Abstract
We recently set up a gamma interferon (IFN-γ) enzyme-linked immunospot assay (ELISPOT), using selected early secreted antigenic target 6 (ESAT-6) peptides, that appears specific for active tuberculosis (A-TB). However, ELISPOT is difficult to automate. Thus, the objective of this study was to determine if the same selected peptides may be used in a technique more suitable for routine work in clinical laboratories, such as whole-blood enzyme-linked immunosorbent assay (WBE). For this purpose, 27 patients with A-TB and 41 control patients were enrolled. Our WBE, using the already described selected peptides from ESAT-6 plus three new ones from culture filtrate protein 10, was performed, and data were compared with those obtained by ELISPOT. Using our selected peptides, IFN-γ production, evaluated by both WBE and ELISPOT, was significantly higher in patients with A-TB than in controls (P < 0.0001). Statistical analysis showed a good correlation between the results obtained by WBE and ELISPOT (r = 0.80, P < 0.001). To substantiate our data, we compared our WBE results with those obtained by QuantiFERON-TB Gold, a whole-blood assay based on region of difference 1 (RD1) overlapping peptides approved for TB infection diagnosis. We observed a slightly higher sensitivity with QuantiFERON-TB Gold than with our WBE (89% versus 81%); however, our test provided a better specificity result (90% versus 68%). In conclusion, results obtained by WBE based on selected RD1 peptides significantly correlate with those generated by ELISPOT. Moreover, our assay appears more specific for A-TB diagnosis than QuantiFERON-TB Gold, and thus it may represent a complementary tool for A-TB diagnosis for routine use in clinical laboratories.
An estimated one-third of the world's population is infected with Mycobacterium tuberculosis, presenting a major impediment to tuberculosis (TB) control (25). Primary infection leads to active tuberculosis (A-TB) in a minority (about 10%) of infected individuals (27), in most cases within 2 years. In the remaining 90% of cases, the immune system contains the infection, and the individual is noninfectious and symptom-free (9). This clinical latency can persist throughout the person's lifetime. However, in some circumstances the host immune response is perturbed and the latent TB infection (LTBI) may develop into a reactivation TB. This process can occur, for example, following human immunodeficiency virus infection, malnutrition, or use of steroids or other immunosuppressive medications or because of advanced age (12). Development of new diagnostic tools would improve the control of TB both by improving diagnosis of A-TB and by allowing a more accurate identification of LTBI (25).
The tuberculin skin test (TST) measures hypersensitivity responses to purified protein derivative, a crude mixture of antigens shared by M. tuberculosis, Mycobacterium bovis bacillus Calmette-Guerin (BCG), and several nontuberculous mycobacteria. The TST has been, until recently, the only available tool for TB diagnosis: however, it provides low sensitivity and specificity (1, 11, 15) and does not allow discrimination between A-TB and LTBI (1).
Advances in genomics and immunology have led to a promising alternative, the in vitro gamma interferon (IFN-γ) assays, based on the concept that T cells of infected individuals release IFN-γ. These assays use early secreted antigenic target 6 (ESAT-6) protein and culture filtrate protein 10 (CFP-10) (3, 17, 19, 24). These proteins, encoded within the region of difference 1 (RD1) of the M. tuberculosis genome, are significantly more specific to M. tuberculosis than purified protein derivative, as they are not shared with BCG substrains or most environmental mycobacteria (with the exception of Mycobacterium kansasii, Mycobacterium szulgai, Mycobacterium flavescens, and Mycobacterium marinum). Peripheral blood mononuclear cells (PBMC) of patients with TB (21, 28) and of household contacts of TB patients (8) release IFN-γ when exposed in vitro to ESAT-6 and CFP-10 intact proteins and to overlapping peptides spanning the length of these antigens. These studies resulted in the development of two commercially available tests (QuantiFERON-TB Gold [Cellestis Limited, Carnagie, Victoria, Australia] and T SPOT-TB [Oxford Immunotec, Oxford, United Kingdom]) approved for TB infection diagnosis (10, 19). Both tests are based on ESAT-6 and CFP-10 overlapping peptides spanning the whole proteins and employ enzyme-linked immunosorbent and enzyme-linked immunospot assays (ELISA and ELISPOT), respectively. These overlapping peptides are 15 amino acids long (16) or have a variable length of 16 to 26 amino acids (10, 17). Although these commercial assays provide an accurate diagnosis of M. tuberculosis infection, they do not discriminate between A-TB and LTBI. Thus, further clinical workup is required to rule out A-TB after a positive response to these tests.
