Abstract
Intracellular accumulation of the protease inhibitors (PIs) saquinavir (SQV), ritonavir (RTV), and indinavir (IDV) was determined in 50 human immunodeficiency virus-positive patients. Following extraction, PIs were quantified by mass spectrometry. Paired plasma and intracellular samples were collected over a full dosing interval from patients (13 on SQV, 6 on RTV, 8 on IDV, 16 on SQV plus RTV, 7 on IDV plus RTV) with a plasma viral load of <400 copies/ml. Data were expressed as intracellular/plasma drug concentration ratios. A hierarchy of intracellular accumulation was demonstrated by the following medians: 9.45 for SQV > 1.00 for RTV > 0.51 for IDV. Coadministration of RTV did not boost ratios of SQV or IDV within the cell or in plasma, although absolute plasma and intracellular SQV concentrations were increased by RTV. Seven individuals receiving SQV in hard-gel capsule form (median, 32 months) had higher intracellular/plasma drug ratios than all other patients receiving SQV (median, 17.62 versus 4.83; P = 0.04), despite consistently low plasma SQV concentrations. How this occurs may provide insight into the mechanisms that limit adequate drug penetration into sanctuary sites.
The failure to achieve and maintain suppression of human immunodeficiency virus (HIV) replication is emerging as a major problem in antiretroviral therapy (ART). One large cohort study has recently reported a mean time before virological failure on first-ART-regimen drugs of 12 months but with markedly decreasing durability for each successive regimen (F. Palella, J. Chmiel, M. Deloria-Knoll, A. Moorman, S. Holmberg, and the HIV Outpatient Investigators, 8th Conf. Retrovir. Opportunistic Infect., abstr. 268B, 2001). Treatment failure is multifactorial and includes viral resistance, poor adherence, and pharmacological and host factors. Much interest has been generated by potential pharmacological mechanisms of failure. HIV replicates within cells; therefore, drugs must penetrate intracellularly at concentrations sufficient to inhibit viral replication. Failure to do so results in the establishment of a sanctuary site where virus may evolve in the absence of selection pressure from the drug (22) or where subtherapeutic levels generate drug-resistant virus with subsequent “seeding” into plasma. Virus from sanctuary sites, such as the central nervous system and seminal fluid, can exhibit genotypic resistance profiles, which differ from peripheral blood isolates (6, 21). Pharmacological studies that examine the cellular and tissue penetration of HIV drugs are crucial to the understanding of sanctuary sites and the subsequent evolution of drug resistance and the failure of ART. This understanding may inform the design of strategies to maximize drug potency. In particular, the roles of cellular efflux transporters such as the P-glycoprotein (P-gp) in limiting the intracellular penetration of drugs and the potential for ritonavir (RTV) to boost intracellular drug accumulation within sanctuary sites deserve further investigation.
We have previously observed a hierarchy in the intracellular accumulation of protease inhibitors (PIs) in vitro in the rank order of nelfinavir > saquinavir (SQV) > RTV > indinavir (IDV) (10). The extent of intracellular drug accumulation was modulated in a stepwise manner by the addition of increasing amounts of α1-acid glycoprotein (the major plasma protein to which PIs bind) or by using cell lines with high-level expression of the efflux transporters P-gp and MRP-1 (8, 9). It is important to determine if these differences in intracellular drug concentrations between PIs are also observed in vivo.
In this report, we confirmed the intracellular localization of the PIs SQV, IDV, and RTV by fractionation experiments in cultured cells and determined the efflux kinetics of SQV in vitro in these cells at different temperatures. These data were utilized to design a method for measuring intracellular PIs in peripheral blood mononuclear cells (PBMCs) from clinical samples. We investigated the intracellular accumulation of SQV, IDV, and RTV and the effect of boosting either SQV or IDV with RTV in HIV-infected patients receiving these drugs as part of a successful antiretroviral regimen.
(Results of this investigation were presented in part previously [S. Khoo, M. Hennessy, F. Mulcahy, S. Clarke, D. J. Back, P. G. Hoggard, J. Tjia, E. G. L. Wilkins, P. Carey, I. Williams, B. Peters, and M. G. Barry, 8th Conf. Retrovir. Opportunistic Infect., abstr. 258, 2001].)
MATERIALS AND METHODS
Materials.
SQV and [14C]SQV (0.06 μCi mmol−1, >99% purity; Roche Pharmaceuticals, Welwyn Garden City, Herts, United Kingdom), RTV (Abbott Laboratories, North Chicago, Ill.), and IDV (Merck, West Point, Pa.) were donated by the manufacturers. [3H]RTV and [3H]IDV were purchased (1.1 Ci mmol−1, >99.9% purity; Moravek Biochemicals, Brea, Calif.). U937 and CEM cells were acquired from the European Collection of Cell Cultures Centre for Applied Microbiology and Research (Salisbury, United Kingdom) and propagated as previously described (9). Lymphoprep was purchased from Nycomed AS (Oslo, Norway). All other compounds were purchased from Sigma Chemical Company (Poole, Dorset, United Kingdom).
Loss of drug during washing.
Intracellular drug loss during the washing procedure was studied in vitro. Cultured cells were incubated with radiolabeled drug to allow drug accumulation, followed by harvesting by two different methods—cell washing and oil stop.
Cell washing experiments.
U937 cells (5 × 106 cells/ml; 5 ml) were incubated with radiolabeled [14C]SQV (1 μM; 18 h), harvested, washed three times in ice-cold phosphate-buffered saline (PBS) with centrifugation (700 × g for 6 min at 4°C), and then extracted in 60% methanol for at least 3 h. This was followed by a final centrifugation (700 × g for 6 min at 4°C), after which the supernatant fraction (containing intracellular drug) was evaporated to dryness and resuspended in 150 μl of 60% methanol. The residual pellet (containing cellular debris) was also resuspended (500 μl of 2 M sodium hydroxide). Supernatant was removed as follows: from culture medium, at the end of each of the three successive washes, from the supernatant fraction, and from the cellular debris. Total radioactivity was determined by liquid scintillation counting.
