Abstract
Yersinia enterocolitica and Yersinia pseudotuberculosis have been identified as causative organisms of reactive arthritis in humans. We evaluated a Western blot assay which uses Yersinia outer membrane proteins as antigens for the detection of Yersinia antibodies as a replacement for the complement fixation (CF) assay. Clinical agreement, sensitivity, and specificity were determined by testing 19 positive and 21 negative serum samples by the CF assay, Western blot assay, and enzyme-linked immunosorbent assay (ELISA). The CF assay and ELISA were compared to the Western blot assay, which was the reference method used in this study. Sera with antibodies that could potentially cross-react with Yersinia were also tested by the Western blot assay. The agreement, sensitivity, and specificity of the CF method were 61%, 26%, and 95%, respectively; and those for the ELISA were 89%, 95%, and 82%, respectively. The prevalences of Yersinia antibodies in 50 healthy donors were 6% for immunoglobulin G (IgG), 2% for IgA, and 2% for IgM. Sera positive for Bartonella henselae, Brucella, Chlamydia pneumoniae, and Rickettsia rickettsii antibodies showed cross-reactivity by the Western blot assay. The highest cross-reactivity was observed with Borrelia burgdorferi; 5 of 11 (45%) specimens were cross-reactive by the IgM-specific assay. Overall, the Western blot assay performs acceptably and is more sensitive than the CF assay, warranting replacement of the CF assay in the laboratory. Due to the evidence of cross-reactivity, particularly with B. burgdorferi, which can cause an oligoarthritis similar to reactive arthritis, the diagnosis of reactive arthritis should be based on clinical findings and complete serologic analysis of the potential causative infectious pathogens.
The genus Yersinia consists of two different gram-negative coccobacillus species that are known to cause enteric infections in humans: Yersinia enterocolitica and Yersinia pseudotuberculosis. Infections with Y. enterocolitica are transmitted primarily to humans through soil, water, animals, and food. Infections with Y. enterocolitica most often occur in young children. The infection manifests in the gastrointestinal tract, causing symptoms of diarrhea; loose, watery, or bloody stools; abdominal pain; and fever (2). Y. pseudotuberculosis is less pathogenic and causes a zoonotic disease with symptoms similar to those caused by Y. enterocolitica. Infections with Y. enterocolitica and Y. pseudotuberculosis can be asymptomatic, mild, or severe and resolve within a few weeks, with or without the use of antibiotics, depending on the severity (14). Complications can occur, however, with the development of an inflammatory arthritis known as reactive arthritis, which can manifest 1 to 4 weeks postinfection. There is an increased risk for the development of reactive arthritis if the individual is positive for the major histocompatibility complex HLA-B27 allele (5).
The incidence of reactive arthritis following Y. enterocolitica infection is very high among adults in Scandinavia, where it is estimated to be 10 to 30% (20). The incidence is much lower in most other countries, including the United States. The most commonly affected joints are the knees and ankles; but other joints, such as the toe, finger, and wrist joints, can be involved. In most cases, two to four joints become involved sequentially and asymmetrically over a period of a few days to 2 weeks. Monoarticular arthritis occurs less commonly. In two-thirds of cases, the acute arthritis persists for 1 to 4 months. Chronic joint disease or ankylosing spondylitis occurs rarely. Subsequent complications of Y. enterocolitica infections that occur less often include reactive uveitis, iritis, conjunctivitis, glomerulonephritis, and urethritis. Reiter's syndrome (arthritis, conjunctivitis, and urethritis) is seen in only 5 to 10% of patients with yersinia-induced arthritis (4).
Serologic tests can be used to support a diagnosis of yersiniosis. With yersiniosis, antibody levels begin to rise within the first week of illness, peak in the second week, and then return to normal within 3 to 6 months. Antibodies may also remain detectable for several years. The isolation of a pathogenic Yersinia strain from feces is the most specific test for the diagnosis of yersiniosis. However, culture is not verysensitive for reactive arthritis, and serologic tests for Yersinia can be helpful diagnostically in cases with a high index of clinical suspicion (4).
Antibodies develop against the Yersinia outer membrane proteins (Yops) and usually persist at high levels for longer periods in cases with associated arthritis and chronic enteritis (7, 26). It has been reported that the assays used to detect antibodies against Yops are more sensitive and specific than stool culture and other serologic methods for the diagnosis of yersinia-associated complications (15). This study was conducted to investigate the utility of a Western blot method that uses Yop antigens for the detection of Yersinia antibodies as a replacement for the complement fixation (CF) method. The cross-reactivity of Yersinia with other bacterial species, such as Borrellia burgdorferi (3, 25), Rickettsia rickettsii (2, 23), and Brucella spp. (2, 17-19), has been reported. Additionally, cross-reactivity between Yersinia and thyroid-stimulating immunoglobulin (TSI) in patients with Graves ' disease has been shown (1, 2, 13, 24). Therefore, this study also examines the extent of cross-reactivity of Yops with these and other related bacterial species.
MATERIALS AND METHODS
Human sera.
This study was approved by the Institutional Review Board (IRB) of the University of Utah (IRB 7275). A total of 149 serum samples were used in this study. The sera were subdivided into three groups.
(i) Group I.
