Abstract
Plant antimicrobials are not used as systemic antibiotics at present. The main reason for this is their low level of activity, especially against gram-negative bacteria. The reported MIC is often in the range of 100 to 1,000 μg/ml, orders of magnitude higher than those of common broad-spectrum antibiotics from bacteria or fungi. Major plant pathogens belong to the gram-negative bacteria, which makes the low level of activity of plant antimicrobials against this group of microorganisms puzzling. Gram-negative bacteria have an effective permeability barrier, comprised of the outer membrane, which restricts the penetration of amphipathic compounds, and multidrug resistance pumps (MDRs), which extrude toxins across this barrier. It is possible that the apparent ineffectiveness of plant antimicrobials is largely due to the permeability barrier. We tested this hypothesis in the present study by applying a combination of MDR mutants and MDR inhibitors. A panel of plant antimicrobials was tested by using a set of bacteria representing the main groups of plant pathogens. The human pathogens Pseudomonas aeruginosa, Escherichia coli, and Salmonella enterica serovar Typhimurium were also tested. The results show that the activities of the majority of plant antimicrobials were considerably greater against the gram-positive bacteria Staphylococcus aureus and Bacillus megaterium and that disabling of the MDRs in gram-negative species leads to a striking increase in antimicrobial activity. Thus, the activity of rhein, the principal antimicrobial from rhubarb, was potentiated 100- to 2,000-fold (depending on the bacterial species) by disabling the MDRs. Comparable potentiation of activity was observed with plumbagin, resveratrol, gossypol, coumestrol, and berberine. Direct measurement of the uptake of berberine, a model plant antimicrobial, confirmed that disabling of the MDRs strongly increases the level of penetration of berberine into the cells of gram-negative bacteria. These results suggest that plants might have developed means of delivering their antimicrobials into bacterial cells. These findings also suggest that plant antimicrobials might be developed into effective, broad-spectrum antibiotics in combination with inhibitors of MDRs.
Plants produce an enormous array of secondary metabolites, and it is commonly accepted that a significant part of this chemical diversity serves to protect plants against microbial pathogens (10). These plant substances are classified as phytoanticipins, which are compounds that are present constitutively, or phytoalexins, whose levels increase strongly in response to microbial invasion. In several well-documented cases, mutant plants that lack the ability to produce a particular phytoalexin had considerably higher levels of sensitivity to microbial pathogens. For example, mutant oats that lack saponin avenacin A-1 became sensitive to a range of fungal pathogens (36). However, the only definitive test for a putative antimicrobial is activity. In this regard, the evidence is often unconvincing. In the example cited above, avenacin A-1 did not actually show antifungal activity in a direct susceptibility test in vitro (3). According to the influential review of Dixon cited above (10), there is only one documented case in which plant antimicrobials were present at a sufficient concentration in vivo to inhibit the growth of a bacterial pathogen (37). In that study, infection of cotton with Xanthomonas campestris elicited the synthesis of a set of sesquiterpenoid phytoalexins, which was followed by a decline in bacterial growth. What remains puzzling is that one of the sesquiterpenoids, 2-hydroxy-7-methoxycadalene, had no antibacterial activity in vitro, yet its expression followed the same pattern as those of the other related phytoalexins.
Plant compounds are routinely classified as “antimicrobial” on the basis of susceptibility tests that produce MICs in the range of 100 to 1,000 μg/ml, orders of magnitude weaker than those of typical antibiotics produced by bacteria and fungi (MICs, 0.01 to 10 μg/ml). A compound that is synthesized in response to pathogen invasion and is required to protect the plant from a pathogen but that shows little activity in an in vitro susceptibility test is not necessarily an antimicrobial. Such a substance might have a regulatory function, indirectly increasing the level of resistance of the plant. This analysis suggests that we lack a solid rationale for making a functional assignment for the vast majority of plant compounds that have been classified as antimicrobials.
One helpful clue regarding the possible function of plant secondary metabolites is that these compounds often show considerable activity against gram-positive bacteria but not against gram-negative species or yeast (26). Both yeast and gram-negative bacteria have evolved significant permeability barriers. In gram-negative species, an outer membrane is a fairly effective barrier for amphipathic compounds, and a set of multidrug resistance pumps (MDRs) extrudes amphipathic toxins across the outer membrane (27, 31, 33, 49). In the yeast Saccharomyces cerevisiae, the presence of ergosterol, which decreases permeability, combined with a set of broad-specificity MDRs also provides an effective barrier (26, 40). By contrast, the single membrane of gram-positive bacteria is considerably more accessible to permeation by amphipathic toxins, and MDRs provide limited protection (26). Several gram-positive bacteria invade plants, but the majority of plant pathogens are gram-negative bacteria or yeast and related fungi.
We proposed that plants produce compounds that can be effective antimicrobials if they find their way into the cell of the pathogen (45). Production of MDR inhibitors by the plant would be one way to ensure delivery of the antimicrobial compound. In our previous studies, we found that Berberis plants which produce a putative antimicrobial, berberine, also make the MDR inhibitors 5′-methoxyhydnocarpin D (5′-MHC-D) and pheophorbide A, which facilitate the penetration of berberine into a model gram-positive bacterium, Staphylococcus aureus (44, 45). Berberis plants do not have known bacterial pathogens, perhaps in part due to their effective chemical defenses. Somewhat similar studies aimed at finding the basis of gram-negative bacterial resistance to plant antimicrobials have not been conclusive. In Rhizobium etli, an operon activated by a number of plant phytoalexins was identified. The operon appeared to code for an RmrAB MDR (13). Mutant rmrAB had a diminished ability for root colonization, and increased susceptibilities to phytoalexins naringenin and coumaric acid were reported. However, the difference in susceptibility between the wild type and mutant was very small, about 30%. Note that the intrinsic variability of the MIC test is twofold. In Agrobacterium tumefaciens, coumestrol, an antifungal phytoalexin of soybeans, was found to induce expression of an LfeAB MDR (35). Mutation in the pump increased the level of accumulation of coumestrol in the pathogen, and the mutant was outcompeted by the wild type in colonizing the plant. However, neither the wild type nor the mutant was sensitive to coumestrol.
