0 (Fig. 1), and four of six piglets were viremic at challenge (Table 1). The maternal antibodies in group B piglets all fell below the S/P ratio cutoff value by 21 dpi, and all but one piglet were seronegative (Fig. 1) and 4 of 5 piglets were viremic at the time of challenge at 42 dpi (Table 1). In group C, at the time of challenge at 42 dpi, four piglets were still positive for PCV2 maternal antibodies with S/P ratios ranging from 0.23 to 0.98; the S/P ratios in the other four pigs were all <0.2 (Fig. 1), and two piglets were viremic at the time of challenge (Table 1).
After challenge, PCV2 antibody levels in piglets from groups A and B continued to rise. In contrast, there was no detectable increase in PCV2 antibody levels in group C piglets after challenge. After challenge, all six piglets in group A were viremic and had serum viral DNA loads ranging from 105 to 109 GC/ml serum at 7 dpc and from 104 to 107 GC/ml serum at 21 dpc. In group B piglets, four of five piglets remained viremic and had serum viral DNA loads ranging from 104 to 107 GC/ml serum at 7 dpc (Table 1). After challenge, two group C piglets remained viremic with no change in the range of serum viral DNA load, and only one additional piglet, which had a very low S/P ratio at the time of challenge, developed viremia (Table 1). Piglet 71 in group B and piglet 54 in group C showed an increase in antibody titer above the cutoff value after challenge, even though viremia remained undetectable in the two pigs (Fig. 1 and Table 1). This could be explained by localized replication of the virus in lymphoid tissues during the early phase of replication, thus resulting in an undetectable level of viremia. Also, piglet 43 in group B had low but persistent viremia since 28 dpi but was seronegative throughout the study. The relatively low level of viremia could explain the absence of a significant increase of antibody in this pig (Table 1). The group D piglets were not exposed to PCV2 until day 42, and their maternal antibody level waned gradually over the course of the experiment (Fig. 1). At the time of challenge, all five group D pigs still had low levels of maternal antibodies, with S/P ratios ranging from 0.24 to 0.33. After challenge, there was no detectable rise of PCV2 antibody titer (Fig. 1), but all five group D piglets developed viremia at 14 dpc.
These results showed that the PCV2-exposed piglets with no or low levels of maternal antibodies (groups A and B) were not protected from the homologous challenge by PCV2, as evidenced by continuous viremia despite rising antibody levels. At the time of challenge, five of six group A piglets had already developed an active serum PCV2 antibody response from the initial exposure. Therefore, it is likely that both the humoral immune response and cell-mediated immune response are required for full protection (11). Future studies with PCV2 challenge beyond 42 dpi are needed to fully evaluate the effects of prior PCV2 exposure on homologous PCV2 challenge.
In conclusion, the results from this study indicated that the levels of PCV2 maternal antibodies are an important determinant of a piglet's response to PCV2 infection. It appears that the higher the levels of maternal antibodies, the more protection the piglets will have. The data from this study have important implications for selecting the optimal timing of vaccination, especially with a live PCV2 vaccine when it becomes available. Since the majority of newborns have PCV2 maternal antibodies, a live PCV2 vaccine will work most efficiently when given to piglets older than 7 to 8 weeks of age, at which time the maternal antibodies have mostly waned.
Acknowledgments
This study was funded in part by a grant from Fort Dodge Animal Health, Inc., and by a grant from USDA-NRI (2004-35204-14213).
We thank S. M. Boyle, L. A. Eng, W. Huckle, S. Tolin, and T. Toth for their support. We also thank Stephen Wu and Mike Gill of Fort Dodge Animal Health for their support.
