Abstract
Tuberculosis (TB) is the most common life-threatening infection in human immunodeficiency virus (HIV)-infected persons and frequently occurs before the onset of severe immunodeficiency. Development of TB is associated with increased HIV type 1 (HIV-1) viral load, a fall in CD4 lymphocyte counts, and increased mortality. The aim of this study was to examine how treatment of pulmonary TB affected HIV-1 activity in HIV-1/TB-coinfected subjects with CD4 cell counts of >100 cells/μl. HIV-1/TB-coinfected subjects were recruited in Kampala, Uganda, and were monitored over time. Based upon a significant (0.5 log10 copies/ml) decrease in viral load by the end of treatment, two patient groups could be distinguished. Responders (n = 17) had more rapid resolution of anemia and pulmonary lesions on chest radiography during TB treatment. This group had a significant increase in viral load to levels not different from those at baseline 6 months after completion of TB treatment. HIV-1 viral load in nonresponders (n = 10) with TB treatment increased and at the 6 month follow-up was significantly higher than that at the time of diagnosis of TB. Compared to baseline levels, serum markers of macrophage activation including soluble CD14 decreased significantly by the end of TB treatment in responders but not in nonresponders. These data further define the impact of pulmonary TB on HIV-1 disease. HIV-1 replication during dual HIV-1/TB infection is not amenable to virologic control by treatment of TB alone. Concurrent institution of highly active antiretroviral treatment needs to be evaluated in patients dually infected with pulmonary TB and HIV-1.
One of every four human immunodeficiency virus type 1 (HIV-1)-infected persons in the world is diagnosed with active tuberculosis (TB) (12), making TB the most frequent life-threatening coinfection in HIV-1-infected patients. Unlike other opportunistic infections (OI), TB develops throughout the course of HIV-1 disease. At the time of development of pulmonary TB, which is the most frequent clinical form of Mycobacterium tuberculosis infection, CD4 cell counts are frequently higher than those of patients with other OI (18). In fact, in areas where TB is endemic, only one-third of HIV-1-infected subjects presenting with TB had CD4 cell counts lower than 200 cells/μl (1). Importantly, the course of HIV-1 infection may be accelerated after TB diagnosis and treatment. In one study, the relative risk of death and the occurrence of new opportunistic infections were increased in HIV-1/TB-coinfected subjects compared to HIV-1-infected control subjects matched for CD4 cell count (27). The risk of death was particularly increased in HIV-1/TB-coinfected patients whose CD4 counts exceeded 200 cells/μl (28). Other OI are also associated with increased HIV-1 viral load levels, but only transiently (25). For example, after treatment for pneumocytis or bacterial pneumonia, increased HIV-1 viral load decreases to levels present prior to the development of infection (3). Also, unlike the effect of TB (11), the increased HIV-1 viral load associated with acute immune activation is not associated with expanded HIV-1 heterogeneity (20). Simultaneous expansion in HIV-1 load and heterogeneity during dual HIV-1/TB infection underscores the chronicity of immune pressure on viral replication. Interestingly, chronic infections other than TB, such as helminthic infestations, have not been found to be associated with increased HIV-1 viral load or worsening of the course of HIV-1 disease (2). Altogether, these data suggest a role for chronic immune stimulation by M. tuberculosis infection in sustained viral activation in HIV-1/TB-infected patients. On the other hand, the significance of comorbidities other than TB for deterioration of HIV-1 disease is less well characterized.
Whether successful treatment of TB averts the effect on HIV-1 replication, and thereby the impact of TB on HIV-1 disease, is unclear. In a study of patients with HIV-1/TB coinfection in the United States, TB-related increases in HIV-1 RNA in plasma decreased after 6 months of antituberculosis treatment (11). In contrast, in a more recent study from South Africa, in subjects with both pulmonary and extrapulmonary TB, inclusive of all levels of CD4 counts, HIV-1 viral loads remained elevated and CD4 counts did not improve after successful antituberculous treatment (19). Sustained activation of the immune system was demonstrable in HIV-1/TB-coinfected patients despite successful antituberculous therapy in this study (19). HIV-1/TB coinfection has long been associated with generalized immune activation (26) involving both lymphocytes and macrophages (16). Activation of mononuclear cells is intense at sites of M. tuberculosis infection, and HIV-1 generated from activated mononuclear cells (16) has an increased capacity to infect uninfected cells (4).
The impact of viral coinfections on HIV-1 disease progression has been investigated heavily in the past. Studies of subjects coinfected with HIV-1 and hepatitis C virus have suggested that HIV-1 disease may progress faster in dually infected patients (21, 24). This observation has not been confirmed by other investigators (22, 31). On the other hand, active infection with GB virus C (GBV-C) may portend a better prognosis in HIV-1 infection, as it appears to be associated with greater CD4 increases and lower HIV-1 viral loads subsequent to highly active antiretroviral treatment (HAART) (23, 29). However, the contribution of these viral cofactors to HIV-1 activity in subjects with dual HIV-1/TB infection is unknown.