In this context, we have recently set up an IFN-γ ELISPOT based on multiepitopic peptides from the ESAT-6 protein, selected by quantitative implemented human leukocyte antigen (HLA) peptide-binding motif analysis. The sensitivity of this assay for A-TB is 74% (29). More importantly, the positive response fell below the detection level after 3 months of anti-TB therapy in those responding to treatment (7). None of the control patients, including those who were TST positive, responded to the selected peptides (29). This test has the potential of being a very useful assay to detect A-TB; however, ELISPOT is difficult to automate because it requires many laboratory steps, such as the separation of cells from blood and the counting of cells and spots. For these reasons, ELISPOT is not suitable for routine use in diagnostic laboratories.
Recently, Schölvinck et al. (23) demonstrated that a sensitive IFN-γ WBE using intact ESAT-6/CFP-10 (RD1) proteins matched the performance of the ELISPOT. We therefore evaluated the performance of our selected RD1 peptides in the identification of A-TB using a WBE and compared the results with those obtained by the ELISPOT. The RD1 peptides used in our test included two that were previously described, selected from ESAT-6 (7, 29), and three that were newly selected from CFP-10. To substantiate our data, the results obtained by the two assays were compared with those obtained with QuantiFERON-TB Gold, a WBE approved for in vitro immunological diagnosis of TB infection, based on the use of RD1 overlapping peptides (10, 19).
MATERIALS AND METHODS
Patient population and study design.
Patients that were admitted to the infectious disease and respiratory disease wards at the National Institute for Infectious Diseases (INMI) L. Spallanzani in Rome, Italy, between April 2004 and November 2004 were evaluated for enrollment. Subjects were included in the study if they tested human immunodeficiency virus negative and were not undergoing cortisone therapy. None of them were undergoing anti-TB therapy at the time of enrollment.
Enrolled patients underwent clinical and microbiological examinations to confirm or exclude the diagnosis of TB. Briefly, sequential respiratory samples (three expectorated or two induced sputa) were collected over the first 7 days following hospital admission. Acid-fast bacillus smear (Ziehl-Neelsen) and culture (on Lowenstein-Jensen and BACTEC 460 media [BD Biosciences Division, Sparks, Md.] and Middlebrook 7H10/7H11 media) were performed with each specimen. Chest X rays were done for each patient. Patients underwent TST administered by the Mantoux method, and those with an induration of ≥10 mm were classified as TST positive (1, 11, 15).
Enrolled patients were classified as “A-TB” if the diagnosis was confirmed by a positive M. tuberculosis culture of biological specimens. For controls, we selected patients with diseases other than A-TB who had come to a resolution of clinical symptoms after antibiotic therapy not involving M. tuberculosis-active drugs and whose sputum culture for M. tuberculosis was negative.
Within 7 days of admission, two blood samples, one in EDTA (for ELISPOT) and one in heparin (for WBE), were drawn from all enrolled individuals. Our WBE and ELISPOT were performed in parallel with a QuantiFERON-TB Gold assay. Clinicians were blinded to the results of in vitro assays, and laboratory personnel were blinded to the status of the patient.
The study was approved by the ethics committee at our institution, and all enrolled patients provided informed written consent.
In vitro assays for TB infection. (i) WBE and ELISPOT based on selected RD1 peptides.
The selection of HLA class II-restricted epitopes of ESAT-6 and CFP-10 M. tuberculosis proteins was performed by quantitative implemented HLA peptide-binding motif analysis as previously described for ESAT-6 (6, 7, 26, 29). Peptides were synthesized as free amino acid termini using 9-fluorenylmethoxy carbonyl chemistry (ABI, Bergamo, Italy). Lyophilized peptides were diluted in dimethyl sulfoxide at stock concentrations of 10 mg/ml for each peptide and stored at −80°C. RD1 peptides used in this study are described in Table 1. Each selected peptide (Table 1) contained one or more epitopes able to bind at least (i) four different HLA-DR, (ii) two different HLA-DP, and (iii) two different HLA-DQ specificities with a putative binding ability of 80% of the maximum binding for any allele belonging to an HLA class II serological specificity. Together, the designed peptide epitopes were able to cover more than 90% of the HLA class II haplotypes present in different human populations (data not shown). Sequence homology searches of all known protein sequences confirmed that the sequences of the selected peptides were uniquely restricted to M. tuberculosis complex proteins.
TABLE 1.