REFERENCES
- 1.Allan, G., B. Meehan, D. Todd, S. Kennedy, F. McNeilly, J. Ellis, E. G. Clark, J. Harding, E. Espuna, A. Botner, and C. Charreyre. 1998. Novel porcine circoviruses from pigs with wasting disease syndromes. Vet. Rec. 142:467-468. [PubMed] [Google Scholar]
- 2.Allan, G. M., and J. A. Ellis. 2000. Porcine circoviruses: a review. J. Vet. Diagn. Investig. 12:3-14. [DOI] [PubMed] [Google Scholar]
- 3.Allan, G. M., F. McNeilly, J. P. Cassidy, G. A. Reilly, B. Adair, W. A. Ellis, and M. S. McNulty. 1995. Pathogenesis of porcine circovirus: experimental infections of colostrum deprived piglets and examination of pig foetal material. Vet. Microbiol. 44:49-64. [DOI] [PubMed] [Google Scholar]
- 4.Allan, G. M., F. McNeilly, J. Ellis, S. Krakowka, B. Meehan, I. McNair, I. Walker, and S. Kennedy. 2000. Experimental infection of colostrum deprived piglets with porcine circovirus 2 (PCV2) and porcine reproductive and respiratory syndrome virus (PRRSV) potentiates PCV2 replication. Arch. Virol. 145:2421-2429. [DOI] [PubMed] [Google Scholar]
- 5.de Boisseson, C., V. Beven, L. Bigarre, R. Thiery, N. Rose, E. Eveno, F. Madec, and A. Jestin. 2004. Molecular characterization of porcine circovirus type 2 isolates from post-weaning multisystemic wasting syndrome-affected and non-affected pigs. J. Gen. Virol. 85:293-304. [DOI] [PubMed] [Google Scholar]
- 6.Ellis, J. A., A. Bratanich, E. G. Clark, G. Allan, B. Meehan, D. M. Haines, J. Harding, K. H. West, S. Krakowka, C. Konoby, L. Hassard, K. Martin, and F. McNeilly. 2000. Coinfection by porcine circoviruses and porcine parvovirus in pigs with naturally acquired postweaning multisystemic wasting syndrome. J. Vet. Diagn. Investig. 12:21-27. [DOI] [PubMed] [Google Scholar]
- 7.Fenaux, M., P. G. Halbur, M. Gill, T. E. Toth, and X. J. Meng. 2000. Genetic characterization of type 2 porcine circovirus (PCV-2) from pigs with postweaning multisystemic wasting syndrome in different geographic regions of North America and development of a differential PCR-restriction fragment length polymorphism assay to detect and differentiate between infections with PCV-1 and PCV-2. J. Clin. Microbiol. 38:2494-2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenaux, M., P. G. Halbur, G. Haqshenas, R. Royer, P. Thomas, P. Nawagitgul, M. Gill, T. E. Toth, and X. J. Meng. 2002. Cloned genomic DNA of type 2 porcine circovirus is infectious when injected directly into the liver and lymph nodes of pigs: characterization of clinical disease, virus distribution, and pathologic lesions. J. Virol. 76:541-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenaux, M., T. Opriessnig, P. G. Halbur, F. Elvinger, and X. J. Meng. 2004. A chimeric porcine circovirus (PCV) with the immunogenic capsid gene of the pathogenic PCV type 2 (PCV2) cloned into the genomic backbone of the nonpathogenic PCV1 induces protective immunity against PCV2 infection in pigs. J. Virol. 78:6297-6303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fenaux, M., T. Opriessnig, P. G. Halbur, F. Elvinger, and X. J. Meng. 2004. Two amino acid mutations in the capsid protein of type 2 porcine circovirus (PCV2) enhanced PCV2 replication in vitro and attenuated the virus in vivo. J. Virol. 78:13440-13446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenaux, M., T. Opriessnig, P. G. Halbur, and X. J. Meng. 2003. Immunogenicity and pathogenicity of chimeric infectious DNA clones of pathogenic porcine circovirus type 2 (PCV2) and nonpathogenic PCV1 in weanling pigs. J. Virol. 77:11232-11243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fenaux, M., T. Opriessnig, P. G. Halbur, Y. Xu, B. Potts, and X. J. Meng. 2004. Detection and in vitro and in vivo characterization of porcine circovirus DNA from a porcine-derived commercial pepsin product. J. Gen. Virol. 85:3377-3382. [DOI] [PubMed] [Google Scholar]
- 13.Hamel, A. L., L. L. Lin, and G. P. Nayar. 1998. Nucleotide sequence of porcine circovirus associated with postweaning multisystemic wasting syndrome in pigs. J. Virol. 72:5262-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harding, J. C., and E. G. Clark. 1997. Recognizing and diagnosing postweaning multisystemic wasting syndrome (PMWS). J. Swine Health Production 5:201-203. [Google Scholar]