To define factors that may affect viral activity during HIV-1/TB infection, we prospectively studied HIV-1-infected adults with CD4 counts of ≥100 cells/μl at the time of diagnosis of TB. In addition, patients were studied after 1 and 6 months ofantituberculous treatment and 6 months after completion of treatment. Changes in plasma HIV-1 RNA and serum activation markers were assessed. Hematologic and radiographic responses to TB treatment were also assessed.
MATERIALS AND METHODS
Study populations.
HIV-1-infected adults with initial episode of sputum smear-positive pulmonary TB were recruited from the National Tuberculosis and Leprosy Programme Clinic at Mulago Hospital in Kampala, Uganda. The diagnosis of pulmonary TB was based on symptoms, chest roentgenography, and sputum smear microscopy. Chest radiographs were interpreted by a single experienced chest physician, who was masked to the patients' clinical status and laboratory studies, using the standard scheme of the U.S. National Tuberculosis and Respiratory Disease Association and were classified as normal, minimal, moderately advanced, or far-advanced disease (8). All subjects had subsequent culture confirmation of TB. Subjects with drug-resistant TB were excluded. No subject had a history of TB or received antituberculous therapy in the preceding 6 months. Subjects with active OI at the time of diagnosis of TB or at follow-up were excluded. Pregnant women and patients with extrapulmonary TB or chronic debilitating diseases, a Karnofsky performance scale score of <60 (unable to care for self and requires the equivalent of hospital or institutional care), or CD4 counts of ≤100 cells/μl were excluded. Febrile (≥38°C) subjects had blood smears examined for malaria parasites. None had a positive malaria smear. The study protocol was approved by the institutional review boards at University Hospitals of Cleveland Case Western Reserve University and the Uganda National AIDS Research Subcommittee. All subjects gave informed consent for study participation. Patients were treated with standard chemotherapy for pulmonary TB (2 months of isoniazid, rifampin, pyrazinamide, and ethambutol treatment followed by 4 months of isoniazid and rifampin treatment). From November 2000 to July 2001, 27 patients meeting the above-mentioned criteria were recruited. Citrated plasma and serum were obtained from subjects at enrollment, at 1 and 6 months after initiation of treatment, and at 6 months of follow-up after completion of anti-TB treatment. Complete blood counts and sputum examination were performed monthly during treatment. Follow-up chest radiographs were obtained at the end of anti-TB treatment.
A group (n = 13) of healthy HIV-1-infected subjects without TB who were positive by purified protein derivative skin test (greater than 5-mm induration) with CD4 counts over 100 cells/μl (median, 388 cells [range, 174 to 860 cells/μl]) were recruited as control subjects to assess changes in viral load over time. Plasma was obtained from these subjects at enrollment and 6 months later. None of HIV-1/TB-coinfected or HIV-infected control subjects had received antiretroviral agents prior to or subsequent to enrollment in the study.
HIV-1 load.
Circulating HIV-1 RNA in citrated plasma was assessed by the COBAS Amplicor HIV-1 Monitor assay (1.5 Test) (Roche, Indianapolis, IN), according to the instructions of the manufacturer.
Quantitation of serum cytokines and activation markers.
Serum concentrations of cytokines and activation markers were assessed using commercially available enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions. ELISA for serum tumor necrosis factor alpha (TNF-α) (BioSource, Nivelles, Belgium) has a sensitivity of 3 pg/ml. ELISAs for interleukin-6 (IL-6), soluble TNF receptor II, and β2-microglobulin (R&D Systems, Minneapolis, MN) have lower sensitivities of 0.7 pg/ml, 0.6 pg/ml, and 0.2 μg/ml, respectively. ELISA for serum neopterin (IBL Immuno Biological Laboratory, Flughafenstrasse, Hamburg, Germany) has a lower level of detection of 0.7 nmol/liter. ELISA for soluble CD14 (sCD14) (R&D Systems, Minneapolis, MN) has a lower sensitivity of 125 pg/ml.
Detection of hepatitis C and GBV-C infection.
Hepatitis C seropositivity was assessed by ELISA (Abbott Laboratories, Abbott Park, IL). GBV-C infection was assessed by reverse transcription-PCR as described previously (30). RNA was extracted from plasma by using a QIAmp viral RNA kit (QIAGEN, Valencia, CA).
Statistical analysis.