Oil stop experiments.
CEM cells (106 cells/ml; 500 μl) were incubated with [14C]SQV (0.1 μCi; 1 μM), [3H]RTV (0.1 μCi; 1 μM), or [3H]IDV (0.1 μCi; 1 μM) for up to 18 h to allow drug accumulation. Cell harvesting by oil stop methodology was performed with 10% n-hexadecane-90% silica oil (400 μl). Cells (400 μl) were layered onto the oil-and-cell suspensions, which had been pelleted by centrifugation (14,000 × g for 30 s). The bottom of the Eppendorf tube (containing the pellet) was clipped off into a scintillation vial. The pellet was reconstituted (100 μl of distilled water), solubilized (tissue solubilizer-glacial acetic acid-hydrogen peroxide, 2:2:1; 100 μl), and incubated for 30 min at 37°C. Scintillation cocktail (4 ml) was added prior to liquid scintillation counting for determination of intracellular drug accumulation as previously described (9).
Intracellular localization of PIs.
Fractionation of U937 cells was performed in an Eppendorf centrifuge as previously described (1, 3). Cells were loaded with [14C]SQV, [3H]RTV, or [3H]IDV (1 μM; 18 h) and centrifuged (700 × g for 6 min at 4°C).
Briefly, this subcellular fractionation procedure used differential centrifugation. This technique included digitonin in the homogenization buffer, shortening the procedure. The cytosolic fraction was isolated in less than 1 min, the washed nuclear fraction was isolated in 4 min, the mitochondrial fraction was isolated in 10 min, and the washed light mitochondrial fraction was isolated in 40 min.
The resulting cell pellet was homogenized and centrifuged in order to separate the cytosolic, nuclear, heavy mitochondrial, light mitochondrial, and microsomal fractions. Protein content in each fraction was measured (14), and drug accumulation was expressed in picomoles of drug per milligram of protein.
Efflux kinetics of SQV with time and at different temperatures.
Drug efflux from U937 cells was assessed at 4 and 37°C. Cells were initially loaded with [14C]SQV (1 μM; 18 h). Drug concentration was measured by scintillography at baseline and then 5, 20, 40, 60, 90, and 120 min after pelleting (700 × g for 6 min at 4°C), followed by resuspension in drug-free medium.
Patient recruitment.
Patients were recruited from four study sites within the United Kingdom. Eligibility criteria for recruitment were as follows: an age of >18 years, having been receiving current ART regimen for ≥6 months, and a plasma viral load of ≤400 copies/ml (Roche Amplicor or Chiron bDNA, version 3). Patients were excluded if they were receiving antituberculous treatment, verapamil, or cytotoxic chemotherapy. All patients received one or two of the following PIs at various doses: SQV (hard- or soft-gel capsule preparations), RTV, or IDV. Sampling of blood for plasma and intracellular drug levels was performed as follows. Study participants attended on the morning of the study day having fasted for a time which had been scheduled to be either 8 h (for three-times-a-day regimens) or 12 h (for twice-a-day regimens). A baseline sample of 20 ml of heparinized blood was collected. ART drug(s) was then ingested under supervision (in accordance with recommendations for food intake), after which further samples were drawn at 2, 4, 8, and 12 h postdose for twice-daily regimens (SQV soft-gel preparation, RTV, and all dual-PI regimens) or 1, 2, 4, and 8 h postdose for three-times-a-day regimens (SQV hard-gel preparation and IDV). All other constituents of the antiretroviral regimen were taken in the usual way. Informed consent was obtained from volunteers, and the study received approval from each local chapter of the Research Ethics Committee.
A total of 50 HIV-positive patients (46 male, 4 female; ages 18 to 64; median, 41 years) were recruited. Plasma viral load was <400 copies/ml in all patients, and CD4 counts ranged from 120 to 1,272 cells/mm3 (median, 370 cells/mm3). The patients in the risk groups for acquisition of HIV were characterized as follows: 37 (74%) homosexual male subjects, 8 (16%) heterosexual subjects, 3 (6%) infected by blood products, 1 (2%) infected through intravenous drug use, and 1 (2%) infected by vertical transmission. There were two black African male subjects and one subject from the Indian subcontinent, and the remainder were Caucasian. Stage of disease (according to the 1993 Centers for Disease Control and Prevention classification) was characterized by 7 (14%) in category A, 21 (42%) in category B, and 22 (44%) in category C.
Patients were divided into five groups: those on single PIs in the form of (i) SQV (n = 13, including 7 patients receiving hard-gel preparation SQV), (ii) RTV (n = 6), or (iii) IDV (n = 8) and those receiving dual-PI regimens with (iv) SQV plus RTV (n = 16) or (v) IDV plus RTV (n = 7). There were no significant differences between the groups with regard to gender, age, ethnicity, CD4 count, or choice of nucleoside backbone. The dosing regimens for single PIs were administered as SQV hard-gel capsules (600 mg every 8 h, n = 6; 1,600 mg every 12 h, n = 1), SQV soft-gel capsules (1,600 mg every 12 h), RTV (500 to 600 mg every 12 h), or IDV (800 to 1,000 mg every 12 h). Dual-PI combinations were administered at the following doses: SQV plus RTV at 400 and 400 mg (n = 9), 200 and 600 mg (n = 1), 800 and 200 mg (n = 2), 1,000 and 100 mg (n = 3), and 1,000 and 200 mg (n = 1), respectively, and IDV plus RTV at 400 and 400 mg (n = 5) and 800 and 100 mg (n = 2), respectively.