Group I contained two samples from patients who tested positive by the CF assay for Yersinia antibodies in the clinical laboratory, nine samples that tested positive by Western blot assay in the clinical laboratory, and eight samples that had previously been characterized as positive for Yersinia antibodies (provided by Viramed Biotech, Munich, Germany). Also in this group were 21 samples from patients who tested negative for Yersinia antibodies by the CF assay in the clinical laboratory.
(ii) Group II.
Group II contained 50 samples from patients with serologic evidence of infection by Brucella (an index value >1.1 is positive; n = 7), Bartonella henselae (immunoglobulin G [IgG] titer, ≥1:256; n = 5), Borrelia burgdorferi (any two IgM Western blot bands from 23, 39, or 41 kDa; n = 11), Chlamydia pneumoniae (IgG titer, ≥1:64; IgM titer, ≥1:20; n = 8), Coxiella burnetii (titer, ≥1:16; n = 3), Francisella tularensis (titer, ≥1:80; n = 9), Mycoplasma pneumoniae (IgG concentration, >0.32 U/liter; IgM concentration, ≥0.95 U/liter; n = 4), and Rickettsia rickettsii (titer, >1.1; n = 3). An additional 9 samples from patients with serologically positive results for TSI (≥130% of the basal activity is positive) were also included, for a total of 59 serum samples in this group. Testing of serum for antibodies against Brucella was performed by an enzyme-linked immunosorbent assay (ELISA; PANBIO Inc., Columbia, MD), testing of serum for antibodies against B. henselae was performed by an immunofluorescence assay (IFA; Focus Technologies, Cypress, CA), testing of serum for antibodies against B. burgdorferi was performed by Western blot assay (MarDx Diagnostics, Inc., Carlsbad, CA), testing of serum for antibodies against C.pneumoniae was performed by an IFA (Focus Technologies), testing of serum for antibodies against C. burnetti was performed by IFA (Focus Technologies), testing of serum for antibodies against F. tularensis was performed by agglutination (Germaine Laboratories, Inc. San Antonio, TX), testing of serum for antibodies against M. pneumoniae was performed by ELISA (GenBio, San Diego, CA), testing of serum for antibodies against R. rickettsii was performed by ELISA (PANBIO), and testing of serum for antibodies against TSI was performed by a radioimmunoassay that measures the amount of cyclic AMP (Amersham LifeScience, Arlington Heights, IL) in CHO cells (Leonard Kohn, NIDDK, NIH)(16).
(iii) Group III.
Group III contained 50 samples obtained from random healthy donors in the Salt Lake City, Utah, area in 2003.
All samples were deidentified and stored at 2 to 8°C until testing was completed. All samples were tested by a CF method (antigen supplied by Virion Inc., Morristown, NJ); the MIKROGEN recomWell Yersinia IgG-, IgA-, and IgM-specific ELISAs (QED Bioscience, Inc., San Diego, CA); and the Viramed Biotech Yersinia ViraBlot IgG-, IgA-, and IgM-specific Western blot assays (Viralab, Inc., San Diego, CA). All tests were performed according to the manufacturers' recommendations.
Complement fixation.
All samples were tested by a CF method, as described previously (6). Antigens specific for Y. pseudotuberculosis and Y. enterocolitica serotypes O3, O8, and O9 were used in each CF reaction for each sample. Samples with antibody titers ≤1:8 for Y. pseudotuberculosis or Y. enterocolitica serotype O3, O8, or O9 were considered negative for Yersinia antibodies. Samples with antibody titers >1:8 for Yersinia were tested for complement antibodies. If there were no complement antibodies present in the sera or if the titer was fourfold higher for Yersinia antibodies than for complement antibodies, the sample was considered positive.
Commercial Western blot test system.
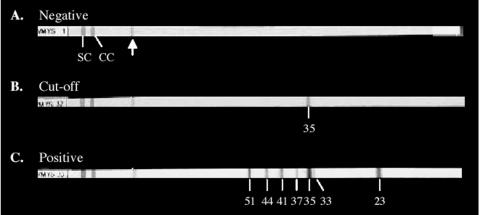
All samples were tested by Western blot assay for IgG-, IgA-, and IgM-specific antibodies (Viralab, Inc.). The assay uses antigens against Yops of pathogenic Y. enterocolitica clinical isolates for the detection of IgG-, IgA-, or IgM-specific antibodies in human serum as an aid in reactive arthritis diagnosis. The test system contains nitrocellulose test strips with Yop antigens that have been electrophoresed on a sodium dodecyl sulfate gel and transferred to the strip. Each strip contains the following Yop antigens at different sizes: YopH (51 kDa), YopM (44 kDa), YopB (41 kDa), LcrV (37kDa), YopD (35 kDa), YopN (33 kDa), and YopE (23 kDa) (12). Testing was performed according to the manufacturer's specifications. All patient serum samples were reacted with individual test strips for 30 min. Following a series of wash steps, diluted alkaline phosphatase-conjugated anti-human IgG, IgA, or IgM was incubated for 15 min on each strip. Following a final wash, chromogen substrate was added and the strips were developed for 5 to 12 min. The test results were analyzed as recommended in the corresponding technical information for each assay. The manufacturer's negative, cutoff, and positive controls (Fig. 1) were tested with each run. For IgG and IgA antibody detection, the intensity of each band appearing on the patient sample strips was compared to the intensity of the 35-kDa control band on the cutoff control strip by using the Virascan software program provided by Viralab, Inc. For IgM antibody detection, the appearance of any bands on the patient sample strips was evaluated visually, as the scanning program for this assay is still under development. The reference ranges for the IgG, IgA, and IgM Western blots are summarized in Table 1.