For all measurements, median and range for the groups were used to summarize the data. Differences between groups were compared by nonparametric tests based on ranks. We used the Mann-Whitney U test for independent groups and the Wilcoxon signed-rank test for related groups (7). To assess the magnitude of associations between HIV RNA response to antituberculous treatment and radiologic response to antituberculous therapy, odds ratios and 95% confidence intervals were calculated (9). For all tests, a P value of <0.05 was considered significant.
RESULTS
HIV-1 viral load in HIV-1/TB infection with pulmonary TB on antituberculous therapy.
After completion of TB treatment, the median viral load in the whole HIV-1/TB-coinfected group (n = 27) decreased by only 0.2 log10 copies/ml (P < 0.001). HIV-1 viral load was 5.5 log10 copies/ml (range, 4.8 to 5.8) at baseline (t0) and 5.3 log10 copies/ml (range, 4.7 to 5.7) upon completion of antituberculous chemotherapy at 6 months (t6). However, the median HIV-1 load at 12 months (i.e., 6 months after completing TB treatment) increased (5.4 log10 copies/ml [range, 4.9 to 5.9]) and was not significantly different than that at t0. Compared to HIV-1 viral load from HIV-infected subjects without TB (median, 4.8 log10 copies/ml [range, 3.4 to 5.9]), the median viral load of the HIV-1/TB-coinfected patients was higher both at t0 (P < 0.02) and at 12 months (P < 0.01). The increase in median viral load of HIV-1 control subjects between t0 and 6 months was not significant (data not shown).
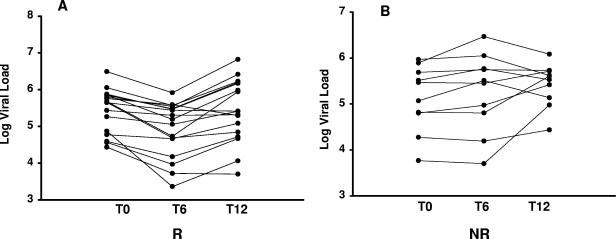
Based upon a significant (0.5 log10 copies/ml) decrease in viral load with treatment of pulmonary TB (from t0 to t6), two groups of HIV-1/TB-coinfected patients could be distinguished (Fig. 1). The first group was comprised of 17 patients, each of whom had a significant drop in viral load by the end of treatment. The median HIV-1 viral load at t0 was 5.7 log10 copies/ml (range, 4.8 to 5.8), and at t6, the median decreased to 5.2 log10 copies/ml (range, 4.4 to 5.5) (P < 0.04) (Fig. 1A). This group will be referred to as responders. The second group (n = 10) was comprised of HIV-1/TB-coinfected patients who had no decrease in viral load upon treatment of TB (nonresponders) (Fig. 1B).
FIG. 1.
Changes in HIV-1 viral load during and after treatment of TB. HIV-1 viral loads in plasma from subjects with pulmonary TB were assessed at time of diagnosis (t0), at the end of 6 months of TB treatment (t6), and 6 months after completion of anti-TB chemotherapy (t12). (A) Viral load changes in responders (R) (n = 17) who had significant (defined as 0.5 log copies/ml) decreases in viral load by the end of TB treatment (P < 0.04). HIV-1 viral load at t12 increased significantly (compared to t6) back to baseline levels (P < 0.003). (B) Viral load in nonresponders (NR) (n = 10) did not decrease with treatment of TB. Viral load was significantly higher at t12 (P < 0.05).
At the 6-month follow-up (t12), HIV-1/TB-coinfected responders had a significant increase of viral load from the end of treatment (t6) back to baseline levels (t0) (P < 0.003). Among the nonresponders, HIV-1 viral load actually increased from t0 by t12 also (P < 0.05).
Clinical characteristics of HIV-1/TB-coinfected patients.
We compared the clinical characteristics of these two groups of HIV-1/TB-coinfected patients (Table 1). Responders were older than nonresponders (median age, 35 versus 27 years, respectively) (P < 0.03). Although plasma HIV viral loads were on average 0.4 logs higher, and CD4 counts were lower among responders at the time of diagnosis of pulmonary TB than among nonresponders, these differences were not significant. The gender composition, baseline hemoglobin levels, and Karnofsky score did not differ between responders and nonresponders. The extent of TB on chest radiographs was similar between the two groups at diagnosis; 83% of responders and 90% of nonresponders had far-advanced (8) pulmonary TB. At the end of treatment, however, there was a trend towards a greater radiologic improvement in the responder group, among whom 75% had radiologic improvement in severity of the lesions. By comparison, only one-third of subjects in the nonresponder group had radiologic improvement (odds ratio [95% confidence interval], 6 [0.76 to 52.6]; P = 0.05). Hemoglobin levels were not different between the responder and nonresponder groups (Table 1). Improvement in hemoglobin levels following TB treatment (t0 to t6) was significant only in responders (P < 0.05). Bacteriologic response to antituberculous therapy was similar between the two groups; sputa from 100% of responders and 90% of nonresponders were culture negative by 3 months.