Whether the in vitro ineffectiveness of plant antimicrobials against gram-negative bacteria is due to poor penetration or efflux by MDRs has remained an open question. In this study, we show that MDR inhibitors can dramatically increase the effectiveness of putative plant antimicrobials against gram-negative bacteria. This finding provides a rationale for an assignment of plant antimicrobials and suggests that plants producing these compounds might have evolved means of delivering them into the cells of pathogens.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used in this study are listed in Table 1. Cells were cultured in Mueller-Hinton broth with aeration at 37 or 30°C.
TABLE 1.
Bacterial strains
| Strain | Genotype | Reference or source |
|---|---|---|
| Staphylococcus aureus 8325-4 | WTa | 22 |
| S. aureus 1758 | S. aureus norA | 22 |
| Escherichia coli K-12 | WT | Wild type |
| E. coli KLE701 | E. coli tolC::tet | K. Lewis |
| Pseudomonas aeruginosa PA767 | WT | 29 |
| P. aeruginosa K1119 | PAO1 ΔmexAB-oprM | 29 |
| Salmonella enterica serovar Typhi- murium ST329 | WT | F. Ausubel |
| P. syringae pv. maculicola ES4326 | WT | F. Ausubel |
| Xanthomonas campestris XCC528 | WT | F. Ausubel |
| Agrobacterium tumefaciens GV3101 | WT | F. Ausubel |
| Erwinia rhapontici Er1 | WT | F. Ausubel |
| E. carotovora ATCC 358 | WT | F. Ausubel |
| Sinorhizobium meliloti Rm1021 | WT | F. Ausubel |
| Bacillus megaterium 11561 | WT | M. Cannon |
WT, wild type.
Antimicrobials.
Asarinin, esculetin, bisnorargemonine, and 13-hydroxylupanine were from F. R. Stermitz's collection. Coumestrol was purchased from Indofine Chemical Co. (Somerville, N.J.). MC207110 was from Microcide Pharmaceuticals Inc. INF271 was kindly provided by Influx Inc. (Chicago, Ill.). Rhein, plumbagin, pyrithione, resveratrol, gossypol, berberine, erythromycin, and tetracycline were purchased from Sigma Chemical Co. (St. Louis, Mo.)
Determination of antimicrobial susceptibility.
Cells (105/ml) were inoculated into Mueller-Hinton broth and dispensed at 0.2 ml/well in 96-well microtiter plates. MICs were determined in triplicate by serial twofold dilution of test compounds, following the recommendations of the National Committee for Clinical Laboratory Standards. The MIC was defined as the concentration of an antimicrobial that completely inhibited cell growth during (i) an 18-h incubation at 37°C or (ii) a 24-h incubation at 30°C. Growth was assayed with a microtiter plate reader (Spectramax PLUS384; Molecular Devices) by monitoring the absorption at 600 nm.
Determination of berberine uptake.
Determination of berberine uptake was performed essentially as described in our previous study (45). Cells were cultured with aeration at 30°C to an optical density at 600 nm (OD600) of 1.8, pelleted, and washed twice with 20 mM HEPES-NaOH (pH 7.0) buffer. The cells were then resuspended to an OD600 of 0.3 in 1 ml of HEPES buffer containing 10 μM glucose, followed by incubation at 37 or 30°C for 1 h. The cells were centrifuged, washed, and resuspended at an OD600 of 0.15 in HEPES buffer. Assays were performed in 96-well flat-bottom black plates (Costar) in a final volume of 200 μl. Berberine was added at 30 μg/ml, and fluorescence was measured with a Spectramax Geminis spectrofluorometer (Molecular Devices) at a 355-nm excitation wavelength and a 517-nm emission wavelength.