FIG. 1.
Western blot nitrocellulose strips reacted with the individual manufacturer's negative, cutoff, and positive control sera. Each strip contains a control section and an analytical section (the separation is indicated by an arrow). The control section contains the strip number; a serum control band (SC); and an IgG, IgA, or IgM conjugate control band (CC). The analytical section of each strip contains Yop antigens of different sizes (in kDa) that develop as dark bands if antibodies against the specific Yop antigens are present in the sera: YopH (51 kDa), YopM (44 kDa), YopB (41 kDa), LcrV (37 kDa), YopD (35 kDa), YopN (33 kDa), and YopE (23 kDa). (A) Negative control showing the appearance of no positive bands in the analytical section of the strip; (B) cutoff control, showing the appearance of the 35-kDa band in the analytical section of the strip; (C) positive control, showing the appearance of 51-, 44-, 41-, 37-, 33-, and 23-kDa bands in the analytical section of the strip.
TABLE 1.
Reference ranges for the Viramed Yersinia IgG, IgA, and IgM Western blot assays
| Ig isotype, band identified, and band size (kDa) | Result | Interpretation |
|---|---|---|
| IgG | ||
| No bands or nonspecific bands | Negative | No detectable IgG antibodies |
| Isolated positive 35-kDa band (intensity, 90 to 300 by Virascan) | Equivocal | IgG-specific antibodies against Yersinia detectable; may be an indication of a recent infection |
| Isolated positive 35-kDa band (intensity, >300 by Virascan) or positivity for at least two bands among bands of 51, 44, 41, 37, 35, 33, or 23 kDa | Positive | IgG-specific antibodies against Yersinia detectable; infection with Yersinia very likely |
| IgA | ||
| No bands or nonspecific bands | Negative | No detectable IgA antibodies |
| Isolated 35-kDa band or positivity for at least two bands among bands of 51, 44, 41, 37, 35, 33, or 23 kDa | Positive | IgA-specific antibodies against Yersinia detectable; infection with Yersinia very likely |
| IgM | ||
| No bands or nonspecific bands | Negative | No detectable IgM antibodies |
| Isolated 35-kDa band or positivity for at least two bands among bands of 51, 44, 41, 37, 35, 33, or 23 kDa | Positive | IgM-specific antibodies against Yersinia detectable; infection with Yersinia very likely |
Commercial ELISAs.
All samples were tested by the MIKROGEN recomWell IgG-, IgA-, and IgM-specific ELISAs. The ELISAs use recombinant Yop antigens that are specific for the genus Yersinia. Testing was performed according to the manufacturer's instructions. All serum samples were diluted 1:100 and reacted with specific antigen in a single well of a 96-well plate at 37°C for 1 h. Following a series of four washes, diluted horseradish peroxidase-conjugated anti-human IgG, IgA, or IgM was reacted with the wells for 30 min at 37°C. All samples were tested for IgG-, IgA-, and IgM-specific antibodies. Following four washes, the wells were developed by using the chromogenic substrate tetramethylbenzidine for 30 min. The reaction was stopped by using 25% phosphoric acid, and the optical density values were measured spectrophotometrically at 450 nm and 650 nm. The test results were analyzed as recommended in the corresponding technical information for each assay. Activity levels for IgG, IgA, and IgM antibodies were reported in units per milliliter. Samples with measured antibody activity levels <20 U/ml were assigned a negative result, samples with measured antibody activity levels ≥20 U/ml but ≤24 U/ml were assigned an equivocal result, and samples with measured antibody activity levels >24 U/ml were assigned a positive result for IgG, IgA, or IgM antibodies.
Statistical analysis.
The agreement, sensitivity, and specificity for each test method were determined by comparing the CF assay and ELISA results to the Western blot assay results by using two-by-two contingency table analysis, where equivocal results were not included in the calculations. The Western blot assay was used as the reference method in this study.
Reproducibility of Western blot assays.
The reproducibility of the IgG, IgA and IgM Western blot assays was examined by using serologically positive and negative sera that were repeatedly tested over 3 days.
Prevalence of Yersinia antibodies in the healthy population.
The prevalence of Yersinia antibodies in the healthy population was examined by each method by testing all samples from group III by the CF assay, the Western blot assays, and the ELISAs.
Cross-reactivity studies.
All samples from group II were tested by the CF assay, the Western blot assays, and the ELISAs to determine the cross-reactivity of each test method. All samples with positive results by the CF assay, the Western blot assays, or the ELISAs were identified as cross-reactive with Yersinia antibodies.