TABLE 1.
Clinical characteristics of responder and nonresponder groups
| Characteristic | Groupa
|
||
|---|---|---|---|
| R | NR | P valuec | |
| VL at baseline (105) (median) | 4.5 (0.27∼31.0)* | 2.0 (0.06∼9.3) | NS |
| VL at 6 mo (105) (median) | 1.6 (0.02∼8.2)* | 3.0 (0.05∼29.0) | NS |
| CD4 at baseline (absolute) (median) | 220 (100∼400) | 310 (100∼500) | NS |
| CD4 at baseline (%) | 12.5 (24.3∼4.4) | 17.8 (24.0∼3) | NS |
| Gender (male/female) | 7/10 | 4/6 | NS |
| Age (yr) (median) | 35 (23∼60) | 27 (23∼37) | <0.03 |
| CXR at t0 (grade 3) (%) | 83 | 90 | NS |
| CXR improvement (grade) (%) | 75 | 33 | 0.05 |
| Hgbb at baseline (median) | 9.9 (8.7∼13.6)** | 10.5 (8.5∼12.7) | NS |
| Hgb at 2 mo (median) | 12.1 (10.1∼13.5)** | 11.3 (9.9∼13.5) | NS |
| Karnofsky score at baseline (median) | 80 (70∼90) | 80 (80∼90) | NS |
R, responder group; NR, nonresponder group. P < 0.001; P < 0.05.
Hgb, hemoglobin.
NS, not significant.
Hepatitis C and GBV-C infection in HIV-1/TB-coinfected patients.
Next, we examined the effect of GBV-C and hepatitis C infection on the course of HIV-1 disease activity in the two groups of HIV-1/TB-coinfected patients. None of the patients in this cohort were seropositive for hepatitis C. Seropositivity for hepatitis C among hospitalized Ugandans is about 6% (personal communications), and presently, there are no data on seropositivity of nonhospitalized people in the community. On the other hand, GBV-C RNA was detected in one-half of study subjects; however, the incidence was identical among responder and nonresponder HIV-1/TB-coinfected patients. The rate of persistence of GBV-C RNA in plasma (assessed in sera at 6 months) was also similar between the two groups. Therefore, the rate and activity of GBV-C coinfection did not differ between these two groups of HIV-1/TB-coinfected subjects, and thus, it is unlikely that the course of HIV-1 disease in patients dually infected with HIV-1 and pulmonary TB was affected by concomitant GBV-C infection.
Relationship of HIV-1 disease makers to treatment of TB.
To better understand the course of HIV disease in the two groups of HIV-1/TB-coinfected patients, we next assessed changes in serum activation markers over time. We assessed markers independently associated with HIV-1 disease progression (β2-microglobulin, neopterin, and soluble TNF receptor II) and the levels of TNF-α and IL-6, which are both elevated during pulmonary TB infection (17).
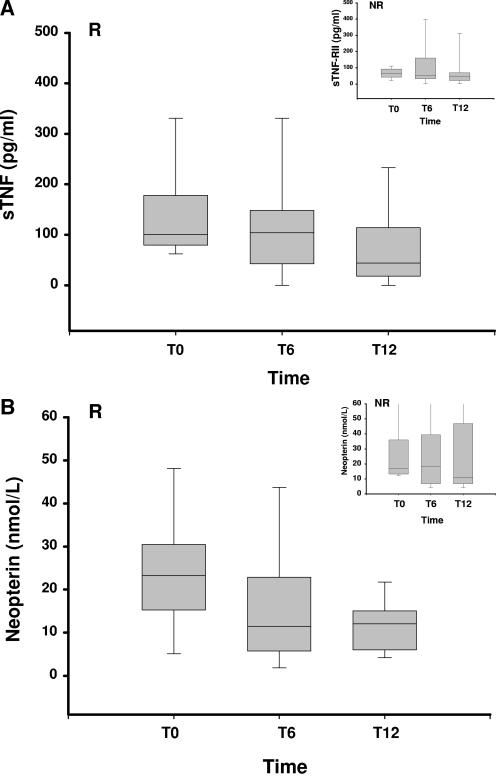
Median values for each of the two groups (responder and nonresponder) at the beginning (t0) and at the end (t6) of antituberculous treatment and at follow-up (t12) were calculated. Changes in β2-microglobulin, TNF receptor II, and IL-6 levels were in parallel between the two groups of HIV-1/TB-coinfected patients and were not different from one another at t0, t6, or t12 (data not shown). Interestingly, trends for higher levels of TNF-α and neopterin (i.e., markers associated with macrophage activation) in serum were found in responders compared to nonresponders at t0. Levels of both of the markers neopterin (P < 0.05) and TNF-α (P < 0.01) decreased significantly in responders alone by 12 months (Fig. 2).