RESULTS
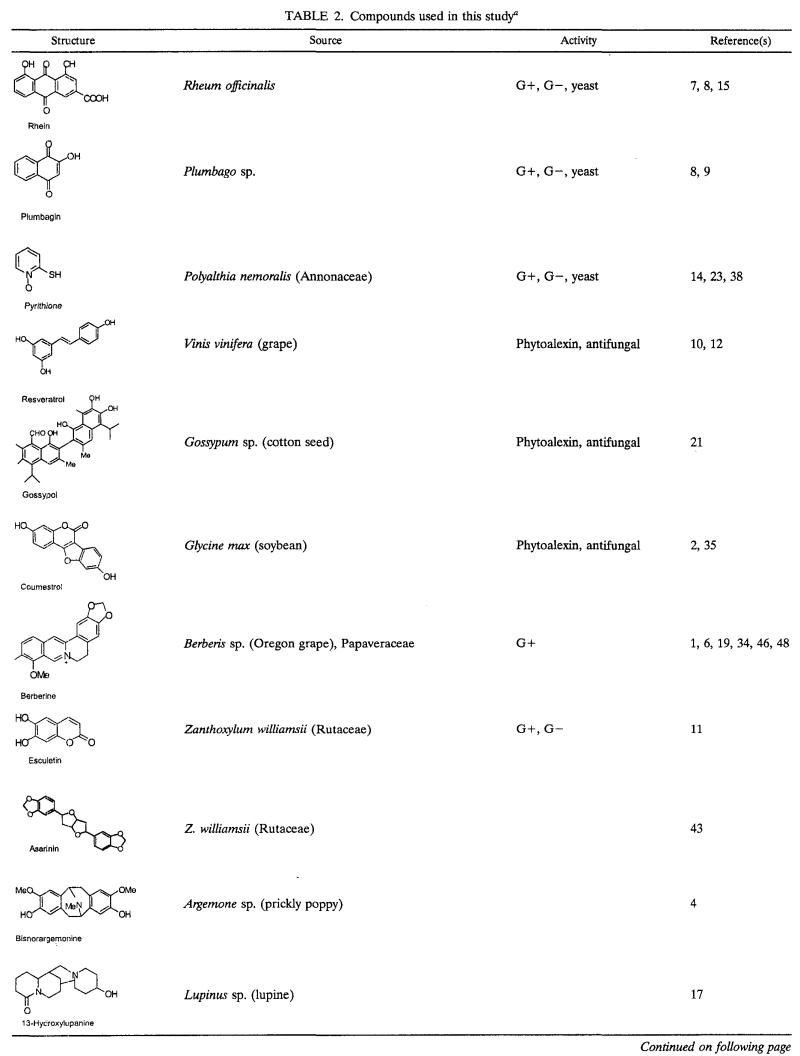
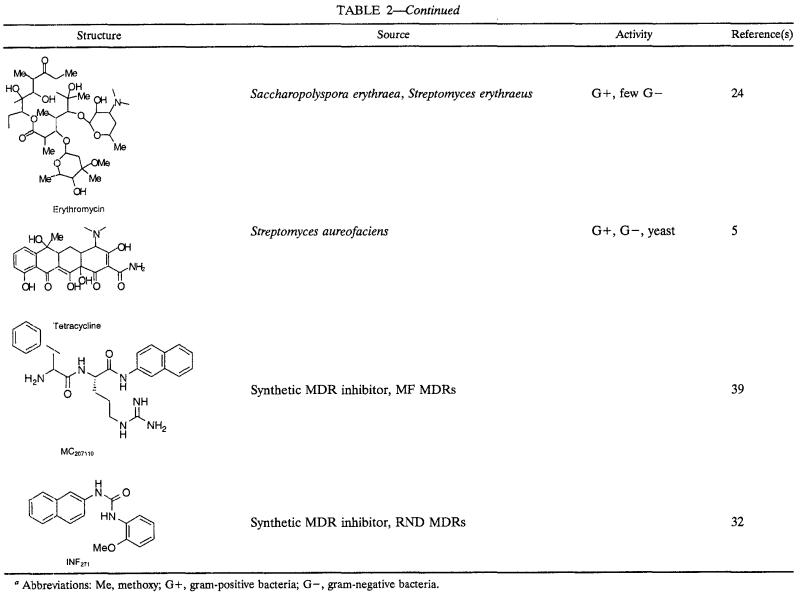
We chose a representative panel of diverse plant antimicrobials (Table 2 and tested their activities against a set of microorganisms. The aim of the experiments was to compare the activities of the antimicrobials against gram-positive bacteria versus those against gram-negative bacteria and to learn whether MDRs were responsible for resistance to the antimicrobial compounds.
TABLE 2.
Compounds used in this studya
Abbreviations: Me, methoxy; G+, gram-positive bacteria; G−, gram-negative bacteria.
Many plant antimicrobials have been reported only once or by a single research group and are not easily accessible. We therefore assembled a panel mainly from commercially available compounds for this study (Table 2). This panel included known phytoalexins, phytoanticipins, and also plant secondary metabolites that have been shown to have little or no antimicrobial activity. For purposes of comparison, we also included conventional antibiotics (tetracycline and erythromycin).
Antimicrobial action against gram-positive bacteria.
Several plant compounds had fairly high levels of activity against Bacillus megaterium, a plant pathogen, and S. aureus, an important human pathogen (Table 3). The MICs of pyrithione, gossypol, plumbagin, and rhein were in the range of 0.6 to 4 μg/ml. Resveratrol (a known antiyeast compound) and berberine had low levels of activity against gram-positive species (MIC range, 30 to 250 μg/ml). A mutant S. aureus isolate lacking the NorA MDR had a considerably higher level of susceptibility to berberine (MIC, 3.25 μg/ml) compared to that of the wild type (MIC, 250 μg/ml). This is in agreement with our previous results (18, 45). NorA belongs to the major facilitator family of transporters that represent the main types of MDRs in gram-positive bacteria (27). These MDRs are believed to extrude primarily amphipathic cations (like berberine) in gram-positive bacteria (18, 26). The norA mutation had little effect on the activities of the other plant antimicrobials tested with the exception of pyrithione, which forms a positively charged zinc complex.
TABLE 3.