RESULTS
By using the samples from group I, 18 of the 19 positive samples were positive by the Western blot IgG (15 samples), IgA (10 samples), or IgM (3 samples) assay. Eighteen of the 21 negative samples were negative for IgG, IgA, and IgM; 1 sample was positive for IgG; and 2 samples were equivocal for IgG by the Western blot assays. By combining the IgG, IgA, and IgM results and excluding the results for the two samples with equivocal results, the Western blot assays showed 95% sensitivity and 95% specificity. By using the 8 previously characterized positive samples (excluding the 11 samples that had tested positive by either the CF or the Western blot assays), all samples tested positive by the combined Western blot assays, showing 100% sensitivity. Only 6 of 19 positive serum samples from group I were positive by the CF method and 21 of 21 negative samples were negative by the CF method, showing 32% sensitivity and 100% specificity. Of the eight previously characterized positive samples, only three samples tested positive by the CF method, for a sensitivity of 38%. For the ELISAs, the combined IgG, IgA, and IgM assay results showed that 18 of 19 positive samples from group I were positive and 14 of 21 negative samples were negative. One negative sample from group I tested equivocal by the IgG ELISA and one sample tested equivocal by the IgA ELISA. Excluding these two samples with equivocal results, the combined ELISAs showed 95% sensitivity and 74% specificity. Based on these results, the agreement, sensitivity, and specificity were determined by comparing the CF and ELISA methods to the Western blot method, which was designated the reference method in this study.
Comparison of CF method with Western blot assay.
CF is a measurement of complement-fixing IgG and IgM antibodies (28), while the Western blot assays measure IgG-, IgA-, and IgM-specific antibodies. The agreement, sensitivity, and specificity of the CF assay were determined by comparing the CF antibody results with the combined IgG, IgA, and IgM Western blot results for all of the samples from group I. The agreement, sensitivity, and specificity were 61%, 26%, and 95%, respectively (Table 2).
TABLE 2.
Agreement, sensitivity, and specificity of the Yersinia CF assay and ELISAs in comparison to the results of the Western blot assays
| Assays compared | Agreement (%) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|
| CF vs Western blota | 61 | 26 | 95 |
| ELISA vs Western blotb | 89 | 95 | 82 |
| ELISA IgG vs Western blot IgG | 86 | 94 | 79 |
| ELISA IgA vs Western blot IgA | 87 | 90 | 86 |
| ELISA IgM vs Western blot IgM | 100 | 100 | 100 |
Comparison of CF IgG and IgM results with IgG-, IgA-, and IgM-specific Western blot assay results combined.
Comparison of IgG-, IgA-, and IgM-specific ELISA results combined with IgG-, IgA-, and IgM-specific Western blot assay results combined.
Comparison of ELISAs with Western blot assay.
The ELISAs measure IgG-, IgA-, and IgM-specific antibodies individually. The agreement, sensitivity, and specificity of the ELISAs were determined by comparing the total-antibody ELISA results to the total-antibody Western blot results for all of the samples from group I. The agreement, sensitivity, and specificity were also determined for the IgG-, IgA-, and IgM-specific ELISAs by comparing their results to those of the IgG-, IgA-, and IgM-specific Western blot assays, respectively. The agreement, sensitivity, and specificity of the total-antibody ELISA results compared to the total-antibody Western blot results were 89%, 95%, and 82%, respectively. The agreement, sensitivity, and specificity of the IgG-, IgA-, and IgM-specific ELISAs compared to the results of the Western blot-specific antibody assays were 86%, 94%, and 79%, respectively, for IgG; 87%, 90%, and 86%, respectively, for IgA; and 100%, 100%, and 100%, respectively, for IgM (Table 2).
Reproducibility studies.
The reproducibilities of the Western blot assays were measured by testing a positive sample and a negative sample in duplicate on three separate runs for each assay. The reproducibility was acceptable, with no qualitative result changes for any of the samples for the IgG, IgA, and IgM assays.
Prevalence of Yersinia antibodies in the healthy population.
By the CF assay, the prevalence of Yersinia antibodies in the 50 subjects tested was 2%. By the IgG, IgA, and IgM Western blot assays, the positivity rates for Yersinia antibodies were 6%, 2%, and 2%, respectively. For the IgG, IgA, and IgM ELISAs, the positivity rates were 18%, 10%, and 4%, respectively.
Cross-reactivity studies.
All samples from group II were assayed by the CF assay, the Western blot assays, and ELISAs. For the CF assay, one of seven (14%) Brucella-positive samples, three of eight (38%) C. pneumoniae-positive samples, one of nine (11%) F. tularensis-positive samples, and one of nine (11%) TSI-positive samples tested positive for Yersinia antibodies (Table 3). For the Western blot assays, cross-reactivity was determined for IgG-, IgA-, and IgM-specific antibody types. Of the samples tested, 3 of 11 (27%) B. burgdorferi-positive samples and 1 of 7 (14%) Brucella-positive samples tested positive for Yersinia IgG antibodies; 4 of 11 (36%) B. burgdorferi-positive samples, 1 of 8 (13%) C. pneumoniae-positive samples, and 1 of 3 (33%) R. rickettsii-positive samples tested positive for Yersinia IgA antibodies; and 1 of 5 (20%) B. henselae-positive samples and 5 of 11 (45%) B. burgdorferi-positive samples tested positive for Yersinia IgM antibodies (Table 3).
TABLE 3.