CD4+ T-cell epitopes in ESAT-6 and CFP-10 proteins selected to be broadly recognized in patients with A-TB
| Protein | Position | Sequence | DR serological specificities covereda |
|---|---|---|---|
| ESAT-6 | 6-28 | WNFAGIEAAASAIQGNVTSIHSL | 1, 3, 4, 8, 11(5), 13(6), 52, 53 |
| ESAT-6 | 66-78 | NALQNLARTISEA | 3, 8, 11(5), 13(6), 15(2), 52 |
| CFP-10 | 18-31 | FERISGDLKTQIDQ | 3, 5, 11(5), 52 |
| CFP-10 | 43-70 | GQWRGAAGTAAQAAVVRFQEAANKQKQE | 1, 3, 4, 7, 8, 11(5), 13(6), 15(2), 52 |
| CFP-10 | 74-86 | TNIRQAGVQYSRA | 3, 4, 7, 8, 11(5), 12(5), 13(6), 15(2) |
HLA class II alleles putatively able to recognize the selected ESAT-6 and CFP-10 peptides are listed according to serological specificity, i.e., the group of alleles recognized by the same allo-antisera used for low-resolution HLA typing. Where necessary, the serological HLA class II subgroup to which the HLA-DR allele that is putatively able to recognize the peptide belongs is shown in parentheses.
Samples were analyzed in parallel by WBE and ELISPOT. For the 40-hour ELISPOT, the PBMC were treated as previously described (29). For WBE, 0.5 ml per well of heparinized blood was seeded in a 48-well plate and treated with selected RD1 peptides and phytohemagglutinin (Sigma, St. Louis, MO) at the same concentration used in ELISPOT (29). Samples were then incubated for 24 h at 37°C in the presence of 5% CO2. IFN-γ levels in culture supernatants were assessed by a commercially available kit (QuantiFERON-CMI kit; Cellestis Limited, Carnegie, Victoria, Australia). Cutoff values were determined by constructing a receiver operator characteristic (ROC) curve by means of LABROC-1 software. The cutoff values that we used in the ELISPOT, expressed as spot-forming cells (SFC)/million PBMC, were 60 for phytohemagglutinin and 34 for selected RD1 peptides, and the cutoff value for all stimuli in WBE experiments was 0.6 IU/ml.
(ii) Commercially available assay.
The QuantiFERON-TB Gold assay (Cellestis Limited, Carnegie, Victoria, Australia) was performed, and its results were scored as indicated by the manufacturer (the cutoff value for a positive test was 0.35 IU/ml).
Statistical analysis.
The means ± standard deviations were calculated. Differences between groups were evaluated by the Mann-Whitney U test. To compare the results of the different assays, we used the McNemar test. Analysis was carried out using the SPSS software, v. 11 for Windows.
RESULTS
Agreement between ELISPOT and WBE using selected RD1 peptides.
To evaluate the efficiency of a WBE based on selected RD1 peptides in diagnosing A-TB, we studied a total of 68 subjects: 27 with microbiologically confirmed TB and 41 controls, 21 of whom were TST positive and 20 of whom were TST negative. Characteristics of all of the subjects are reported in Table 2.
TABLE 2.
Epidemiological and demographic characteristics of enrolled subjectsa
| Characteristic | Value for group
|
||
|---|---|---|---|
| Active TB (microbiological diagnosis) | Controls
|
||
| TST positive | TST negative | ||
| Total no. of patients | 27 | 21 | 20 |
| Mean age (yr) ± SE | 37 ± 2 | 43 ± 2 | 40 ± 3 |
| Sex | |||
| Females | 7 | 9 | 8 |
| Males | 20 | 12 | 12 |
| Ethnicity | |||
| Western Europe | 5 | 17 | 13 |
| Non-Western Europe | 22 | 4 | 7 |
| Diseases other than TB | |||
| Bacterial pneumonia | 8 | 8 | |
| Bronchitis | 5 | 6 | |
| Fever of unknown origin | 2 | 3 | |
| Bacterial abscess | 1 | 1 | |
| Bacterial pleuritis | 1 | ||
| Pulmonary carcinoma | 3 | 2 | |
| Pseudotumor | 1 | ||
Abbreviations: TB, tuberculosis; TST, tuberculin skin test; SE, standard error; BCG, bacillus Calmette et Guerin.
Samples were analyzed by WBE and ELISPOT using the same selected RD1 peptides: three were newly synthesized from CFP-10 (Table 1), and two were the peptides from ESAT-6 that were already described (29).