Susceptibility of bacteria to plant antimicrobials
| Antimicrobial and additiona | MIC (μg/ml)
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | S. aureus norA | B. mega- teriuma | E. coli | E. coli tolC | P. aeru- ginosa PAO1 | P. aeru- ginosa mexAB | S. enterica serovar Typhi- murium | P. syrin- gae | X. cam- pestrisb | A. tume- faciansc | E. rha- pontici | E. caro- tovorac | S. meli- lotib | |
| Resveratrol | 125 | 250 | 250 | 500 | 125 | 1000 | 500 | 500 | 500 | 250 | 500 | 500 | 250 | 125 |
| MC207110 | 125 | 250 | 125 | 125 | 0.49 | 62.5 | 31.25 | 3.91 | 31.25 | 7.81 | 125 | 62.5 | 1.95 | 15.62 |
| INF271 | 62.5 | 62.5 | 62.5 | 250 | 0.49 | 250 | 250 | 250 | 250 | 125 | 125 | 125 | 62.5 | 62.5 |
| MC207110 + INF271 | 62.5 | 62.5 | 31.25 | 31.25 | 0.24 | 62.5 | 31.25 | 3.91 | 15.6 | 7.81 | 62.5 | 31.25 | 0.98 | 3.91 |
| Gossypol | 3.12 | 1.95 | 3.91 | 250 | 125 | 1000 | 500 | 500 | 31.25 | 125 | 250 | 500 | 250 | 3.91 |
| MC207110 | 3.12 | 1.95 | 3.91 | 62.5 | 7.81 | 250 | 250 | 125 | 31.25 | 7.81 | 125 | 15.65 | 125 | 0.98 |
| INF271 | 32 | 7.81 | 15.65 | 250 | 62.5 | 500 | 500 | 500 | 31.25 | 62.5 | 125 | 125 | 125 | 0.98 |
| MC207110 + INF271 | 6.25 | 6.25 | 7.81 | 31.25 | 31.25 | 250 | 250 | 62.5 | 15.75 | 3.12 | 62.5 | 62.5 | 62.5 | 0.98 |
| Coumestrol | 250 | 250 | 250 | 500 | 1.95 | 1000 | 500 | 500 | 500 | 250 | 500 | 250 | 250 | 250 |
| MC207110 | 250 | 250 | 250 | 62.5 | 1.95 | 500 | 62.5 | 62.5 | 250 | 125 | 125 | 250 | 15.62 | 125 |
| INF271 | 125 | 250 | 250 | 250 | 31.25 | 500 | 250 | 250 | 250 | 250 | 250 | 250 | 62.5 | 62.5 |
| MC207110 + INF271 | 62.5 | 62.5 | 125 | 31.25 | 0.92 | 500 | 3.91 | 62.5 | 62.5 | 125 | 125 | 125 | 15.62 | 125 |
| Rhein | 4 | 4 | 4 | 500 | 4 | >500 | 500 | 100 | 64 | 100 | 100 | 100 | 100 | 64 |
| MC207110 | 2 | 0.62 | 2 | 6.25 | 0.5 | 250 | 125 | 20 | 1.25 | 1.25 | 50 | 6.25 | 6.25 | 6.25 |
| INF271 | 0.62 | 0.62 | 0.98 | 100 | 2 | 250 | 500 | 20 | 2.5 | 2.5 | 50 | 50 | 50 | 16 |
| MC207110 + INF271 | 0.31 | 0.31 | 0.49 | 0.62 | 0.25 | 5 | 6.25 | 5 | 0.62 | 0.62 | 5 | 3.12 | 3.12 | 3.12 |
| Plumbagin | 0.78 | 0.31 | 2 | 100 | 2 | >500 | >500 | 100 | 2 | 25 | 12.5 | 32 | 12.5 | 3.25 |
| MC207110 | 0.31 | 0.31 | 0.5 | 5 | 0.78 | 31.25 | 31.25 | 10 | 0.39 | 0.39 | 1.56 | 3.2 | 0.78 | 0.39 |
| INF271 | 0.31 | 0.31 | 0.25 | 50 | 2 | 250 | 250 | 50 | 0.78 | 12 | 6.25 | 6.25 | 3.12 | 1.56 |
| MC207110 + INF271 | 0.15 | 0.15 | 0.25 | 2.5 | 0.19 | 31.25 | 31.25 | 2.5 | 0.19 | 0.39 | 0.78 | 0.78 | 0.39 | 0.19 |
| Pyrithione | 0.62 | 0.16 | 0.98 | 2 | 2 | 8 | 4 | 2 | 0.25 | 0.25 | 2 | 2 | 2 | 64 |
| MC207110 | 0.16 | 0.08 | 0.24 | 0.16 | 0.08 | 1.25 | 0.62 | 0.62 | 0.16 | 0.08 | 0.16 | 0.32 | 0.16 | 5 |
| INF271 | 0.16 | 0.08 | 0.49 | 1 | 1 | 2.5 | 2 | 2 | 0.25 | 0.16 | 0.64 | 2 | 1 | 32 |
| MC207110 + INF271 | 0.08 | 0.04 | 0.24 | 0.04 | 0.04 | 0.62 | 0.62 | 0.25 | 0.04 | 0.08 | 0.08 | 0.08 | 0.08 | 1.25 |
| Berberine | 250 | 3.25 | 125 | >1,000 | 62.5 | 1,000 | 500 | 1000 | 100 | 100 | 200 | 200 | 200 | 200 |
| MC207110 | 31.25 | 1.62 | 125 | 250 | 62.5 | 500 | 500 | 250 | 25 | 50 | 100 | 100 | 50 | 0.9 |
| INF271 | 1.95 | 0.98 | 0.98 | 500 | 31.25 | 500 | 500 | 500 | 50 | 50 | 100 | 100 | 50 | 100 |
| MC207110 + INF271 | 1.95 | 0.39 | 0.98 | 15.6 | 6.25 | 500 | 500 | 1.9 | 7.8 | 25 | 100 | 50 | 0.9 | 0.9 |
| Esculetin | 31.25 | 15.62 | 250 | 1,000 | 62.5 | >1,000 | >1,000 | 1,000 | 500 | 500 | 500 | 500 | 500 | 500 |
| MC207110 | 31.25 | 15.62 | 250 | 250 | 15.62 | 500 | 500 | 250 | 125 | 125 | 250 | 125 | 125 | 125 |
| INF271 | 15.62 | 15.62 | 250 | 500 | 31.25 | 500 | 500 | 500 | 250 | 250 | 250 | 250 | 250 | 250 |