Other components of the combination regimen included zidovudine in 19 (38%) patients, stavudine in 25 (50%) patients, lamivudine in 31 (62%) patients, didanosine in 12 (24%) patients, abacavir in 6 (12%) patients, zalcitabine in 1 (2%) patient, nevirapine in 2 (4%) patients, and efavirenz in 1 (2%) patient. In addition, 9 (18%) patients received cotrimoxazole, 9 (18%) received acyclovir, 7 (14%) received loperamide, 6 (12%) were on statins and/or fibrates for hyperlipidemia, 5 (10%) received temazepam, 3 (6%) received nonsteroidal anti-inflammatory agents (ibuprofen, indomethacin, and diclofenac), and 1 (2%) was on ranitidine, lansoprazole, and venlafaxine.
Isolation of PBMCs and drug extraction.
Based on data from the efflux studies described above, drug loss from cells was minimized by ensuring that cells were separated immediately after venipuncture and that cell washing, counting, and methanol extraction took no longer than a further 30 min. Prior to separation, an aliquot of 5 ml was removed and centrifuged in order to determine plasma drug concentrations. PBMCs were extracted from the remainder of blood by density cushion centrifugation (Lymphoprep; Nycomed AS). The cells were washed three times in ice-cold PBS, centrifuged (700 × g for 6 min at 4°C), and counted with a hemocytometer before a final overnight extraction in 1 ml of 60% methanol. On the following day, samples were centrifuged (700 × g for 6 min at 4°C) and the supernatant fraction was transferred to a glass tube prior to evaporation to dryness.
Internal standard (Ro 31-9564) was added to standard curves (for SQV, between 0 and 50 to 10,000 ng ml−1; for RTV, between 0 and 100 to 20,000 ng ml−1; for IDV, between 0 and 75 to 15,000 ng ml−1), heat-inactivated plasma (40 min at 58°C), and dried PBMC extracts (resuspended in 200 μl of distilled water) prior to further extraction with diethyl ether. The aqueous layer was frozen, and the organic layer was transferred into a clean tube and evaporated to dryness. Samples were reconstituted in mobile phase (10 mM ammonium formate buffer-acetonitrile, 30:70 [vol/vol]) prior to injection on the column. PIs were eluted on a Hypurity Elite 5C18 column (5 μM; 250 by 4.6 mm) with mobile phase maintained at 1.2 ml min−1. Quantification of ions resulting from fragmentation of parent compound (PI) was analyzed by electrospray ionization mass spectrometry (ThermoQuest Duo; ThermoQuest Ltd., Manchester, United Kingdom) using Xcalibur software. The ion source parameters used were a heated capillary tube temperature of 270°C, a source voltage of 4.5 kV, a capillary voltage of 3V, a sheath gas flow of 60 U, an auxiliary gas flow of 50 U, and tube lens offset at −40 V. The following parent compounds/two fragment ions (m/z) were used in mass spectrometry/mass spectrometry quantification of the PIs: IDV, 614.4/475.3 (596.3); RTV, 721.4/426.1 (296.0); SQV, 671.4/570.3 (433.2); internal standard, 674.4/573.3 (388.2).
Data analysis.
The intracellular drug concentration was derived from a measured volume of each PBMC of 0.4 pl, a quantity that is consistent with published data (4).
Drug exposure was expressed as the area under the concentration-time curve from 0 to 24 h (AUC0-24). Noncompartmental modeling (Topfit software, version 2.0; Gustav Fischer Verlag, Stuttgart, Germany) was utilized to derive the AUC over the dosing interval (8 or 12 h) by using the linear trapezoid rule for the plasma and intracellular drug concentrations for each patient. Intracellular accumulation was expressed as the ratio of AUC0-24 for intracellular drug/AUC0-24 for drug in plasma. These ratios were compared between different groups of patients by nonparametric analysis with the Mann-Whitney U test. Correlation between sets of data was assessed using Spearman's rank correlation coefficient (ρ).
RESULTS
The recovery of radiolabeled PIs is shown in Table 1. Drug recovered from supernatant after extraction in 60% methanol was assumed to have been distributed within the cells. These experiments confirmed that three washes with ice-cold PBS were required to remove any contaminating media containing SQV.
TABLE 1.
Recovery of the radiolabeled PIs following incubation in U937 cellsa
| Treatment stepb | SQV | RTV | IDV |
|---|---|---|---|
| RPMI medium | 86.5 ± 6.51 | 77.5 ± 0.84 | 81.3 ± 5.42 |
| Wash 1 | 4.45 ± 1.92 | 8.04 ± 0.85 | 4.63 ± 2.10 |
| Wash 2 | 1.90 ± 0.56 | 3.73 ± 0.57 | 2.77 ± 1.46 |
| Wash 3 | 0.82 ± 0.17 | 2.02 ± 1.30 | 1.83 ± 0.54 |
| 60% Methanol | 5.71 ± 0.64 | 8.37 ± 0.43 | 9.34 ± 2.49 |
| Cell pelleting | 0.57 ± 0.34 | 0.32 ± 0.09 | 0.18 ± 0.14 |
Radiolabeled PIs were administered in 1 μM concentrations following an 18-h incubation in 10 ml of 106 U937 cells/ml. Data are expressed in percentages as means ± SD (n ≥ 4).
Recovery was from the supernatants of RPMI and washes in PBS.