Cross-reactivity observed for the Yersinia CF assay, Western blot assays, and ELISAs with samples positive for antibodies to various organisms
| Organism | Total no. of samples tested | No. of samples positive bya:
|
||||||
|---|---|---|---|---|---|---|---|---|
| CF assay | Western blotting
|
ELISA
|
||||||
| IgG | IgA | IgM | IgG | IgA | IgM | |||
| B. henselae | 5 | 1 | 1 | 2 | ||||
| B. burgdorferi | 11 | 3 | 4 | 5 | 6 | 5 | 1 | |
| Brucella | 7 | 1 | 1 | 4 | ||||
| C. pneumoniae | 8 | 3 | 1 | 2 | 2 | |||
| C. burnetii | 3 | |||||||
| F. tularensis | 9 | 1 | 2 | 3 | ||||
| M. pneumoniae | 4 | |||||||
| R. rickettsii | 3 | 1 | 1 | 1 | ||||
| TSI-positive organism | 9 | 1 | 3 | 1 | ||||
Serum Yersinia IgG and IgM antibodies by CF assay; serum IgG, IgA, and IgM antibodies by Western blot assay; and serum IgG, IgA, and IgM antibodies by ELISA.
The cross-reactivity for the ELISA was also determined for IgG-, IgA-, and IgM-specific antibodies. Of the samples tested, 1 of 5 (20%) B. henselae-positive samples, 6 of 11 (55%) B.burgdorferi-positive samples, 4 of 7 (57%) Brucella-positive samples, 2 of 8 (25%) C. pneumoniae-positive samples, 2 of 9 (22%) F. tularensis-positive samples, 1 of 3 (33%) R. rickettsii-positive samples, and 3 of 9 (33%) TSI-positive samples tested positive for Yersinia IgG antibodies; 5 of 11 (45%) B. burgdorferi-positive samples, 2 of 8 (25%) C. pneumoniae-positive samples, 3 of 9 (33%) F. tularensis-positive samples, 1 of 3 (33%) R. rickettsii-positive samples, and 1 of 9 (11%) TSI-positive samples tested positive for Yersinia IgA antibodies; and 2 of 5 (40%) B. henselae-positive samples and 1 of 11 (9%) B. burgdorferi-positive samples tested positive for Yersinia IgM antibodies (Table 3).
The cross-reactivity of the Yersinia Western blot assay with B. burgdorferi occurred most frequently with the Yersinia YopD antigen. All samples observed to have cross-reactivity between B. burgdorferi and Yersinia except one Yersinia IgM positive-sample showed cross-reactivity with the YopD antigen of the Western blot assay. Five samples observed to have cross-reactivity between Yersinia and B. burgdorferi were retested for antibodies against B. burgdorferi by a Western blot method (MarDx Diagnostics) to identify the specific B.burgdorferi- positive antigens. All five samples were positive for IgG antibodies against the 41-kDa flagellar (Fla) protein and for IgG and IgM antibodies against the 23-kDa outer surface protein C (OspC) of B. burgdorferi (Table 4).
TABLE 4.
B. burgdorferi Western blot results for B. burgdorferi-positive samples showing cross-reactivity with Yersinia
| Sample no. |
B. burgdorferi Western blot results for the following isotype and antigens of the indicated size (kDa)a
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgM
|
IgG
|
||||||||||||
| 23 | 39 | 41 | 18 | 23 | 28 | 30 | 39 | 41 | 45 | 58 | 66 | 93 | |
| 1 | + | + | + | + | + | + | + | + | |||||
| 2 | + | + | + | + | |||||||||
| 3 | + | + | + | + | |||||||||
| 4 | + | + | + | + | |||||||||
| 5 | + | + | + | + | + | ||||||||
Positive bands on the B. burgdorferi Western blot are indicated by a plus sign. Western blot antigens against specific B. burgdorferi antibodies are indicated by their sizes (in kilodaltons).
DISCUSSION
The traditional diagnosis of Yersinia infection has been based on stool culture examination and less sensitive serology methods, such as agglutination and the CF assay. Currently, a “gold standard” is lacking for the proper diagnosis of reactive arthritis, and there are no accepted criteria for the identification of causative organisms (10). Where the traditional diagnostic methods have proven to be useful only for the detection of acute infection, an assay must be highly sensitive and specific to detect antibodies that circulate following the acute phase.
By using as the comparison method the Viramed Western blot assay, which uses Yops as antigens for antibody detection, poor agreement and sensitivity were observed for the CF assay, with 61% agreement, 26% sensitivity, and 95% specificity. The poor sensitivity of the CF method compared to the Western blot assay may be attributed to the fact that the two methods detect antibodies against different antigens. The CF method detects antibodies against Y. pseudotuberculosis and Y.enterocolitica lipopolysaccharide O3, O8, and O9 antigens, whereas Western blotting detects Yop antibodies. Yops are produced by all strains of Y. enterocolitica and Y. psuedotuberculosis.
The combined results for the IgG-, IgA-, and IgM-specific ELISAs, which also use Yops as antigens, showed better agreement and sensitivity (89% and 95%, respectively) but lower specificity (82%) compared to the combined IgG, IgA, and IgM Western blot assay results. The individual specific ELISAs exhibited low specificities of 79% for IgG and 86% for IgA compared to the results of the specific Western blot assays; the IgM-specific ELISA, however, was 100% specific. Similarly, previous reports have indicated an increased sensitivity and a lack of specificity, which led to a higher number of false-positive results, by an ELISA technique for Yersinia antibody detection (2, 29).