| MC207110 + INF271 | 15.62 | 3.91 | 250 | 250 | 15.62 | 500 | 500 | 125 | 31.25 | 31.25 | 31.25 | 62.5 | 31.25 | 31.25 |
| Asarinin | 62.5 | 31.25 | 250 | >1,000 | 62.5 | >1,000 | >500 | 1000 | 500 | 500 | 500 | 500 | 500 | 500 |
| MC207110 | 31.25 | 15.62 | 250 | 250 | 15.62 | 500 | 500 | 250 | 125 | 125 | 250 | 125 | 125 | 125 |
| INF271 | 15.62 | 15.62 | 250 | 500 | 31.25 | 500 | 500 | 500 | 250 | 250 | 250 | 250 | 250 | 250 |
| MC207110 + INF271 | 15.62 | 7.81 | 250 | 250 | 15.62 | 500 | 500 | 125 | 62.5 | 62.5 | 31.25 | 62.5 | 31.25 | 62.5 |
| Bisnorargemonine | 1,000 | 1,000 | >500 | >1,000 | 1,000 | >1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 |
| MC207110 | 1,000 | 1,000 | >500 | >1,000 | 1,000 | >1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 |
| INF271 | 500 | 500 | >500 | 500 | 500 | 1,000 | 1,000 | >500 | 1,000 | 1,000 | 1,000 | 1,000 | >500 | >500 |
| MC207110 + INF271 | 500 | 500 | >500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | >500 | >500 |
| 13-Hydroxylupanine | 1,000 | 1,000 | >500 | >1,000 | 1,000 | >1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 |
| MC207110 | 1,000 | 1,000 | >500 | >1,000 | 1,000 | >1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 |
| INF271 | 500 | 500 | >500 | >1,000 | 1,000 | 1,000 | 500 | 500 | 500 | 500 | >500 | 1000 | >500 | >500 |
| MC207110 + INF271 | 500 | 500 | >500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | >500 | >500 | >500 | >500 |
| Tetracycline | 2 | 0.62 | 12.5 | 12.5 | 1.25 | 250 | 125 | 12.5 | 12.5 | 12.5 | 25 | 31.25 | 150 | 0.24 |
| MC207110 | 1 | 0.62 | 1.56 | 1.25 | <0.49 | 62.5 | 12.5 | 1.91 | 3.25 | 3.25 | 7.81 | 7.81 | 7.81 | NDd |
| INF271 | 0.24 | 0.24 | <0.24 | 12.5 | 0.62 | 250 | 125 | 12.5 | 6.25 | 6.25 | 12.5 | 15.62 | 1.91 | ND |
| MC207110 + INF271 | <0.24 | <0.24 | <0.24 | 0.62 | <0.49 | 31.25 | 12.5 | 1.9 | 3.25 | 3.25 | 3.91 | 3.91 | 1.91 | ND |
| Erythromycin | 0.39 | 0.18 | 2 | 125 | 1.95 | 250 | 50 | 50 | 6.25 | 6.25 | 50 | 25 | 50 | 0.78 |
| MC207110 | 0.18 | 0.18 | 0.64 | 3.91 | 0.39 | 25 | 5 | 1.56 | 0.19 | 0.19 | 1.25 | 0.39 | 0.39 | 0.19 |
| INF271 | 0.18 | 0.18 | <0.24 | 31.25 | 1.95 | 100 | 25 | 50 | 3.25 | 3.25 | 25 | 12.5 | 12.5 | 0.39 |
| MC207110 + INF271 | 0.09 | 0.09 | <0.24 | 0.18 | 0.18 | 12.5 | 5 | 1.56 | 0.09 | 0.19 | 0.39 | 0.09 | 0.09 | 0.09 |
MC207110 was added at a final concentration of 20 μg/ml, and INF271 was added at a final concentration of 10 μg/ml except where stated otherwise. All MIC determinations were performed in triplicate.
The final concentration of MC207110 at 10 μg/ml and the final concentration INF271 at 5 μg/ml were at least two- to four-fold lower than those inhibiting growth by these compounds alone.
MC207110 at 5 μg/ml and INF271 at 2.5 μg/ml.
ND, not determined.
In addition, antimicrobials were tested in the presence of well-characterized MDR inhibitors. INF271 is a neutral inhibitor of MF MDRs (32), and MC207110 is an effective inhibitor of RND MDRs of gram-negative species (39). Unexpectedly, both INF271 and MC207110 significantly potentiated the actions of most antimicrobials in the panel against B. megaterium and S. aureus. The presence of other, unidentified MDRs (22) is likely responsible for this potentiation. Resveratrol and coumestrol showed little direct activity, and the MDR inhibitors caused little potentiation of activity by these antimicrobials. Gossypol presented an interesting anomaly: the activity of this antimicrobial decreased about 10-fold in the presence of INF271 in the wild type, and a 4-fold decrease in activity was observed in the norA strain.
Antimicrobial action against gram-negative species.