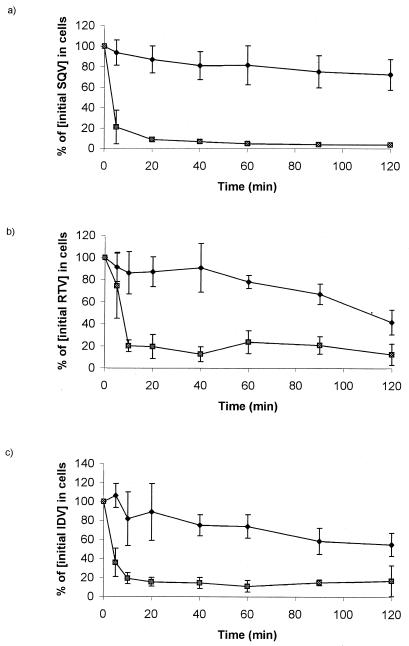
Under oil stop conditions, the intracellular drug concentrations of SQV, RTV, and IDV were 29.3 ± 5.9, 7.9 ± 4.7, and 0.80 ± 0.4 μM (mean ± standard deviation [SD]; n = 9), respectively. After cell washing (three times), the SQV, RTV, and IDV concentrations were 25.8 ± 7.0, 6.8 ± 4.9, and 0.7 ± 0.2 μM (mean ± SD; n = 9), respectively. This illustrates that approximately 12, 14, and 12% of SQV, RTV, and IDV, respectively, were lost during the washing and extraction procedure. These data are consistent with those from validation studies on temperature effects and SQV loss from cultured cells, where, although >50% of PI was lost over 1 h at 20°C, only ∼10% was lost at 4°C (Fig. 1).
FIG. 1.
Effect of temperature on the efflux of SQV (a), RTV (b), and IDV (c) from U937 cells. Data are expressed as means ± SD (n = 3). The SQV efflux at 4°C (⧫) was less than that at 37°C (▩).
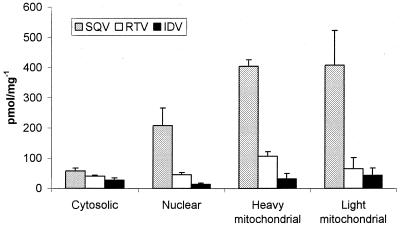
The presence of drug was detected in all subcellular fractions (cytosolic, nuclear, and mitochondrial) studied (Fig. 2). The same rank order of accumulation (SQV > RTV > IDV) was observed in each fraction as has previously been observed for whole U937 and CEM cells (8, 9).
FIG. 2.
Accumulation of the HIV PIs SQV, RTV, and IDV following the fractionation of U937 cells. Data are expressed as means ± SD (n = 4). The accumulation of drug is standardized to picomoles per milligram of protein in each particular fraction.
Efflux of [14C]SQV at 37°C was rapid (Fig. 1), with loss of ∼80% of the drug within the first 5 min. However, the use of ice-cold PBS and centrifugation at 4°C resulted in the retention of >80% of cell-associated drug at 60 min. These results are consistent with data from the oil stop experiments described above, where drug loss associated with washing and extraction was 12 to 14%. Drug efflux from cells was therefore critically dependent upon both time and, more importantly, assay temperature. All future experiments assessing intracellular drug accumulation in PBMCs utilized ice-cold (4°C) reagents and refrigerated centrifugation.
The lower limits of detection for SQV and RTV on columns were less than 5 and 10 pg, respectively. These correspond to plasma drug concentrations of 375 and 750 pg ml−1 or PBMC (10 × 106 cells) drug concentrations of 10 and 20 ng ml−1, respectively. The lower limit of detection for IDV on the column was 5 pg, corresponding to the lower limits of quantification of 125 pg ml−1 for plasma and 40 pg for a PBMC extract from 10 × 106 cells.
The interassay coefficients of variation (CV) for SQV were 9.7, 3.9, and 7.1% at concentrations of 100 ng ml−1 and 5 and 15 μg ml−1, respectively. The intra-assay CV were 2.0, 3.5, and 6.1% at the same concentrations. The interassay CV for RTV were 8.5, 7.0, and 4.9% at concentrations of 250 ng ml−1 and 2 and 8 μg ml−1, respectively. The intra-assay CV were 5.5, 5.8, and 5.7% at the same concentrations. The interassay CV for IDV were 6.9 and 1.5% at concentrations of 150 and 3,000 ng ml−1, respectively. The intra-assay CV were 4.5 and 4.7%, respectively, at the same IDV concentrations.
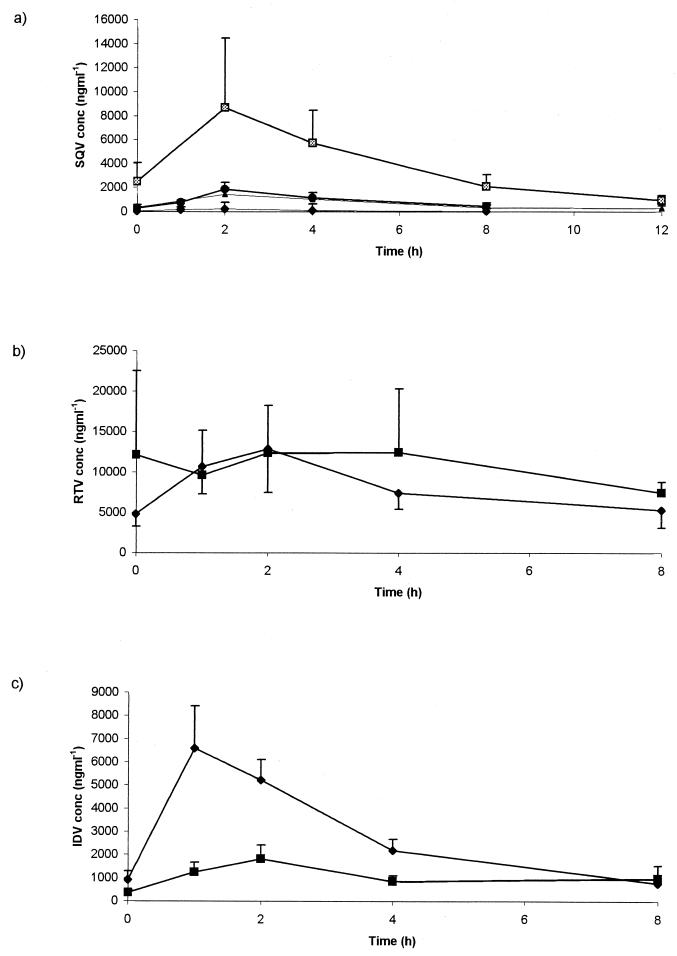