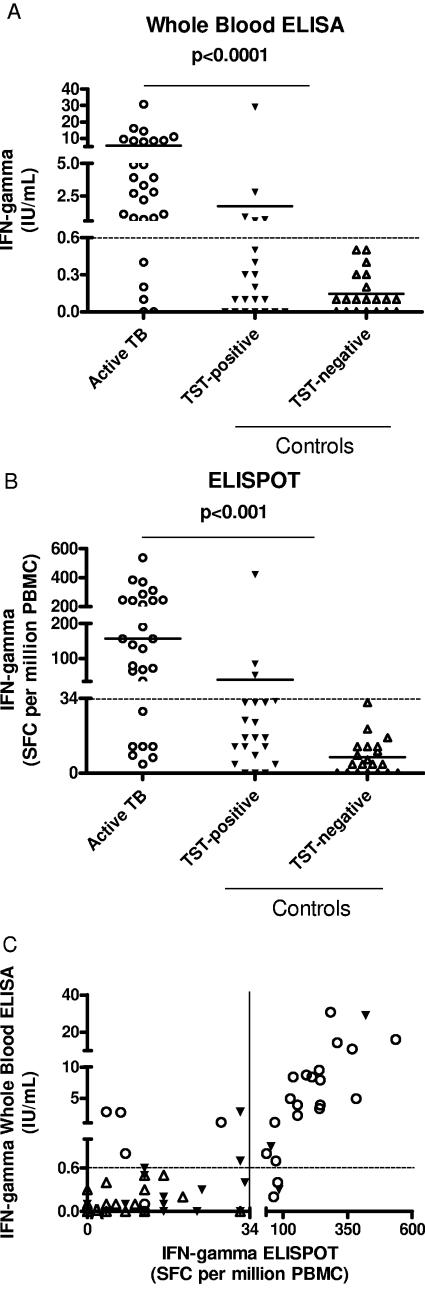
IFN-γ production (IU/ml) in response to selected RD1 peptides evaluated by WBE was significantly higher in patients with A-TB (5.5 ± 1.3 IU/ml) than in controls (0.95 ± 0.7 IU/ml) (P < 0.0001). This difference was statistically significant independently of the TST status of controls, either positive (1.7 ± 1.3 IU/ml, P < 0.0001 versus A-TB) or negative (0.1 ± 0.03 IU/ml, P < 0.0001 versus A-TB) (Fig. 1A). Similarly, the IFN-γ production in response to the same peptides, evaluated by ELISPOT and expressed as SFC per million PBMC, was significantly higher in patients with A-TB (156.8 ± 26.8 SFC) than in controls (23.8 ± 10 SFC, P < 0.0001). This difference also was statistically significant independently of the TST status of controls, either positive (39.7 ± 19.5 SFC, P < 0.0001 versus A-TB) or negative (7.2 ± 1.8 SFC, P < 0.0001 versus A-TB) (Fig. 1B). When the response to selected RD1 peptides obtained by WBE was compared to that found by ELISPOT, the number of SFC per million PBMC counted by ELISPOT correlated significantly with the IU/ml measured by WBE (r = 0.80, P < 0.001) (Fig. 1C).
FIG.1.
IFN-γ response to selected RD1 peptides in A-TB patients and controls by WBE and ELISPOT. Detection of IFN-γ was performed by WBE (A), ELISPOT (B), and by both WBE and ELISPOT (C). The highest response to selected RD1 peptides in terms of IFN-γ is reported in IU/ml or SFC per million PBMC for each individual. Horizontal bars represent the mean IU/ml or SFC per million PBMC values for each group of patients. The P value denotes the difference between the responders in each group. Symbols: ○, active TB; ▾, TST-positive controls; ▵, TST-negative controls.
In terms of results scoring, in WBE experiments the response to selected RD1 peptides was scored positive in 22/27 (81%) A-TB patients and in 4/41 (10%, P < 0.0001) controls (4/21 among TST-positive individuals, P < 0.0001; 0/20 among TST-negative individuals, P < 0.0001). Thus, in the population studied, the sensitivity of the WBE for A-TB was 81% and the specificity was 90% (Table 3). ELISPOT responses were scored positive in 20/27 (74%) patients with A-TB and in 3/41 (7%, P < 0.0001) controls (3/21 among TST positives, P < 0.0001; 0/20 among TST negatives, P < 0.0001), with resulting sensitivity and specificity values for A-TB of 74% and 93%, respectively (Table 3). We observed 86.7% concordance of positive and negative results between the two tests (k = 0.71; P < 0.001).
TABLE 3.
Comparison of the different assays to perform immune diagnosis of TB
| Peptides | Test | No. of patients positive/total no. (%)
|
|||
|---|---|---|---|---|---|
| Active TB patients | Control subjects
|
||||
| TST positive | TST negative | Totala | |||
| Selected RD1 | WBE | 22/27 (81) | 4/21 (19) | 0/20 (0) | 4/41 (10) |
| ELISPOT | 20/27 (74) | 3/21 (14) | 0/20 (0) | 3/41 (7) | |
| RD1 overlapping | QuantiFERON-TB-Gold | 24/27 (89) | 11/21 (52) | 2/20 (10) | 13/41 (32) |
Total, TST-positive and TST-negative control subjects.