FIG. 2.
Changes in TNF-α and neopterin levels in sera of HIV-1/TB-coinfected patients. Medians and ranges of each serum marker for HIV-1/TB-coinfected subjects over time are shown. In HIV-1/TB-coinfected responders (R), both TNF-α (A) and serum neopterin (B) decreased 6 months after completion of TB treatment. Inserts show changes in nonresponders (NR). sTNF, soluble TNF.
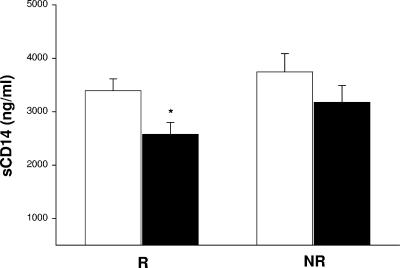
To further assess how changes in macrophage activation correlate with the course of HIV disease in these two groups of HIV-1/TB-coinfected patients, we next assessed changes in sCD14, a more specific marker of macrophage activation (32), in plasma obtained at the beginning and after the completion of antituberculous treatment. Levels of sCD14 are particularly elevated in subjects with dual HIV-1/TB infection (15). We chose to examine plasma samples obtained from HIV-1/TB-coinfected subjects at 1 month of TB treatment, rather than at t0. The rationale was to obviate the high sCD14 levels at the time of diagnosis of TB in HIV-1/TB-coinfected patients (15), which likely reflect intense macrophage activation due to uncontrolled M. tuberculosis infection. Comparison of sCD14 levels of the whole group of HIV-1/TB-coinfected patients to those of HIV-infected control Ugandans without TB indicated that sCD14 levels were significantly higher (3,523 ± 188 ng/ml versus 2,228 ± 176 ng/ml; P < 0.005). Initial levels of sCD14 were not different between the responders and nonresponders. However, levels of sCD14 resolved to a significant degree by the end of treatment in responders alone (Fig. 3) (P < 0.01). No correlation between the plasma sCD14 level and HIV-1 viral load was observed.
FIG. 3.
Changes in sCD14 in HIV-1/TB-coinfected patients. Concentrations of sCD14 in HIV-1/TB-coinfected responders (R) (left) and nonresponders (NR) (right) were measured in plasma at 1 month and at 6 months. Reduction in sCD14 in responders alone was significant (marked by an asterisk) (P < 0.05).
DISCUSSION
Unlike other OI occurring during HIV-1 infection (25), TB is associated with sustained and intense viral replication for prolonged periods of time. The expanded viral heterogeneity in HIV-1 obtained from sites of M. tuberculosis infection (6), or systemically in patients with pulmonary TB (5), likely reflects chronic immune pressure on the virus. Our data indicate that the adverse effect of TB on HIV-1 load is altered little by antituberculous therapy alone in patients with pulmonary TB with CD4 counts above 100 cells/μl. Data reported previously by Morris et al. from a larger cohort of HIV-1/TB-coinfected patients from South Africa that included various forms of TB and was unrestricted for CD4 counts also showed no effect of anti-TB therapy on HIV-1 replication (19). Data from Africa contrast with data from the United States (11) and Europe (personal communications), where heightened HIV-1 activity in subjects dually infected with HIV-1 and pulmonary TB decreased significantly after completion of antituberculous chemotherapy. Differences between African and non-African HIV-1-infected cohorts with pulmonary TB maybe due to more timely access to medical care in the latter cohort. In this scenario, effects of differences in the length of “immune” pressure due to uncontrolled M. tuberculosis infection on HIV-1 replication may account for the differences between the two cohorts. Importantly, here, viral loads of HIV-1/TB-coinfected subjects remained high 6 months after completion of chemotherapy. These data further underscore the deleterious effect of pulmonary TB on the course of HIV-1 disease.
Here, we distinguish two groups of HIV-1/TB-coinfected patients based on reduction in HIV-1 viral load by the end of anti-TB treatment. The group with a significant drop in HIV-1 viral load by 6 months (i.e., responders) was older. This is in contrast to previously published data where age has been identified as a negative prognosticator of progression of HIV-1 disease (13). The two groups (responders and nonresponders) were equally comprised of men and women. Importantly, in responders, viral loads returned to baseline levels 6 months after treatment of TB. In nonresponders, HIV-1 viral load increased significantly during follow-up. In contrast, the changes in HIV-1 viral load between plasma obtained 6 months apart in HIV-1-infected control subjects was insignificant. These data indicate that a history of TB is associated with significant expansion of HIV-1 replication within a 6-month interval. Additionally, these data from the HIV-1/TB-coinfected responder group suggest that reservoirs of latent HIV-1 infection are likely already established prior to TB treatment. The subsequent loss of control of latent reservoirs in this group and the significant increase in viral loads to pretreatment levels at follow-up need to be addressed further in future studies.