Intracellular and plasma drug profiles of SQV, IDV, and RTV are shown in Fig. 3. Target plasma AUC0-24s have previously been defined at 20,000 ng · h ml−1 for SQV (5) and between 20,000 and 55,000 ng · h ml−1 for IDV (11; D. M. Burger, A. M. C. van Rossum, P. W. H. Hugen, S. P. M. Geelen, R. De Groot, and the Dutch Study Group for Children with HIV Infection, 1st Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 5.3, 2000) based upon virological responses in ART-naïve patients commencing therapy, and the requirement to maintain trough plasma drug concentrations above the MEC for wild-type HIV. The median AUC0-24s for SQV in plasma were as follows: the SQC hard-gel preparation, 1,815 ng · h ml−1 (all patients below the target AUC); the SQV soft-gel preparation, 4,197 ng · h ml−1 (4 of 6 below the target AUC); SQV boosted with RTV, 30,762 ng · h ml−1 (7 of 16 below the target AUC). The median AUC0-24 for IDV in plasma was 87,832 ng · h ml−1 (one of eight below the target AUC), and the median AUC0-24 for IDV boosted with RTV was 55,854 ng · h ml−1 (all patients were above the target AUC). The proportion of patients with suboptimal SQV exposure was higher than that observed in previous unselected clinic cohorts (D. M. Burger, R. M. W. Hoetelmans, P. W. H. Hugen, R. P. G. Van Heeswijk, A. I. Veldkamp, and the Athena Study Group, 1st Int. Workshop Clin. Pharmacol. HIV Ther., abstr. 6.6, 2000) or in samples sent to our laboratory for therapeutic drug monitoring (12) and reflected the poor bioavailability of hard-gel capsules of SQV.
FIG. 3.
Concentrations of SQV (± RTV) (a), RTV (b), and IDV (± RTV) (c) within cells (▩ [soft gel] and • [hard gel] in panel a, ▪ in panel b, and ⧫ in panel c) and in plasma (▴ [soft gel] and ⧫ [hard gel] in panel a, ⧫ in panel b, and ▪ in panel c) throughout the dosing interval in HIV-positive patients.
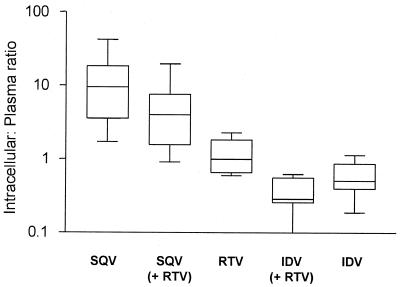
The median concentration ratios (95% confidence intervals) for each drug within cells and in plasma (all patients receiving a single PI or one PI in combination with another) were 6.73 (5.36 to 12.18) for SQV, 1.31 (1.12 to 1.86) for RTV, and 0.47 (0.33 to 0.65) for IDV. These are shown in Table 2 and Fig. 4. Both SQV (P < 0.0001) and RTV (P < 0.0001) exhibited higher intracellular/plasma drug concentration ratios than to IDV. In patients receiving only one PI, the ratio for SQV within the cell and in plasma was also significantly higher than that for RTV (median, 9.45 versus 1.00; P = 0.0001).
TABLE 2.
Intracellular accumulation of SQV, RTV, and IDV given either singly or in combination
| Drug(s) | n | Median AUC0-24 (ng · h ml−1) (range) |
Intracellular/plasma drug concn ratio |
|||
|---|---|---|---|---|---|---|
| Plasma | Intracellular | Median | 95% CIa | Range | ||
| SQV | ||||||
| All | 29 | 14,688 (147-104,034) | 36,676 (4,180-1,087,956) | 6.73 | 5.36-12.18 | 0.91-42.04 |
| Sole PI | 13 | 3,190 (147-57,876) | 31,380 (5,370-301,660) | 9.45 | 5.84-19.20 | 1.71-42.04 |
| Hard gel | 7 | 1,815 (147-16,038) | 27,480 (5,370-40,000) | 17.62 | 4.35-29.45 | 1.71-42.04 |
| Soft gel | 6 | 4,197 (3,190-57,876) | 35,408 (18,320-301,060) | 7.50 | 3.49-11.35 | 2.76-13.14 |
| SOV + RTV | 16 | 30,762 (1,972-104,034) | 126,778 (4,180-1,087,956) | 4.01 | 2.79-8.65 | 0.91-19.50 |
| RTV | ||||||
| All | 29 | 105,010 (12,480-491,680) | 94,440 (26,160-1,119,220) | 1.31 | 1.12-1.86 | 0.20-4.19 |
| Sole PI | 6 | 246,338 (79,460-491,680) | 181,601 (94,400-1,119,220) | 1.00 | 0.51-1.81 | 0.60-2.28 |
| RTV + SQV | 16 | 69,650 (12,480-234,220) | 54,646 (26,160-671,900) | 1.25 | 0.92-2.08 | 0.20-4.19 |
| RTV + IDV | 7 | 115,240 (22,116-224,680) | 151,100 (27,808-357,280) | 1.8 | 0.85-2.63 | 0.41-3.45 |
| IDV | ||||||
| All | 15 | 79,404 (17,583-190,014) | 24,834 (10,482-94,080) | 0.47 | 0.33-0.65 | 0.10-1.14 |
| Sole PI | 8 | 87,832 (17,583-190,014) | 39,561 (17,682-94,080) | 0.51 | 0.34-0.88 | 0.19-1.14 |
| IDV + RTV | 7 | 55,854 (27,880-155,944) | 14,220 (10,482-72,400) | 0.29 | 0.19-0.52 | 0.10-0.63 |
CI, confidence interval.
FIG. 4.
Ratio of SQV (± RTV), RTV, and IDV (± RTV) within the cells and in the plasma of 50 HIV-positive patients. The horizontal line, box, and whiskers represent the median, lower and upper quartiles, and range, respectively.