WBE and ELISPOT proved to have good internal reproducibility, with less than 10% difference between duplicate samples. The ELISPOT was always run in duplicate, while duplicate samples were assayed for 10 patients with the WBE (data not shown). Moreover, samples from five patients were retested after a week, and again, less than 10% difference among the results obtained on the two different experiment days was found (data not shown).
In conclusion, these data indicate that the response to selected RD1 peptides is associated with A-TB and that WBE results correlate significantly with ELISPOT data.
Comparison between our WBE for selected RD1 peptides and the QuantiFERON-TB Gold assay.
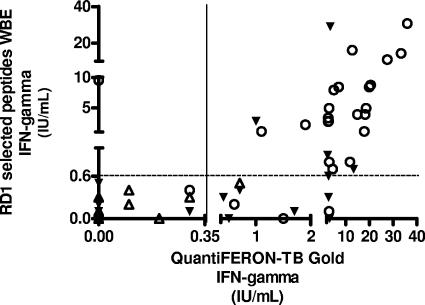
We next compared the results obtained by our above-described WBE with those obtained by QuantiFERON-TB Gold, a whole-blood assay approved for the diagnosis of TB infection, based on RD1 overlapping peptides (Table 3). In the QuantiFERON-TB Gold assay, IFN-γ production in response to TB antigens was significantly higher in patients with A-TB (10.01 ± 2.04 IU/ml) than in controls (0.84 ± 0.34 IU/ml, P < 0.0001), and this difference was statistically significant independently of the TST status of controls, either positive (1.5 ± 0.6 IU/ml, P < 0.0001 versus A-TB) or negative (0.1 ± 0.04 IU/ml, P < 0.0001 versus A-TB) (Fig. 2). Responses were scored positive in 24/27 patients with A-TB (89%) and in 13/41 controls (32%, P < 0.0001; 11/21 among TST positives, P = 0.008; 2/20 among TST negatives, P < 0.0001). Thus, in the population studied, the sensitivity of this assay for A-TB was 89% and the specificity was 68% (Table 3). In a comparison of the results generated by QuantiFERON-TB Gold with those obtained by our WBE, no difference was found in the proportion of positive responses among patients with A-TB (P = 0.6), but a significantly higher response by TST-positive controls was observed in the QuantiFERON-TB Gold assay (11/21 versus 4/21, P = 0.016).
FIG. 2.
Correlation between WBE and QuantiFERON-TB Gold results. The highest IFN-γ-secreting T-cell response is reported in IU/ml for each individual. Symbols: ○, active TB; ▾, TST-positive controls; ▵, TST-negative controls.
In conclusion, these data indicate that in patients with A-TB, responses to selected RD1 peptides detected by WBE correlate with those obtained by QuantiFERON-TB Gold; however, the different results of TST-positive controls in the two assays suggest that our test provides a higher specificity.
DISCUSSION
We recently set up an IFN-γ ELISPOT, using selected ESAT-6 peptides, that appears to be specific for A-TB (7, 29). However, ELISPOT is difficult to automate. Thus, the objective of this study was to develop a test to detect A-TB that is suitable for routine use in diagnostic laboratories, such as an ELISA performed with whole blood. By using selected peptides, three of which were new multiepitopic peptides of CFP-10 protein and two of which were already characterized peptides of ESAT-6 protein (7, 29), we found that the response was significantly higher in those with A-TB than in controls by both WBE and ELISPOT. Data obtained by the two tests correlated significantly. To substantiate our data, we compared WBE results with those obtained by QuantiFERON-TB Gold, which is a whole-blood ELISA based on RD1 overlapping peptides approved for TB infection diagnosis. Among patients with A-TB, the results obtained by WBE did not differ significantly from those obtained by QuantiFERON-TB Gold. Conversely, a higher proportion of TST-positive controls were scored positive by QuantiFERON-TB Gold than by our WBE.
Using our selected RD1 peptides, we found that IFN-γ production was significantly higher in patients with A-TB than in controls. WBE showed a slightly higher sensitivity for A-TB than ELISPOT (81% versus 71%), although the difference was not statistically significant. The higher sensitivity may likely be due to the fact that blood cells are incubated with the reagents without any prior manipulation. Thus, such a technique, in addition to being easier to automate, may also be more sensitive. Regarding the sensitivity issue, it is important to note that three of those five A-TB patients who did not respond in vitro to the selected peptides in the WBE test were probably in a condition of “M. tuberculosis-specific unresponsiveness,” since they did not respond in assays with QuantiFERON-TB Gold, either. This condition could be a consequence of the intrinsic limits of such immune diagnostic assays, which is in line with data recently published showing only 70% positive responses to RD1 ELISPOT in children with active pulmonary TB (18).