A lack of specificity for the ELISA was further observed with the sera from 50 healthy subjects. The IgG, IgA, and IgM ELISAs had positivity rates of 18%, 10%, and 4%, respectively, whereas the IgG, IgA, and IgM Western blot assays had positivity rates of 6%, 2%, and 2%, respectively. The CF assay had a positivity rate of 2% for the healthy population tested. Various studies have reported that the incidences of Yersinia antibodies in the healthy population in Western Europe are 20 to 40% for IgG and 3 to 10% for IgA. It was suggested that the high prevalence could be due to either a high proportion of asymptomatic carriage or the fact that the specificities of the available serological tests used in the study were insufficient (22). Our results for the ELISA were similar, suggesting a lack of specificity that resulted in a high number of false-positive results. The low incidence in the healthy population measured by the CF assay may be attributed to its lack of sensitivity, which was demonstrated in the studies comparing the CF assay and the Western blot assays and ELISAs. The Western blot assays had acceptable sensitivities and the highest specificities of the methods evaluated.
The ability to differentiate between IgG, IgA, and IgM antibodies compared to IgG and IgM detection by the CF method is of significant importance in the diagnosis of yersinia-associated complications. Antibodies against Yersinia Yops develop after infection and often persist at high levels in cases of reactive arthritis (7, 26). IgA antibodies have been shown to persist for 14 to 16 months following the onset of infection, with peak levels correlating directly with the severity of arthritis. This is in contrast to the persistence of IgA antibodies for only 5 months in cases of yersiniosis without subsequent complications (5). In cases of chronic enteritis, IgA antibodies develop against YopE (23 kDa), YopD (35 kDa), and YopB (41 kDa). IgA antibodies against YopD develop in 90% of reactive arthritis cases (7, 26, 27). IgG antibodies develop against all outer membrane proteins of Yersinia; but they develop more frequently against YopE (23 kDa), YopB (41 kDa), YopD (35 kDa), and YopH (51 kDa) (8, 11, 21). IgG antibodies can persist longer in cases of reactive arthritis, but not as consistently as IgA antibodies. IgM antibodies persist for only 1 to 3 months following the onset of infection and are not as useful for the diagnosis of reactive arthritis (5). As observed in this study, both the Western blot assays and the ELISAs allow the differentiation of specific antibody isotypes, including the specific detection of IgA antibodies, which are the most important for the diagnosis of reactive arthritis. The CF assay detects only IgG and IgM antibodies and does not differentiate between specific antibody isotypes.
Cross-reactivity was observed with all assays evaluated. The CF assay exhibited the lowest amount of cross-reactivity with other organisms among the methods evaluated, although this may be attributed to the poor sensitivity of the assay. The highest cross-reactivity for the CF assay was shown with C.pneumoniae, with three of eight C. pneumoniae-positive samples testing positive for Yersinia. Cross-reactivity was also observed with Brucella, F. tularensis, and TSI by the CF assay. One sample from each category for B. henselae, Brucella, C.pneumoniae, and R. rickettsii showed cross-reactivity by Western blot assay. Of the methods evaluated, the ELISA exhibited the highest cross-reactivity with other organisms. All organisms and antibodies tested, excluding C. burnetti and M.pneumoniae, showed cross-reactivity by the ELISA. This could be attributed to the lower specificity of the ELISA. Previous studies have reported cross-reactivity between Yersinia, Brucella (2,17-19), R. rickettsii (2, 23), and TSI (1, 2, 13, 24). Cross-reactivity between F. tularensis and Brucella spp. has been reported (9), indicating a possible explanation for the cross-reactivity observed between F. tularensis and Yersinia by the CF assays and the ELISAs. However, it is possible that some of the samples showing cross-reactivity with Yersinia are actually true positives. There are no known reports of cross-reactivity between Yersinia, B. henselae, and C. pneumoniae.
B. burgdorferi exhibited the largest amount of cross-reactivity with Yersinia by both the Western blot assays and the ELISAs. In the Western blot assay, 3 of 11 B. burgdorferi-positive samples tested positive for Yersinia IgG, 4 of 11 samples tested positive for Yersinia IgA, and 5 of 11 samples tested positive for Yersinia IgM. For the ELISA, 6 of 11 samples tested positive for Yersinia IgG, 5 samples tested positive for Yersinia IgA, and 1 sample tested positive for Yersinia IgM. Similarly, previous studies have shown evidence of cross-reactivity between Yersinia and B. burgdorferi (3, 25). One such study analyzed sera from 30 patients diagnosed with reactive arthritis for the occurrence of B. burgdorferi-specific antibodies (25). Twenty of the 30 (66.6%) reactive arthritis-positive samples tested positive for antibodies against B. burgdorferi by Western blot assay, and 10% of these were positive for Yersinia antibodies by the agglutination technique. As a control, 4 of 30 (13%) samples from healthy donors tested positive for B. burgdorferi antibodies, of which 0% were positive for Yersinia. It was reported that the cross-reactivity between the two organisms could possibly be due to the antigenic similarity of the 60-kDa common antigen of B. burgdorferi to that of other bacterial species. While this may be a viable explanation for the cross-reactivity observed in the measurement of Yersinia antibodies by CF or agglutination techniques, where whole-cell antigens are used, it does not explain the high cross-reactivity of the Western blot assays and ELISAs that use Yop antigens that are specific for yersinial virulence. Interestingly, our study did not reveal cross-reactivity between Yersinia and B.burgdorferi by the CF assay.