Of the 13 patients receiving SQV as the sole PI, 7 were prescribed the hard-gel formulation, which, in 6 cases, was given at the original licensed dose of 600 mg every 8 h. This mode of dispensing SQV has since been superseded by soft-gel capsules given at higher doses. However, all six patients on the SQV hard-gel formulation remained virologically suppressed and preferred to remain on their original regimen. These patients had taken hard-gel capsules of SQV for a median of 32 months (range, 24 to 43 months). Although this study was not primarily designed to examine for differences between hard- and soft-gel SQV formulations, it was interesting to note that patients on hard-gel SQV capsules had high ratios of drug within cells and in plasma. For example, five of seven patients on hard-gel SQV capsules had high ratios (>10) compared to one of six patients on soft-gel SQV capsules (Table 3). We observed a significant difference in median intracellular/plasma drug ratio between the seven patients receiving SQV in hard-gel capsule form and all other patients receiving SQV (17.62 versus 4.83; P = 0.04). It is probable that the six patients on hard-gel SQV capsules represented a highly selective unusual subset of patients who achieved virological suppression despite having received lower doses of a formulation with limited (∼5%) bioavailability. All these patients had very low plasma SQV AUC0-24s, as would be expected (Table 2).
TABLE 3.
Intracellular accumulation of SQV in patients receiving hard-gel versus soft-gel formulations of the drug
| Patient no. | Capsule form | AUC0-24 (ng · h ml−1) |
Intracellular/plasma drug concn ratio | |
|---|---|---|---|---|
| Plasma | Intracellular | |||
| 35 | Hard gel | 16,038 | 27,480 | 1.71 |
| 36 | Hard gel | 1,821 | 5,370 | 2.95 |
| 39 | Hard gel | 1,060 | 13,620 | 12.85 |
| 40 | Hard gel | 1,815 | 40,000 | 22.03 |
| 51 | Hard gel | 1,781 | 31,380 | 17.62 |
| 53 | Hard gel | 147 | 6,180 | 42.04 |
| 54 | Hard gel | 1,833 | 34,995 | 19.09 |
| 2 | Soft gel | 5,450 | 36,676 | 6.73 |
| 3 | Soft gel | 3,190 | 26,340 | 8.26 |
| 13 | Soft gel | 4,384 | 18,320 | 4.18 |
| 14 | Soft gel | 57,876 | 159,936 | 2.76 |
| 41 | Soft gel | 3,614 | 34,140 | 9.45 |
| 52 | Soft gel | 22,910 | 301,060 | 13.14 |
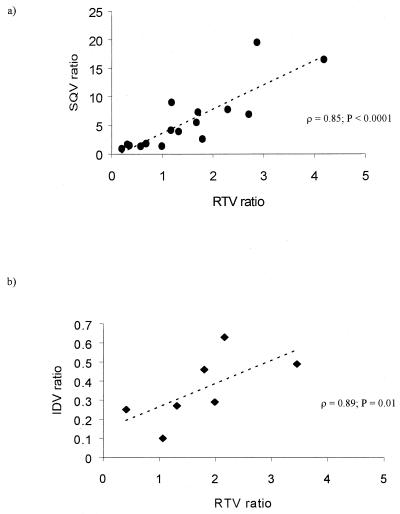
Plasma SQV concentrations were enhanced as expected by RTV, resulting in an approximately ninefold higher median AUC0-24. Coadministration of RTV did not result in boosting of the intracellular/plasma SQV concentration ratio. Instead, a higher median intracellular/plasma SQV ratio was noted in the group receiving SQV (9.45) versus SQV plus RTV (4.01; P = 0.03), which was influenced by the high ratios observed in patients receiving SQV in hard-gel capsule form. After excluding the seven patients on hard-gel SQV capsules from analysis, there was still no significant boosting of intracellular/plasma SQV ratios by RTV (P = 0.58). There was no significant correlation between the intracellular/plasma SQV ratio and either the dose of RTV (ρ = −0.08; P = 0.76) or the plasma RTV concentration (ρ = −0.11; P = 0.69). However, a strong correlation in the ratio between SQV and RTV within the cell and in plasma was observed in patients receiving both drugs (ρ = 0.85; P < 0.0001) (Fig. 5a).
FIG. 5.
Relationship between the intracellular accumulation of each PI in patients receiving SQV plus RTV (a) and those receiving IDV plus RTV (b). Correlation was assessed by using Spearman's rank correlation coefficient (ρ).
Similarly, RTV did not boost the median intracellular/plasma drug ratio of IDV, which was 0.51 in patients receiving IDV, compared to 0.29 in patients receiving IDV plus RTV (P = 0.09). A similar correlation was observed between the intracellular/plasma IDV and RTV concentration ratio in patients receiving both drugs (ρ = 0.89; P = 0.01) (Fig. 5b).
DISCUSSION
Our findings suggest that the recovery of PIs associated with the cell pelleting represents drug that is present within cell compartments, such as the cytoplasm and mitochondria, rather than that bound to the cell surface membrane, since radiolabeled compound was detected in all subcellular fractions. This study therefore provides the rationale for further studies in order to investigate the subcellular localization of PIs. We have shown that the significant drug efflux out of cells that can occur during laboratory manipulation is both time and temperature dependent. One of our main recommendations, therefore, is that all such assays should be performed under the stringent laboratory conditions we have proposed, particularly with regard to working temperature and rapidity of processing. Failure to adhere to these conditions is likely to generate spurious data.
We have previously observed a hierarchy of intracellular accumulation of PIs (SQV > RTV > IDV) in vitro, and in this study, we have demonstrated the same phenomenon in vivo. Another study assessing the restoration of HIV infectivity in cells pretreated with PIs also observed a similar hierarchy of persistence of antiviral effect following removal from drug-treated cultures (15). The clinical significance of this is unclear at present, since clinical trials directly comparing different PIs have not demonstrated any major differences in therapeutic efficacy.