The fact that ELISPOT and whole-blood assays correlate well has already been described by Schölvinck et al., although in a different experimental setting (23). In fact, our WBE differs from those described by others (10, 16-18, 20-23, 30) in terms of the reagents used (selected RD1 peptides, overlapping peptides, and intact proteins), methodology, and the results obtained. It could be argued that the incubation times rather than the reagents used could account for the difference in specificities of our test and others. However, this is not the case, since incubation times varied in different studies from 1 day (our WBE and those described previously [10, 16, 17, 20-22]), 2 days (the ELISPOT described here and those described previously [7, 29]), and 3 days (18, 23, 30). In this report, our WBE differed from the QuantiFERON-TB Gold test only in the use of the selected peptides, and this was the only factor that could account for the difference in terms of assay specificity for active disease (90% versus 68%). At present, the reason(s) for the discrepancy of the responses to selected peptides (our WBE and 7, 29), overlapping peptides (10, 16, 17, 20), and intact proteins (18, 21-23, 30) remains unclear. Studies are ongoing in our laboratory to explain such an interesting phenomenon. A possible explanation for the specificity of the selected RD1 peptide response for A-TB may rely on both the different immunological status of the patients with A-TB and LTBI and the selection of a cutoff by ROC analysis able to maximize the accuracy of the diagnosis of A-TB. Regarding the immune status of the patients, it must be considered that metabolically active and viable bacilli secrete proteins, such as ESAT-6 and CFP-10 (2). Consequently, IFN-γ-producing T cells specific to the selected epitopes are also present at high frequency only during the active replication of mycobacteria. Previous papers have demonstrated that lymphocytes with immediate effector memory function circulate for a limited time, until the antigen has been cleared (20). It can be hypothesized that the frequency of T cells specific to the single epitopes contained in the selected peptides might dramatically decrease during latent infection in the absence of bacterial replication and continuous exposure to M. tuberculosis-secreted antigen. Thus, in the presence of a small antigenic load, as in patients with LTBI or with A-TB after efficacious treatment, the limited number of effector cells specific to the selected peptides in the peripheral blood could fall below the cutoff, while the number of T cells specific to intact proteins or to the overlapping peptides covering the whole proteins, which contain multiple epitopes, could remain high enough to be detected. Moreover, it is worth noting that our cutoff value was found by ROC analysis and was selectively chosen to maximize the accuracy of the diagnosis of A-TB, whereas that of the commercially available assays was probably chosen to maximize the accuracy of detection of TB infection (10, 16). This different statistical approach may, in part, explain the different results obtained.
Some limitations of the present study need to be mentioned. First, the study was performed with a small population, and thus, the estimates for sensitivity and specificity may be imprecise. Second, the positive in vitro response to RD1 antigens could be related to infection with mycobacteria other than M. tuberculosis, such as M. kansasii, M. szulgai, M. flavescens, M. marinum, and Mycobacterium leprae, because the ESAT-6 gene or a homologue is also present in these mycobacteria (13, 14). Third, we did not assess the role of different HLA backgrounds in response to selected peptides. In fact, although the population evaluated here is heterogeneous, as the patients came from different areas, the fact that subjects sharing the same HLA alleles could not respond to the same HLA-restricted epitopes (4) may represent a limit when using selected peptides. Therefore, to confirm the specificity of our assay, it will be important to evaluate a larger population in future studies, including patients with mycobacterial diseases other than TB, and also to assess the response to peptides in the context of the patients' HLA class II background.
In conclusion, this whole-blood assay based on selected RD1 peptides may be a potentially useful and easy-to-use tool for TB control. In fact, this assay may facilitate the diagnosis of active TB in the clinical setting. Moreover, in the context of a screening program, our assay using selected RD1 peptides may help to rule out A-TB in individuals with positive results to either the TST or an assay of RD1 overlapping peptides. The simple assay format would be readily adaptable to routine laboratories, even in medically underserved environments where the research application of such whole-blood assays to populations has been shown to be entirely feasible (5).
Acknowledgments
We are grateful to all patients and nursing staff who took part in this study. We thank C. Nisii, G. M. Fimia, and T. Alonzi for critical review of the paper.
The study was supported by a grant from the Italian Ministry of Health (RF 04.126).
D. Goletti, S. Carrara, D. Vincenti, M. Amicosante, and E. Girardi have a patent pending on a T-cell assay based on selected RD1 peptides (PCT international patent no., PCT/EP2005/050728; priority date, 19 February 2004).