Macrophages contribute significantly to viral load during HIV-1/TB coinfection (15), particularly at sites of M. tuberculosis infection (16). In this regard, the role of macrophage activation in HIV-1/TB coinfection has been noted previously (15). Here, the role of macrophages in supporting viral production is particularly notable in responders who concomitantly correct both their serum viral loads and sCD14 by the end of TB treatment. In the nonresponders, an insignificant drop in sCD14 by the end of treatment, i.e., persistent macrophage activation, may be based on continuous CD4-T-cell/macrophage interaction.
The reason(s) for differences in viral replication between HIV-1/TB-coinfected responders and nonresponders is unclear and may relate to the duration of TB-related immune pressure. Responders may have been subjected to shorter periods of immune pressure, as diagnosis of progressive primary pulmonary TB is more common at lower CD4 counts (14). By contrast, nonresponders likely may include more subjects with reactivation of pulmonary TB and may therefore be subjected to prolonged immune activation. As noted above, even in responders, HIV-1 viral load increased back to baseline after successful completion of TB treatment and to levels higher than those in the HIV-1 control subjects. These data suggest that HIV-1 reservoirs maybe large and saturated in HIV-1/TB-coinfected subjects. Thus, regardless of the initial response in lowering the viral load, sustained generalized immune activation (11) may promote viral replication after successful treatment of TB. Whether persistent cellular activation in HIV-1/TB-coinfected patients underlies the loss of control of HIV-1 replication is not known but may be responsible for the excessive mortality associated with dual infection (27).
In summary, HIV-1 replication during dual HIV-1/TB coinfection is less amenable to virologic control by treatment of TB compared to other OI. HAART needs to be evaluated for patients dually infected with HIV-1 and pulmonary TB in both anti-TB responders and nonresponders, as both groups are subjected to high viral replication at follow-up. Clinicians treating HIV-1/TB coinfection face numerous challenges. Currently, many of the antiretroviral drugs have important interactions with rifampin, a first-line drug for the treatment of TB. Key issues that should be addressed in future studies include both optimal regimens and time to start HAART in HIV-1/TB-coinfected patients. As earlier institution of HAART may effectively abrogate the risk of development of TB (10), and thereby the deleterious impact of TB on HIV-1 disease, this strategy should be considered in developing countries where the incidence of M. tuberculosis infection remains rampant.
Acknowledgments
This study was supported by grants HL 51636 and AI-36219, Tuberculosis Prevention and Control Research Unit (AI-95383), and by the Center for AIDS Research at Case Western Reserve University, Cleveland, Ohio.
We acknowledge Roche pharmaceuticals for donation of Amplicor kits. We thank the patients who participated in the study.
We do not have any conflicts of interest with the contents of the manuscript.
REFERENCES
- 1.Badri, M., R. Ehrlich, R. Wood, T. Pulerwitz, and G. Maartens. 2001. Association between tuberculosis and HIV disease progression in a high tuberculosis prevalence area. Int. J. Tuberc. Lung Dis. 5:225-232. [PubMed] [Google Scholar]
- 2.Brown, M., M. Kizza, C. Watera, M. A. Quigley, S. Rowland, P. Hughes, J. A. Whitworth, and A. M. Elliott. 2004. Helminth infection is not associated with faster progression of HIV disease in coinfected adults in Uganda. J. Infect. Dis. 190:1869-1879. [DOI] [PubMed] [Google Scholar]
- 3.Bush, C. E., R. M. Donovan, N. P. Markowitz, P. Kvale, and L. D. Saravolatz. 1996. A study of HIV RNA viral load in AIDS patients with bacterial pneumonia. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 13:23-26. [DOI] [PubMed] [Google Scholar]
- 4.Cantin, R., J. F. Fortin, G. Lamontagne, and M. Tremblay. 1997. The presence of host-derived HLA-DR1 on human immunodeficiency virus type 1 increases viral infectivity. J. Virol. 71:1922-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins, K. R., H. Mayanja-Kizza, B. A. Sullivan, M. E. Quinones-Mateu, Z. Toossi, and E. J. Arts. 2000. Greater diversity of HIV-1 quasispecies in HIV-infected individuals with active tuberculosis. J. Acquir. Immune Defic. Syndr. 24:408-417. [DOI] [PubMed] [Google Scholar]
- 6.Collins, K. R., M. E. Quinones-Mateu, M. Wu, H. Luzze, J. L. Johnson, C. Hirsch, Z. Toossi, and E. J. Arts. 2002. Human immunodeficiency virus type 1 (HIV-1) quasispecies at the sites of Mycobacterium tuberculosis infection contribute to systemic HIV-1 heterogeneity. J. Virol. 76:1697-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Conover, W. 1980. Practical nonparametric statistics, 2nd ed., p. 213-344. John Wiley & Sons, New York, N.Y.