Cross-reactivity between B. burgdorferi and Yersinia was observed with the YopD antigen in all but one of the B. burgdorferi samples tested by the Western blot assays. All samples with observed cross-reactivity tested positive for IgG antibodies against the Fla antigen, as well as IgG and IgM antibodies against the OspC antigen for B. burgdorferi. A possible explanation for the extensive cross-reactivity between the Yersinia YopD antigen with B. burgdorferi antibodies may be possible antigenic similarity between the OspC and Fla antigens of B.burgdorferi and YopD of Yersinia, although further studies would need to be performed.
Based on our findings, the Viramed Western blot assay is useful as an aid in the diagnosis and management of enteric infection and could be a valuable tool in identifying Yersinia as a causative organism in reactive arthritis cases. However, because cross-reactivity exists between Yersinia and other bacterial species, particularly B. burgdorferi, which causes symptoms similar to those of yersinia-associated reactive arthritis, clinical diagnosis should be based on the complete clinical picture and laboratory findings.
Acknowledgments
This work was supported by the ARUP Institute for Clinical and Experimental Pathology.
Special thanks go to Barry Menefee at Viralab, Inc., and Martin Kintrup at Viramed Biotech for supplying the characterized positive samples and all of the Western blot reagents used in this study.
REFERENCES
- 1.Benvenga, S., F. Guarneri, M. Vaccaro, L. Santarpia, and F. Trimarchi. 2004. Homologies between proteins of Borrelia burgdorferi and thyroid autoantigens. Thyroid 14:964-966. [DOI] [PubMed] [Google Scholar]
- 2.Bottone, E. J. 1997. Yersinia enterocolitica: the charisma continues. Clin. Microbiol. Rev. 10:257-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruckbauer, H. R., V. Preac-Mursic, R. Fuchs, and B. Wilske. 1992. Cross-reactive proteins of Borrelia burgdorferi. Eur. J. Clin. Microbiol. Infect. Dis. 11:224-232. [DOI] [PubMed] [Google Scholar]
- 4.Campbell, E., and D. T. Dennis. 2001. Plague and other Yersinia infections, p. 993-1001. In E. Braunwald, A. S. Fauci, D. L. Kasper, S. L. Hauser, D. L. Longo, and J. L. Jameson (ed.), Harrison’s principles of internal medicine, 15th ed. McGraw-Hill, New York, N.Y.
- 5.Colmegna, I., R. Cuchacovich, and L. R. Espinoza. 2004. HLA-B27-associated reactive arthritis: pathogenetic and clinical considerations. Clin. Microbiol. Rev. 17:348-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Constantine, N. T., and D. P. Lana. 2003. Immunoassays for the diagnosis of infectious diseases, p. 218-233. In P. R. Murray, E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.), Manual of clinical microbiology, 8th ed. ASM Press, Washington, D.C.
- 7.Cremer, J., M. Putzker, M. Faulde, and L. Zoller. 1993. Immunoblotting of Yersinia plasmid-encoded released proteins: a tool for serodiagnosis. Electrophoresis 14:952-959. [DOI] [PubMed] [Google Scholar]
- 8.de Koning, J., J. Heesemann, J. A. Hoogkamp-Korstanje, J. J. Festen, P. M. Houtman, and P. L. van Oijen. 1989. Yersinia in intestinal biopsy specimens from patients with seronegative spondyloarthropathy: correlation with specific serum IgA antibodies. J. Infect. Dis. 159:109-112. [DOI] [PubMed] [Google Scholar]
- 9.Erdenebaatar, J., B. Bayarsaikhan, M. Watarai, S. Makino, and T. Shirahata. 2003. Enzyme-linked immunosorbent assay to differentiate the antibody responses of animals infected with Brucella species from those of animals infected with Yersinia enterocolitica O9. Clin. Diagn. Lab. Immunol. 10:710-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fendler, C., S. Laitko, H. Sorensen, C. Gripenberg-Lerche, A. Groh, J. Uksila, K. Granfors, J. Braun, and J. Sieper. 2001. Frequency of triggering bacteria in patients with reactive arthritis and undifferentiated oligoarthritis and the relative importance of the tests used for diagnosis. Ann. Rheum. Dis. 60:337-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granfors, K., M. Viljanen, A. Tiilikainen, and A. Toivanen. 1980. Persistence of IgM, IgG, and IgA antibodies to Yersinia in yersinia arthritis. J. Infect. Dis. 141:424-429. [DOI] [PubMed] [Google Scholar]