We began the analysis with rhein, a fairly well characterized antimicrobial of rhubarb reported to have activity against bacteria and yeast (7, 8, 15). The main pathogen of rhubarb, however, is a gram-negative organism, Erwinia rhapontici (16), but we were unable to find data in the literature discussing the action of rhein against this bacterium. Rhein was fairly ineffective against E. rhapontici (MIC, 100 μg/ml) (Table 3). The MDR inhibitor MC207110 significantly potentiated the action of rhein, decreasing the MIC to 6.25 μg/ml. MDRs of this bacterium have not been reported at the time of this writing. However, all gram-negative species tested (or sequenced) so far have RND MDRs that are the target of MC207110 (27). INF271 had a minor effect on the activity of rhein. This experiment shows that rhein is potentially a very effective antimicrobial against the major pathogen of rhubarb, and the plant would clearly benefit from disabling of the efflux resistance mechanism of its pathogen. It is possible that the potential activity of rhein against gram-negative bacteria is even greater than that suggested by our experiments, since we do not know whether MC207110 completely inhibits efflux in E. rhapontici.
Rhein was ineffective against Escherichia coli (MIC, 500 μg/ml). The TolC porin is a component of E. coli transenvelope MDRs (27, 49), and the rhein MIC for a tolC mutant was 4 μg/ml. Addition of MC207110 and INF271 further increased the activity of rhein (MIC, 0.25 μg/ml). This striking 2,000-fold increase in activity demonstrates the potential of rhein as an effective antimicrobial against gram-negative bacteria. Note that MC207110 also increased the activity of a conventional antibiotic, erythromycin, against the tolC mutant. It appears that the presence of MDRs not dependent on TolC might be responsible for this observation. Rhein was completely ineffective against the ubiquitous animal and plant pathogen Pseudomonas aeruginosa, with no MIC detected at the limit of solubility (>500 μg/ml). This is not surprising, since P. aeruginosa has a very high level of intrinsic resistance to virtually all known antimicrobials and antibiotics due to a combination of a very restrictive outer membrane barrier and transenvelope MDRs (33). A combination of MC207110 and INF271 strongly potentiated the activity of rhein, resulting in a low MIC of 5 μg/ml. Rhein was ineffective against the other plant pathogens tested, but again, its activity was strongly potentiated either by MC207110 alone or by a combination of MC207110 and INF271. For example, the rhein MIC for Pseudomonas syringae, the broad-host-range plant pathogen, was 64 μg/ml, and in the presence of MDR inhibitors the rhein MIC was 0.62 μg/ml.
Plumbagin, resveratrol, gossypol, coumestrol, and berberine all showed similar patterns of activity against gram-negative species. All were relatively ineffective against most species in the panel, but the activity of each compound was strongly potentiated by MDR inhibitors against several of the species. This suggests that a high level of activity of an antimicrobial against a given species was achieved when the inhibitors happened to block the MDRs responsible for efflux of the tested compound. The actions of these compounds are similar to that of the model antibiotic erythromycin used in this study for comparative purposes. Erythromycin has a narrow spectrum of activity, limited primarily to gram-positive species. It is known to be effectively extruded by MDRs in gram-negative species. Disabling of MDRs broadened the spectrum of erythromycin activity to include all gram-negative species tested. Tetracycline, another common antibiotic tested for comparison, has a broader spectrum of activity that includes such common gram-negative pathogens as E. coli and Salmonella enterica serovar Typhimurium.
Pyrithione was the only plant antimicrobial tested that showed a high level of direct activity against gram-negative bacteria (Table 3). This observation confirms previous reports in the literature (14, 23, 38). Interestingly, the potency of this antimicrobial was further increased by MDR inhibitors, with the resulting MIC being as low as 40 ng/ml in several cases.
Resveratrol presented a notable exception: this compound was relatively ineffective against the gram-positive species B. megaterium and S. aureus, with little of its activity potentiated by the MDR inhibitors, but it was very effective against some gram-negative species in the presence of MDR inhibitors. Compare the resveratrol MIC for S. aureus of 62.5 μg/ml (with MC207110 and INF271) versus a resveratrol MIC of 1 μg/ml (with MC207110 and INF271) for E. carotovora or the resveratrol MIC of 0.24 μg/ml for E. coli tolC. It is possible that the resveratrol target(s) in gram-negative species is more sensitive to the action of this antimicrobial.
Actions of compounds with uncertain antibacterial action.
Disabling of MDRs may uncover the antimicrobial activities of compounds that have little or no activity on their own. With this possibility in mind, we chose a sampling of plant secondary metabolites for which antibacterial action is weak or absent (Table 2). These are asarinin, esculetin, and the basic alkaloids bisnorargemonine and 13-hydroxylupanine.
Asarinin and esculetin had low levels of activity against B. megaterium (MIC, 250 μg/ml) and fairly low levels of activity against S. aureus (MIC range, 30 to 60 μg/ml) (Table 3). Their activities against all gram-negative bacteria tested were very low indeed, with MICs in the range of 500 to 1, 000 μg/ml. However, disabling of the MDRs significantly increased the levels of activity in some cases. In the presence of MC207110 and INF271, the asarinin and esculetin MICs for S. aureus norA were 7.8 and 3.9 μg/ml, respectively. Activity against gram-negative bacteria also increased significantly when MDR activity was disabled, and the resulting MICs were mostly in the range of 15 to 60 μg/ml. P. aeruginosa presented a notable exception: there was virtually no increase in activity against the mutants lacking the constitutively expressed MexAB-OprM MDR or in the presence of MDR inhibitors. In general, these results suggest that asarinin and esculetin are plant antimicrobial compounds.
Bisnorargemonine and 13-hydroxylupanine had virtually no activity against the panel of bacteria tested, and the use of mutants lacking MDRs or MDR inhibitors had no effect.
Disabling of MDRs leads to accumulation of a model plant antimicrobial.