REFERENCES
- 1.American Thoracic Society. 2000. Diagnostic standards and classification of tuberculosis in adults and children. Am. J. Respir. Crit. Care Med. 161:1376-1395. [DOI] [PubMed] [Google Scholar]
- 2.Andersen, A. B., and P. Brennan. 1994. Proteins and antigens of Mycobacterium tuberculosis, p. 307-332. In B. Bloom (ed.), Tuberculosis. ASM Press, Washington, D.C.8189684
- 3.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 4.Betts, M. R., J. P. Casazza, B. A. Patterson, S. Waldrop, W. Trigona, T. M. Fu, F. Kern, L. J. Picker, and R. A. Koup. 2000. Putative immunodominant human immunodeficiency virus-specific CD8+ T-cell responses cannot be predicted by major histocompatibility complex class I haplotype. J. Virol. 74:9144-9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Black, G. F., R. E. Weir, S. Floyd, L. Bliss, D. K. Warndorff, A. C. Crampin, B. Ngwira, L. Sichali, B. Nazareth, J. M. Blackwell, K. Branson, S. D. Chaguluka, L. Donovan, E. Jarman, E. King, P. E. Fine, and H. M. Dockrell. 2002. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet 359:1393-1401. [DOI] [PubMed] [Google Scholar]
- 6.Bossuyt, P. M., J. B. Reitsma, D. E. Bruns, C. A. Gatsonis, P. P. Glasziou, L. M. Irwig, J. G. Lijmer, D. Moher, D. Rennie, H. C. de Vet, and Standards for Reporting of Diagnostic Accuracy. 2003. The STARD statement for reporting studies of diagnostic accuracy: explanation and elaboration. Ann. Intern. Med. 138:W1-W12. [DOI] [PubMed] [Google Scholar]
- 7.Carrara, S., D. Vincenti, N. Petrosillo, M. Amicosante, E. Girardi, and D. Goletti. 2004. Use of a T-cell-based assay for monitoring efficacy of antituberculosis therapy. Clin. Infect. Dis. 38:754-756. [DOI] [PubMed] [Google Scholar]
- 8.Doherty, T. M., A. Demissie, J. Olobo, D. Wolday, S. Britton, T. Eguale, P. Ravn, and P. Andersen. 2002. Immune responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 signal subclinical infection among contacts of tuberculosis patients. J. Clin. Microbiol. 40:704-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dye, C., S. Scheele, P. Dolin, V. Pathania, and M. C. Raviglione. 1999. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence and mortality by country. JAMA 282:677-686. [DOI] [PubMed] [Google Scholar]
- 10.FDA. 2005. Approval for the use of synthetic peptide antigens used in the QuantiFERON-TB Gold. P10033/S0006. [Online.] www.fda.gov/cdrh/pma/pmadec04.html. Accessed 14 April 2005.
- 11.Fine, P. E., J. Bruce, J. M. Ponnighaus, P. Nkhosa, A. Harawa, and E. Vynnycky. 1999. Tuberculin sensitivity: conversions and reversions in a rural African population. Int. J. Tuberc. Lung Dis. 3:962-975. [PubMed] [Google Scholar]
- 12.Flynn, J. L., and J. Chan. 2001. Immunology of tuberculosis. Annu. Rev. Immunol. 19:93-129. [DOI] [PubMed] [Google Scholar]
- 13.Geluk, A., K. E. van Meijgaarden, K. L. M. C. Franken, Y. W. Subronto, B. Wieles, S. M. Arend, E. P. Sampaio, T. de Boer, W. R. Faber, B. Naafs, and T. H. Ottenhoff. 2002. Identification and characterization of the ESAT-6 homologue of Mycobacterium leprae and T-cell cross-reactivity with Mycobacterium tuberculosis. Infect. Immun. 70:2544-2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harboe, M., T. Oettinger, H. G. Wiker, I. Rosenkrands, and P. Andersen. 1996. Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect. Immun. 64:16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huebner, R. E., M. F. Schein, and J. B. Bass, Jr. 1993. The tuberculin skin test. Clin. Infect. Dis. 17:968-975. [DOI] [PubMed] [Google Scholar]
- 16.Lalvani, A., A. Pathan, H. Durkan, K. A. Wilkinson, A. Whelan, J. J. Deeks, W. H. Reece, M. Latif, G. Pasvol, and A. V. Hill. 2001. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet 357:2017-2021. [DOI] [PubMed] [Google Scholar]
- 17.Mori, T., M. Sakatani, F. Yamagishi, T. Takashima, Y. Kawabe, K. Nagao, E. Shigeto, N. Harada, S. Mitarai, M. Okada, K. Suzuki, Y. Inoue, K. Tsuyuguchi, Y. Sasaki, G. H. Mazurek, and I. Tsuyuguchi. 2004. Specific detection of tuberculosis infection with an interferon-gamma-based assay using new antigens. Am. J. Respir. Crit. Care Med. 170:59-64. [DOI] [PubMed] [Google Scholar]