- 15.Krakowka, S., J. A. Ellis, B. Meehan, S. Kennedy, F. McNeilly, and G. Allan. 2000. Viral wasting syndrome of swine: experimental reproduction of postweaning multisystemic wasting syndrome in gnotobiotic swine by coinfection with porcine circovirus 2 and porcine parvovirus. Vet. Pathol. 37:254-263. [DOI] [PubMed] [Google Scholar]
- 16.Lekcharoensuk, P., I. Morozov, P. S. Paul, N. Thangthumniyom, W. Wajjawalku, and X. J. Meng. 2004. Epitope mapping of the major capsid protein of type 2 porcine circovirus (PCV2) by using chimeric PCV1 and PCV2. J. Virol. 78:8135-8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nawagitgul, P., P. A. Harms, I. Morozov, B. J. Thacker, S. D. Sorden, C. Lekcharoensuk, and P. S. Paul. 2002. Modified indirect porcine circovirus (PCV) type 2-based and recombinant capsid protein (ORF2)-based enzyme-linked immunosorbent assays for detection of antibodies to PCV. Clin. Diagn. Lab. Immunol. 9:33-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Opriessnig, T., S. Yu, E. L. Thacker, and P. G. Halbur. 2004. Derivation of porcine circovirus type 2-negative pigs from positive sow herds. J. Swine Health Production 12:186-191. [Google Scholar]
- 19.Opriessnig, T., M. Fenaux, S. Yu, R. B. Evans, D. Cavanaugh, J. M. Gallup, F. J. Pallares, E. L. Thacker, K. M. Lager, X. J. Meng, and P. G. Halbur. 2004. Effect of porcine parvovirus vaccination on the development of PMWS in segregated early weaned pigs coinfected with type 2 porcine circovirus and porcine parvovirus. Vet. Microbiol. 98:209-220. [DOI] [PubMed] [Google Scholar]
- 20.Opriessnig, T., E. L. Thacker, S. Yu, M. Fenaux, X. J. Meng, and P. G. Halbur. 2004. Experimental reproduction of postweaning multisystemic wasting syndrome in pigs by dual infection with Mycoplasma hyopneumoniae and porcine circovirus type 2. Vet. Pathol. 41:624-640. [DOI] [PubMed] [Google Scholar]
- 21.Parreno, V., D. C. Hodgins, L. de Arriba, S. Y. Kang, L. Yuan, L. A. Ward, T. L. To, and L. J. Saif. 1999. Serum and intestinal isotype antibody responses to Wa human rotavirus in gnotobiotic pigs are modulated by maternal antibodies. J. Gen. Virol. 80:1417-1428. [DOI] [PubMed] [Google Scholar]
- 22.Rovira, A., M. Balasch, J. Segales, L. Garcia, J. Plana-Duran, C. Rosell, H. Ellerbrok, A. Mankertz, and M. Domingo. 2002. Experimental inoculation of conventional pigs with porcine reproductive and respiratory syndrome virus and porcine circovirus 2. J. Virol. 76:3232-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segales, J., C. Rosell, and M. Domingo. 2004. Pathological findings associated with naturally acquired porcine circovirus type 2 associated disease. Vet. Microbiol. 98:137-149. [DOI] [PubMed] [Google Scholar]
- 24.Suradhat, S., and S. Damrongwatanapokin. 2003. The influence of maternal immunity on the efficacy of a classical swine fever vaccine against classical swine fever virus, genogroup 2.2, infection. Vet. Microbiol. 92:187-194. [DOI] [PubMed] [Google Scholar]
- 25.Tischer, I., H. Gelderblom, W. Vettermann, and M. A. Koch. 1982. A very small porcine virus with circular single-stranded DNA. Nature 295:64-66. [DOI] [PubMed] [Google Scholar]
- 26.Tischer, I., W. Mields, D. Wolff, M. Vagt, and W. Griem. 1986. Studies on epidemiology and pathogenicity of porcine circovirus. Arch. Virol. 91:271-276. [DOI] [PubMed] [Google Scholar]
- 27.Todd, D., M. S. McNulty, A. Mankertz, P. D. Lukert, J. W. Randles, and J. L. Dale. 2000. Family Circoviridae. In M. H. V. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carstens, M. K. Estes, S. M. Lemon, J. Maniloff, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy: classification and nomenclature of viruses. Seventh report of the International Committee on Taxonomy of Viruses. Academic Press, San Diego, Calif.
- 28.Ward, L. A., E. D. Rich, and T. E. Besser. 1996. Role of maternally derived circulating antibodies in protection of neonatal swine against porcine group A rotavirus. J. Infect. Dis. 174:276-282. [DOI] [PubMed] [Google Scholar]