- 8.Falk, A., J. B. O'Connor, P. C. Pratt, W. R. Webb, J. A. Wier, and E. Wolinsky. 1969. Classification of pulmonary tuberculosis, p. 68-76. In Diagnostic standards and classification of tuberculosis. National Tuberculosis and Respiratory Disease Association, New York, N.Y.
- 9.Fleiss, J. L. 1981. Statistical methods for rates and proportions, 2nd ed., p. 61-82. John Wiley & Sons, New York, N.Y.
- 10.Girardi, E., G. Antonucci, P. Vanacore, M. Libanore, I. Errante, A. Matteelli, and G. Ippolito. 2000. Impact of combination antiretroviral therapy on the risk of tuberculosis among persons with HIV infection. AIDS 14:1985-1991. [DOI] [PubMed] [Google Scholar]
- 11.Goletti, D., D. Weissman, R. W. Jackson, N. M. Graham, D. Vlahov, R. S. Klein, S. S. Munsiff, L. Ortona, R. Cauda, and A. S. Fauci. 1996. Effect of Mycobacterium tuberculosis on HIV replication. Role of immune activation. J. Immunol. 157:1271-1278. [PubMed] [Google Scholar]
- 12.Harries, A. D., N. J. Hargreaves, R. Chimzizi, and F. M. Salaniponi. 2002. Highly active antiretroviral therapy and tuberculosis control in Africa: synergies and potential. Bull. W. H. O. 80:464-469. [PMC free article] [PubMed] [Google Scholar]
- 13.Kalayjian, R. C., A. Landay, R. B. Pollard, D. D. Taub, B. H. Gross, I. R. Francis, A. Sevin, M. Pu, J. Spritzler, M. Chernoff, A. Namkung, L. Fox, A. Martinez, K. Waterman, S. A. Fiscus, B. Sha, D. Johnson, S. Slater, F. Rousseau, and M. M. Lederman. 2003. Age-related immune dysfunction in health and in human immunodeficiency virus (HIV) disease: association of age and HIV infection with naive CD8+ cell depletion, reduced expression of CD28 on CD8+ cells, and reduced thymic volumes. J. Infect. Dis. 187:1924-1933. [DOI] [PubMed] [Google Scholar]
- 14.Keiper, M. D., M. Beumont, A. Elshami, C. P. Langlotz, and W. T. Miller, Jr. 1995. CD4 T lymphocyte count and the radiographic presentation of pulmonary tuberculosis. A study of the relationship between these factors in patients with human immunodeficiency virus infection. Chest 107:74-80. [DOI] [PubMed] [Google Scholar]
- 15.Lawn, S. D., M. O. Labeta, M. Arias, J. W. Acheampong, and G. E. Griffin. 2000. Elevated serum concentrations of soluble CD14 in HIV− and HIV+ patients with tuberculosis in Africa: prolonged elevation during anti-tuberculosis treatment. Clin. Exp. Immunol. 120:483-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawn, S. D., T. L. Pisell, C. S. Hirsch, M. Wu, S. T. Butera, and Z. Toossi. 2001. Anatomically compartmentalized human immunodeficiency virus replication in HLA-DR+ cells and CD14+ macrophages at the site of pleural tuberculosis coinfection. J. Infect. Dis. 184:1127-1133. [DOI] [PubMed] [Google Scholar]
- 17.Lawn, S. D., D. Rudolph, S. Wiktor, D. Coulibaly, A. Ackah, and R. B. Lal. 2000. Tuberculosis (TB) and HIV infection are independently associated with elevated serum concentrations of tumour necrosis factor receptor type 1 and beta2-microglobulin, respectively. Clin. Exp. Immunol. 122:79-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lucas, S., and A. M. Nelson. 1994. Pathogenesis of tuberculosis in human immunodeficiency virus-infected people, p. 503-513. In B. R. Bloom (ed.), Tuberculosis: pathogenesis, protection, and control. ASM Press, Washington, D.C.