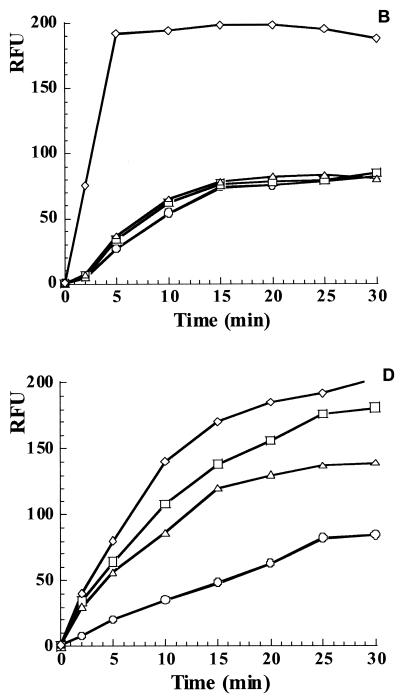
The accumulation of berberine was used to directly measure the possible effects of MDR inhibitors. This was especially important for the bacterial species used in this study for which MDRs have not yet been identified. Berberine is a useful model plant antimicrobial whose accumulation in microbial cells can be conveniently monitored by measuring the fluorescence of berberine bound to DNA (45). In this regard, berberine behaves analogously to ethidium bromide.
In previous studies, we found that both INF271 and the plant MDR inhibitor 5′-MHC-D strongly increased the rate of penetration and the level of accumulation of berberine in S. aureus cells (45). This experiment described the nature of synergy between berberine and 5′-MHC-D produced by the same Berberis plants. Similarly, the effects of INF271 and MC207110 on berberine accumulation in gram-negative bacteria were examined in this study (Fig. 1). Several species for which susceptibility data indicated a distinct potentiating effect of MDR inhibitors were chosen for this experiment: S. enterica serovar Typhimurium, P. syringae, Xanthomonas campestris, Erwinia carotovora, and Sinorhizobium meliloti. The results show that for the species examined, either MC207110 or INF271, or both, strongly increased the rate of accumulation of berberine in the cells (Fig. 1), consistent with the strong potentiation by MDR inhibitors of growth inhibition by berberine. This experiment suggests that for the other antimicrobials tested, MDR inhibitors similarly exerted their potentiating action through inhibition of MDRs.
FIG. 1.
Accumulation of berberine by gram-negative bacteria. The uptake of berberine with no addition (○) or with the addition of MC207110 (□), INF271 (▵), or MC207110 plus INF271 (⋄) by cells of S. typhimurium (A), P. syringae (B), X. campestris (C), E. carotovora (D) and S. meliloti (E) was measured by determination of the increase in fluorescence following binding to DNA and is expressed as relative fluorescence units (RFU). Berberine was present at a concentration of 30 μg/ml, and MC207110 and INF271 were added at the same final concentrations used for the MIC determinations.
DISCUSSION
A vast majority of small-molecule plant antimicrobials are agents with weak or narrow-spectrum activities, while bacteria, yeast, and fungi produce antibiotics that both are often effective and have broad spectra of activity. The nature of this disparity is puzzling. One radical possibility is that plant “antimicrobials” actually have other functions in the plant and their low level of antimicrobial activity is accidental and largely irrelevant. An even more extreme opinion, not uncommon and stemming from the same puzzle, is that plants happen to make many secondary metabolites for no good reason, and some of them will inevitably have antimicrobial properties, very much like random substances from a library of synthetic compounds. Our recent work with berberine, a cationic alkaloid, offered a possible explanation for the apparent ineffectiveness of plant antimicrobials (45, 46). Berberine is a weak antimicrobial produced by a wide variety of plant species. It is an amphipathic cation that resembles quaternary ammonium antiseptics in its chemical properties and possibly in its mechanism of action as well. The likely targets of berberine are the cytoplasmic membrane and DNA, into which it intercalates (20). Amphipathic cations are the preferred substrates of most MDRs (26), and we reasoned that the low level of activity of berberine might result from effective efflux. We found this to be true with a model bacterial pathogen, S. aureus: both disruption of the main MDR (NorA) and application of an MDR inhibitor strongly potentiated the antimicrobial action of berberine (18). Next, we wondered whether plants producing berberine have also evolved an MDR inhibitor to disable the resistance mechanism of bacteria. We found that Berberis plants produce 5′-MHC-D, which completely blocked NorA and acted in synergy with berberine (45, 46). In the absence of efflux, berberine, a hydrophobic cation, accumulates in the cells of microbial pathogens, and the accumulation is driven by the membrane potential (41). Active accumulation in the pathogen is an attractive property for an antimicrobial, and berberine inspired the design of a cationic polymer with a “sterile” surface (47) that is not subject to MDR-dependent resistance (30).
The finding of natural synergy between berberine and an MDR inhibitor leads to a general possibility that plant antimicrobials are potentially effective, with the apparent in vitro weakness resulting from MDR-dependent efflux. This hypothesis was tested in the present study.