The mechanism of intracellular accumulation of PIs such as SQV is unknown but may be due to either active transport of the drug into cells or else intracellular sequestration due to protein binding or ion trapping. Whether or not intracellular drug remains unbound or else complexed to proteins (and thus without pharmacological activity) is an important but unanswered question. It is interesting to note that the extent of plasma protein binding differs between SQV (∼97%), RTV (∼98%), and IDV (∼60%). We have previously demonstrated that intracellular drug accumulation in vitro is influenced by the amount of α1-acid glycoprotein, with IDV being less affected than RTV or SQV (9).
The accumulation of PIs within HIV-infected cells is not a static phenomenon but rather a dynamic event representing a balance between passive diffusion, sequestration, and active influx and/or efflux across the cell surface membrane. Some of these processes may be amenable to therapeutic manipulation. The use of RTV to boost levels of SQV and IDV in plasma is thought to result from the potent inhibition of cytochrome P450 CYP3A4 in the gastrointestinal tract and liver. In this study, we sought to investigate whether inhibition of P-gp by RTV was able to boost intracellular concentrations of SQV or IDV over and above elevation of the levels of either drug in plasma. The ability to achieve this would have important consequences for drug delivery into sanctuary sites and allow the design of strategies to maximize the potency of existing ART. Unfortunately, coadministration of RTV did not result in an increase in the concentration ratio for either SQV or IDV within cells or in plasma, although the absolute concentration of intracellular SQV was increased. This observation is consistent with other published studies. The proportion of SQV accumulating in seminal fluid was not boosted by the addition of RTV (18) or nelfinavir (M. Reijers, R. Van Heeswijk, S. Jurrians, H. Schuittemaker, P. Portegies, G. J. Weverling, J. Lange, and R. Hoetelmans, 7th Conf. Retrovir. Opportunistic Infect., abstr. 316, 2000). Coadministration of RTV increased concentrations of IDV in plasma and correspondingly within cerebrospinal fluid (2.4-fold) and semen (8.2-fold) (20), but there was no clear evidence of preferential targeting of drug into these sanctuary sites.
Failure by RTV to boost the penetration of SQV and IDV into cells and sanctuary sites could be explained if RTV was a poor inhibitor of P-gp (exerting most of its boosting effect through the inhibition of P450 CYP3A4) or if P-gp did not have a major function in limiting the penetration of PIs into these sites. The latter seems less likely, based on animal experiments. P-gp knockout mice demonstrated increased bioavailability of PIs by two- to fivefold (13) and increased penetration of PIs into the brain by 7- to 36-fold (16; R. B. Kim, 8th Conf. Retrovir. Opportunistic Infect., abstr. S1, 2001). Animals given the specific P-gp inhibitors GF-120918 and LY-335979 had increased the penetration of amprenavir and nelfinavir into the brain (13- to 37-fold) and testes (4-fold) (2, 16). If RTV is a relatively weak inhibitor of P-gp, as has been suggested by other studies (7), then targeting of the drug to sanctuary sites might be more effectively achieved by specific inhibitors of P-gp, such as those listed above.
This study was not powered to assess for differences in intracellular accumulation between hard-gel and soft-gel capsule formulations of SQV. Nevertheless, the high intracellular/plasma SQV concentration ratios observed in many of the patients receiving hard-gel SQV capsules was striking. As previously discussed, these patients probably represent a highly selected group with very low AUC0-24s of SQV in plasma. The median and highest recorded AUC0-24s of 1,798 ng · h ml−1 and 16,038 ng · h ml−1, respectively, were well short of the target AUC of 20,000 ng · h ml−1 (5) for SQV but typical of values previously observed with hard-gel SQV formulations (17). Previous studies have illustrated long-term suppression of viral replication despite low plasma SQV concentrations (19). The authors suggested that the satisfactory antiviral response might possibly be explained by the intracellular pharmacokinetics of SQV. We propose, therefore, that increased accumulation of SQV within infected cells might explain how apparently subtherapeutic plasma drug concentrations can still successfully suppress HIV replication in some individuals. These findings may prove to be of particular significance when investigating the pharmacology of sanctuary sites for HIV.
In conclusion, the differences we observed in intracellular drug accumulation between PIs was striking but no clinical correlation can be made until these pharmacological observations are linked to therapeutic outcomes in future studies. One of the more interesting observations, that increased intracellular drug accumulation occurs with patients achieving durable virological suppression with hard-gel capsules of SQV despite universally low plasma drug concentrations, lends weight to the notion that intracellular penetration of PIs may be clinically important.
Acknowledgments
This study was funded in part by an unrestricted educational grant from Roche Pharmaceuticals Ltd., Welwyn Garden City, Herts, United Kingdom. Patrick Hoggard is grateful to BSAC for financial support.
We gratefully acknowledge the advice and assistance of Ian Weller, David Cornforth, Diana Aldam, Helen Reynolds, Emma MacFarlane, Ruth Johnstone, Christine Whitehead, and Elaine Stockwell.