- 12.Heesemann, J., U. Gross, N. Schmidt, and R. Laufs. 1986. Immunochemical analysis of plasmid-encoded proteins released by enteropathogenic Yersinia sp. grown in calcium-deficient media. Infect. Immun. 54:561-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heyma, P., L. C. Harrison, and R. Robins-Browne. 1986. Thyrotrophin (TSH) binding sites on Yersinia enterocolitica recognized by immunoglobulins from humans with Graves' disease. Clin. Exp. Immunol. 64:249-254. [PMC free article] [PubMed] [Google Scholar]
- 14.Hill Gaston, J. S., and M. S. Lillicrap. 2003. Arthritis associated with enteric infection. Best Pract. Res. Clin. Rheumatol. 17:219-239. [DOI] [PubMed] [Google Scholar]
- 15.Hoogkamp-Korstanje, J. A., and V. M. Stolk-Engelaar. 1995. Yersinia enterocolitica infection in children. Pediatr. Infect. Dis. J. 14:771-775. [DOI] [PubMed] [Google Scholar]
- 16.Kim, W. B., B. Y. Cho, H. Y. Park, H. K. Lee, L. D. Kohn, K. Tahara, and C. S. Koh. 1996. Epitopes for thyroid-stimulating antibodies in Graves' sera: a possible link of heterogeneity to differences in response to antithyroid drug treatment. J. Clin. Endocrinol. Metab. 81:1758-1767. [DOI] [PubMed] [Google Scholar]
- 17.Kittelberger, R., F. Hilbink, M. F. Hansen, M. Penrose, G. W. de Lisle, J. J. Letesson, B. Garin-Bastuji, J. Searson, C. A. Fossati, A. Cloeckaert, et al. 1995. Serological crossreactivity between Brucella abortus and Yersinia enterocolitica O:9. I. Immunoblot analysis of the antibody response to Brucella protein antigens in bovine brucellosis. Vet. Microbiol. 47:257-270. [DOI] [PubMed] [Google Scholar]
- 18.Kittelberger, R., F. Hilbink, M. F. Hansen, G. P. Ross, M. A. Joyce, S. Fenwick, J. Heesemann, H. Wolf-Watz, and K. Nielsen. 1995. Serological crossreactivity between Brucella abortus and Yersinia enterocolitica O:9. II. The use of Yersinia outer proteins for the specific detection of Yersinia enterocolitica infections in ruminants. Vet. Microbiol. 47:271-280. [DOI] [PubMed] [Google Scholar]
- 19.Kittelberger, R., M. P. Reichel, M. A. Joyce, and C. Staak. 1997. Serological crossreactivity between Brucella abortus and Yersinia enterocolitica O:9. III. Specificity of the in vitro antigen-specific gamma interferon test for bovine brucellosis diagnosis in experimentally Yersinia enterocolitica O:9-infected cattle. Vet. Microbiol. 57:361-371. [DOI] [PubMed] [Google Scholar]
- 20.Leirisalo-Repo, M. 1987. Yersinia arthritis. Acute clinical picture and long-term prognosis. Contrib. Microbiol. Immunol. 9:145-154. [PubMed] [Google Scholar]
- 21.Maki-Ikola, O., J. Heesemann, R. Lahesmaa, A. Toivanen, and K. Granfors. 1991. Combined use of released proteins and lipopolysaccharide in enzyme-linked immunosorbent assay for serologic screening of Yersinia infections. J. Infect. Dis. 163:409-412. [DOI] [PubMed] [Google Scholar]
- 22.Putzker, M., H. Sauer, and D. Sobe. 2001. Plague and other human infections caused by Yersinia species. Clin. Lab. 47:453-466. [PubMed] [Google Scholar]
- 23.Schlesinger, M., S. Pollak, and P. A. Vardy. 1979. Cross-antigenicity between Yersinia and Rickettsia. Isr. J. Med. Sci. 15:612-613. [PubMed] [Google Scholar]
- 24.Shenkman, L., and E. J. Bottone. 1976. Antibodies to Yersinia enterocolitica in thyroid disease. Ann. Intern. Med. 85:735-739. [DOI] [PubMed] [Google Scholar]
- 25.Sobieszczanska, B., and A. Przondo-Mordarska. 1996. Cross-reactivity between Borrelia burgdorferi and “arthrogenic” bacteria in sera from patients with reactive arthritis. Rocz. Akad. Med. Bialymst. 41:90-95. [PubMed] [Google Scholar]
- 26.Stahlberg, T. H., K. Granfors, and A. Toivanen. 1987. Immunoblot analysis of human IgM, IgG and IgA responses to plasmid-encoded antigens of Yersinia enterocolitica serovar O3. J. Med. Microbiol. 24:157-163. [DOI] [PubMed] [Google Scholar]
- 27.Stahlberg, T. H., J. Heesemann, K. Granfors, and A. Toivanen. 1989. Immunoblot analysis of IgM, IgG, and IgA responses to plasmid encoded released proteins of Yersinia enterocolitica in patients with or without yersinia triggered reactive arthritis. Ann. Rheum. Dis. 48:577-581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turgeon, M. L. 1990. Immunology and serology in laboratory medicine. The C.V. Mosby Company, St. Louis, Mo.
- 29.Vesikari, T., K. Granfors, M. Maki, and P. Gronroos. 1980. Evaluation of ELISA in the diagnosis of Yersinia enterocolitica diarrhoea in children. Acta Pathol. Microbiol. Scand. Sect. B 88:139-142. [DOI] [PubMed] [Google Scholar]