- 18.Nicol, M. P., D. Pienaar, K. Wood, B. Eley, R. J. Wilkinson, H. Henderson, L. Smith, S. Samodien, and D. Beatty. 2005. Enzyme-linked immunospot assay responses to early secretory antigenic target 6, culture filtrate protein 10, and purified protein derivative among children with tuberculosis: implications for diagnosis and monitoring of therapy. Clin. Infect. Dis. 40:1301-1308. [DOI] [PubMed] [Google Scholar]
- 19.Pai, M., L. W. Riley, J. M. Colford, Jr. 2004. Interferon-gamma assays in the immunodiagnosis of tuberculosis: a systematic review. Lancet Infect. Dis. 4:761-776. [DOI] [PubMed] [Google Scholar]
- 20.Pathan, A. A., K. A. Wilkinson, P. Klenerman, H. McShane, R. N. Davidson, G. Pasvol, A. V. Hill, and A. Lalvani. 2001. Direct ex vivo analysis of antigen-specific IFN-gamma-secreting CD4+ T cells in Mycobacterium tuberculosis-infected individuals: association with clinical disease state and effect of treatment. J. Immunol. 167:5217-5225. [DOI] [PubMed] [Google Scholar]
- 21.Ravn, P., A. Demissie, T. Eguale, H. Wondwosson, D. Lein, H. A. Amoudy, A. S. Mustafa, A. K. Jensen, A. Holm, I. Rosenkrands, F. Oftung, J. Olobo, F. von Reyn, and P. Andersen. 1999. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J. Infect. Dis. 179:637-645. [DOI] [PubMed] [Google Scholar]
- 22.Ravn, P., E. M. Munk, A. B. Andersen, B. Lundgren, J. D. Lundgren, L. N. Nielsen, A. Kok-Jensen, P. Andersen, and K. Weldingh. 2005. Prospective evaluation of a whole-blood test using Mycobacterium tuberculosis-specific antigens ESAT-6 and CFP-10 for diagnosis of active tuberculosis. Clin. Diagn. Lab. Immunol. 12:491-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schölvinck, E., K. A. Wilkinson, A. O. Whelan, A. R. Martineau, M. Levin, R. J. Wilkinson. 2004. Gamma interferon-based immunodiagnosis of tuberculosis: comparison between whole-blood and enzyme-linked immunospot methods J. Clin. Microbiol. 42:829-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sorensen, A. L., S. Nagai, G. Houen, P. Andersen, and A. B. Andersen. 1995. Purification and characterization of a low-molecular-mass T-cell antigen secreted by Mycobacterium tuberculosis. Infect. Immun. 63:1710-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart, G. R., B. D. Robertson, and D. B. Young. 2003. Tuberculosis: a problem with persistence. Nat. Rev. Microbiol. 1:97-105. [DOI] [PubMed] [Google Scholar]
- 26.Sturniolo, T., E. Bono, J. Ding, L. Raddrizzani, O. Tuereci, U. Sahin, M. Braxenthaler, F. Gallazzi, M. P. Protti, F. Sinigaglia, and J. Hammer. 1999. Generation of tissue-specific and promiscuous HLA ligand database using DNA microarrays and virtual HLA class II matrices. Nat. Biotechnol. 17:555-561. [DOI] [PubMed] [Google Scholar]
- 27.Styblo, K. 1980. Recent advances in epidemiological research in tuberculosis. Adv. Tuberc. Res. 20:1-63. [PubMed] [Google Scholar]
- 28.Ulrichs, T., M. E. Munk, H. Mollenkopf, S. Behr-Perst, R. Colangeli, M. L. Gennaro, and S. H. Kaufmann. 1998. Differential T cell responses to Mycobacterium tuberculosis ESAT-6 in tuberculosis patients and healthy donors. Eur. J. Immunol. 28:3949-3958. [DOI] [PubMed] [Google Scholar]
- 29.Vincenti, D., S. Carrara, P. De Mori, L. P. Pupillo, N. Petrosillo, F. Palmieri, O. Armignacco, G. Ippolito, E. Girardi, M. Amicosante, and D. Goletti. 2003. Identification of ESAT-6 epitopes for the immunodiagnosis of active tuberculosis. Mol. Med. 19:105-111. [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkinson, K. A., M. Simsova, E. Scholvinch, P. Sebo, C. Leclerc, H. M. Vordermeier, S. J. Dickson, J. R. Brown, R. N. Davidson, G. Pasvol, M. Levin, and R. J. Wilkinson. 2005. Efficient ex vivo stimulation of Mycobacterium tuberculosis-specific T-cells by genetically detoxified Bordetella pertussis adenylate cyclase antigen toxoids. Infect. Immun. 73:2991-2998. [DOI] [PMC free article] [PubMed] [Google Scholar]