- 19.Morris, L., D. J. Martin, H. Bredell, S. N. Nyoka, L. Sacks, S. Pendle, L. Page-Shipp, C. L. Karp, T. R. Sterling, T. C. Quinn, and R. E. Chaisson. 2003. Human immunodeficiency virus-1 RNA levels and CD4 lymphocyte counts, during treatment for active tuberculosis, in South African patients. J. Infect. Dis. 187:1967-1971. [DOI] [PubMed] [Google Scholar]
- 20.Ostrowski, M. A., D. C. Krakauer, Y. Li, S. J. Justement, G. Learn, L. A. Ehler, S. K. Stanley, M. Nowak, and A. S. Fauci. 1998. Effect of immune activation on the dynamics of human immunodeficiency virus replication and on the distribution of viral quasispecies. J. Virol. 72:7772-7784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Piroth, L., M. Duong, C. Quantin, M. Abrahamowicz, R. Michardiere, L. S. Aho, M. Grappin, M. Buisson, A. Waldner, H. Portier, and P. Chavanet. 1998. Does hepatitis C virus co-infection accelerate clinical and immunological evolution of HIV-infected patients? AIDS 12:381-388. [DOI] [PubMed] [Google Scholar]
- 22.Quan, C. M., M. Krajden, J. Zhao, and A. W. Chan. 1993. High-performance liquid chromatography to assess the effect of serum storage conditions on the detection of hepatitis C virus by the polymerase chain reaction. J. Virol. Methods 43:299-307. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez, B., I. Woolley, M. M. Lederman, D. Zdunek, G. Hess, and H. Valdez. 2003. Effect of GB virus C coinfection on response to antiretroviral treatment in human immunodeficiency virus-infected patients. J. Infect. Dis. 187:504-507. [DOI] [PubMed] [Google Scholar]
- 24.Sabin, C. A., P. Telfer, A. N. Phillips, S. Bhagani, and C. A. Lee. 1997. The association between hepatitis C virus genotype and human immunodeficiency virus disease progression in a cohort of hemophilic men. J. Infect. Dis. 175:164-168. [DOI] [PubMed] [Google Scholar]
- 25.Sulkowski, M. S., R. E. Chaisson, C. L. Karp, R. D. Moore, J. B. Margolick, and T. C. Quinn. 1998. The effect of acute infectious illnesses on plasma human immunodeficiency virus (HIV) type 1 load and the expression of serologic markers of immune activation among HIV-infected adults. J. Infect. Dis. 178:1642-1648. [DOI] [PubMed] [Google Scholar]
- 26.Vanham, G., K. Edmonds, L. Qing, D. Hom, Z. Toossi, B. Jones, C. L. Daley, B. Huebner, L. Kestens, P. Gigase, and J. J. Ellner. 1996. Generalized immune activation in pulmonary tuberculosis: co-activation with HIV infection. Clin. Exp. Immunol. 103:30-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whalen, C., C. R. Horsburgh, D. Hom, C. Lahart, M. Simberkoff, and J. Ellner. 1995. Accelerated course of human immunodeficiency virus infection after tuberculosis. Am. J. Respir. Crit. Care Med. 151:129-135. [DOI] [PubMed] [Google Scholar]
- 28.Whalen, C. C., P. Nsubuga, A. Okwera, J. L. Johnson, D. L. Hom, N. L. Michael, R. D. Mugerwa, and J. J. Ellner. 2000. Impact of pulmonary tuberculosis on survival of HIV-infected adults: a prospective epidemiologic study in Uganda. AIDS 14:1219-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams, C. F., D. Klinzman, T. E. Yamashita, J. Xiang, P. M. Polgreen, C. Rinaldo, C. Liu, J. Phair, J. B. Margolick, D. Zdunek, G. Hess, and J. T. Stapleton. 2004. Persistent GB virus C infection and survival in HIV-infected men. N. Engl. J. Med. 350:981-990. [DOI] [PubMed] [Google Scholar]
- 30.Wong, S. B., S. H. Chan, and E. C. Ren. 1999. Diversity of GB virus C/hepatitis G virus isolates in Singapore: predominance of group 2a and the Asian group 3 variant. J. Med. Virol. 58:145-153. [DOI] [PubMed] [Google Scholar]
- 31.Wright, T. L., H. Hollander, X. Pu, M. J. Held, P. Lipson, S. Quan, A. Polito, M. M. Thaler, P. Bacchetti, and B. F. Scharschmidt. 1994. Hepatitis C in HIV-infected patients with and without AIDS: prevalence and relationship to patient survival. Hepatology 20:1152-1155. [PubMed] [Google Scholar]
- 32.Ziegler-Heitbrock, H. W., and R. J. Ulevitch. 1993. CD14: cell surface receptor and differentiation marker. Immunol. Today 14:121-125. [DOI] [PubMed] [Google Scholar]