REFERENCES
- 1.Bronfman, M., G. Loyola, and C. S. Koenig. 1998. Isolation of intact organelles by differential centrifugation of digitonin-treated hepatocytes using a table Eppendorf centrifuge. Anal. Biochem. 255:252-256. [DOI] [PubMed] [Google Scholar]
- 2.Choo, E. F., B. Leake, C. Wandel, H. Imamura, A. J. Wood, G. R. Wilkinson, and R. B. Kim. 2000. Pharmacological inhibition of P-glycoprotein transport enhances the distribution of HIV-1 protease inhibitors into brain and testes. Drug Metab. Dispos. 28:655-660. [PubMed] [Google Scholar]
- 3.Cui, L., L. Locatelli, M. Y. Xie, and J. P. Sommadossi. 1997. Effect of nucleoside analogs on neurite regeneration and mitochondrial DNA synthesis in PC-12 cells. J. Pharmacol. Exp. Ther. 280:1228-1234. [PubMed] [Google Scholar]
- 4.Furman, P. A., J. A. Fyfe, M. H. St. Clair, K. Weinhold, J. L. Rideout, G. A. Freeman, S. N. Lehrman, D. P. Bolognesi, S. Broder, H. Mitsuya, et al. 1986. Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proc. Natl. Acad. Sci. USA 83:8333-8337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gieschke, R., B. Fotteler, N. Buss, and J. L. Steimer. 1999. Relationships between exposure to saquinavir monotherapy and antiviral response in HIV-positive patients. Clin. Pharmacokinet. 37:75-86. [DOI] [PubMed] [Google Scholar]
- 6.Gupta, P., C. Leroux, B. K. Patterson, L. Kingsley, C. Rinaldo, M. Ding, Y. Chen, K. Kulka, W. Buchanan, B. McKeon, and R. Montelaro. 2000. Human immunodeficiency virus type 1 shedding pattern in semen correlates with the compartmentalization of viral quasi species between blood and semen. J. Infect. Dis. 182:79-87. [DOI] [PubMed] [Google Scholar]
- 7.Huisman, M. T., J. W. Smit, H. R. Wiltshire, R. M. Hoetelmans, J. H. Beijnen, and A. H. Schinkel. 2001. P-Glycoprotein limits oral availability, brain, and fetal penetration of saquinavir even with high doses of ritonavir. Mol. Pharmacol. 59:806-813. [DOI] [PubMed] [Google Scholar]
- 8.Jones, K., P. G. Bray, S. H. Khoo, R. A. Davey, E. R. Meaden, S. A. Ward, and D. J. Back. 2001. P-Glycoprotein and transporter MRP1 reduce HIV protease inhibitor uptake in CD4 cells: potential for accelerated viral drug resistance? AIDS 15:1353-1358. [DOI] [PubMed] [Google Scholar]
- 9.Jones, K., P. G. Hoggard, S. Khoo, B. Maher, and D. J. Back. 2001. Effect of alpha1-acid glycoprotein on the intracellular accumulation of the HIV protease inhibitors saquinavir, ritonavir and indinavir in vitro. Br. J. Clin. Pharmacol. 51:99-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones, K., P. G. Hoggard, S. D. Sales, S. Khoo, R. Davey, and D. J. Back. 2001. Differences in the intracellular accumulation of HIV protease inhibitors in vitro and the effect of active transport. AIDS 15:675-681. [DOI] [PubMed] [Google Scholar]
- 11.Kakuda, T. N., L. M. Page, P. L. Anderson, K. Henry, T. W. Schacker, F. S. Rhame, E. P. Acosta, R. C. Brundage, and C. V. Fletcher. 2001. Pharmacological basis for concentration-controlled therapy with zidovudine, lamivudine, and indinavir. Antimicrob. Agents Chemother. 45:236-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khoo, S. H., S. E. Gibbons, and D. J. Back. 2001. Therapeutic drug monitoring as a tool in treating HIV infection. AIDS 15(Suppl. 4):S171-S181. [DOI] [PubMed] [Google Scholar]
- 13.Kim, R. B., M. F. Fromm, C. Wandel, B. Leake, A. J. Wood, D. M. Roden, and G. R. Wilkinson. 1998. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J. Clin. Investig. 101:289-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lowry, O. H., N. J. Rosenborough, A. L. Farr, and R. J. Randall. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 15.Nascimbeni, M., C. Lamotte, G. Peytavin, R. Farinotti, and F. Clavel. 1999. Kinetics of antiviral activity and intracellular pharmacokinetics of human immunodeficiency virus type 1 protease inhibitors in tissue culture. Antimicrob. Agents Chemother. 43:2629-2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palella, F. J., Jr., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, S. D. Holmberg, and HIV Outpatient Study Investigators. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 17.Schapiro, J. M., M. A. Winters, F. Stewart, B. Efron, J. Norris, M. J. Kozal, and T. C. Merigan. 1996. The effect of high-dose saquinavir on viral load and CD4+ T-cell counts in HIV-infected patients. Ann. Intern. Med. 124:1039-1050. [DOI] [PubMed] [Google Scholar]
- 18.Taylor, S., and A. S. Pereira. 2001. Antiretroviral drug concentrations in semen of HIV-1 infected men. Sex. Transm. Infect. 77:4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Heeswijk, R. P., J. W. Cohen Stuart, D. M. Burger, J. H. Beijnen, J. C. Borleffs, and R. M. Hoetelmans. 2002. Long-term suppression of viral replication despite low plasma saquinavir concentrations in the CHEESE study. Br. J. Clin. Pharmacol. 53:211-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Praag, R. M., S. Repping, J. W. de Vries, J. M. Lange, R. M. Hoetelmans, and J. M. Prins. 2001. Pharmacokinetic profiles of nevirapine and indinavir in various fractions of seminal plasma. Antimicrob. Agents Chemother. 45:2902-2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venturi, G., M. Catucci, L. Romano, P. Corsi, F. Leoncini, P. E. Valensin, and M. Zazzi. 2000. Antiretroviral resistance mutations in human immunodeficiency virus type 1 reverse transcriptase and protease from paired cerebrospinal fluid and plasma samples. J. Infect. Dis. 181:740-745. [DOI] [PubMed] [Google Scholar]
- 22.Zhang, L., B. Ramratnam, K. Tenner-Racz, Y. He, M. Vesanen, S. Lewin, A. Talal, P. Racz, A. S. Perelson, B. T. Korber, M. Markowitz, and D. D. Ho. 1999. Quantifying residual HIV-1 replication in patients receiving combination antiretroviral therapy. N. Engl. J. Med. 340:1605-1613. [DOI] [PubMed] [Google Scholar]