Testing of known plant antimicrobials with a representative panel of bacteria, including gram-positive and gram-negative human and plant pathogens, showed that mutations in MDRs or application of MDR inhibitors dramatically increased their levels of activity. The weakness of antimicrobials is primarily their lack of activity against the major plant pathogens, gram-negative bacteria. Interestingly, the most striking increase in activity observed in this study was against gram-negative bacteria. For example, rhein, a well-characterized antimicrobial isolated from rhubarb, had an MIC of 100 μg/ml for E. rhapontici, the primary pathogen of rhubarb. Application of MDR inhibitors increased the activity of rhein 30-fold. Rhein showed no activity whatsoever against P. aeruginosa (MIC, >500 μg/ml, which was above the limit of solubility), but its activity in the presence of MDR inhibitors was significant, with an MIC of 5 μg/ml. Berberine had no activity against S. enterica serovar Typhimurium (MIC, >1,000 μg/ml), but its activity was potentiated more than 500-fold by MDR inhibitors. This survey shows that plant antimicrobials are potentially as effective as conventional antibiotics produced by bacteria and fungi if they are delivered into the pathogen cell. Do plants have MDR inhibitors that act in synergy with antimicrobials against gram-negative bacteria? This remains an intriguing question that we are examining.
The marked increases in the levels of activity of plant antimicrobials observed in this study suggest a rational approach to a functional assignment for these compounds. One can now take any plant secondary metabolite and learn whether the substance is a potential antimicrobial by testing it in the presence of MDR inhibitors. We performed such a test, selecting compounds with uncertain or unknown antibacterial activities. Of the four compounds chosen, asarinin and esculetin showed very little if any activity against gram-negative species (MICs, ≥500 μg/ml), but their activities were strongly potentiated by MDR inhibitors, resulting in reasonable activity (MICs, 15 to 60 μg/ml). Asarinin and esculetin are therefore likely plant antimicrobials. Bisnorargemonine and 13-hydroxylupanine had virtually no antibacterial activity, and disabling of the MDRs had no effect. This suggests that the two compounds are not antibacterials. Based on our data, the approach described in this paper provides a straightforward means of functional assignment for antimicrobials. We have focused on bacteria in this study, but it will be necessary to complete similar experiments with wild-type and MDR-deficient yeast strains to properly designate a plant compound as an antimicrobial.
The approach described here might be useful in elucidating some puzzling observations regarding the apparent lack of in vitro activity of putative phytoalexins. Indirect evidence strongly suggests that RmrAB and LfeAB MDRs play an important role in protection of R. etli and A. tumefaciens, respectively, from plant phytoalexins. However, in vitro susceptibility tests showed that these MDRs play little if any role in resistance. Specifically, there was no difference in susceptibility to coumestrol between the wild type and the lfeAB mutant of A. tumefaciens. Our experiments with coumestrol show that it indeed has very little activity against A. tumefaciens (MIC, 500 μg/ml) or other bacteria (MICs, 250 to 500 μg/ml). In the presence of the MDR inhibitors INF271 and MC207110, the MIC of coumestrol for A. tumefaciens decreased to 125 μg/ml, which is still fairly high. However, for some species we observed a dramatic increase in coumestrol activity. For example, INF271 and MC207110 increased the activity of coumestrol 500-fold against E. coli tolC and 300-fold against P. aeruginosa mexAB-oprM. This shows that coumestrol is potentially a very effective antibacterial compound and that its activity depends on accessibility to the cell. It seems possible that several MDRs protect A. tumefaciens from coumestrol, and disabling of only one of them has little effect. It would be interesting to retest the activity of coumestrol against an A. tumefaciens lfeAB strain in the presence of MDR inhibitors. Similar logic may be applied to elucidate the role of RmrAB in the phytoalexin resistance of R. etli.
Apart from the interesting basic science question regarding the function of plant secondary metabolites, there is an urgent need to develop new antibiotics (25, 28). This need results from the rapid rise of multidrug-resistant pathogens such as S. aureus, Enterococcus faecalis, P. aeruginosa, and many others. Plant antimicrobials have not been used as systemic antibiotics so far. Those rare plant antimicrobials that are effective and that have broad-spectrum activities, like pyrithione of Polyalthea nemoralis (14) (known better to us as an antiseptic discovered independently by chemists and a compound in Head and Shoulders shampoo), are fairly toxic antiseptics. Those that do not have high levels of toxicity are ineffective or highly specific. Our finding of considerable potentiation of the activities of plant antimicrobials by MDR inhibitors opens the possibility for the development of combination therapy. For example, rhein becomes an effective broad-spectrum antibiotic in the presence of MDR inhibitors, and it is approved for systemic use for the treatment of osteoarthritis (administered as a prodrug, diacerein [42]).
Another useful application stemming from knowledge of MDR-based resistance is in drug discovery, including the finding of new plant antimicrobials. We proposed some time ago that target microorganisms lacking MDRs can be used for highly sensitive drug screening (18), and this method is used in the industry (for example, see http://www.phytera.com/Press/012301.htm).
It seems that efflux by MDRs does provide a satisfactory explanation for the apparent ineffectiveness of many plant antimicrobials in vitro. This leads us to the next interesting puzzle: why have plants not evolved self-sufficient broad-spectrum antibiotics like tetracycline or aminoglycosides that are not easily extruded by MDRs of pathogens?
Acknowledgments
We thank Frederick M. Ausubel, Glenn Kaatz, Maura Cannon, and Keith Poole for bacterial strains and Margaret Essenberg for helpful discussion.
This work was supported by NIH grant RO1GM59903.
REFERENCES
- 1.Amin, A. H., T. V. Subbaiah, and K. M. Abbasi. 1969. Berberine sulfate: antimicrobial activity, bioassay, and mode of action. Can. J. Microbiol. 15:1067-1076. [DOI] [PubMed] [Google Scholar]
- 2.Boue, S. M., C. H. Carter, K. C. Ehrlich, and T. E. Cleveland. 2000. Induction of the soybean phytoalexins coumestrol and glyceollin by Aspergillus. J. Agric. Food Chem. 48:2167-2172. [DOI] [PubMed] [Google Scholar]
- 3.Carter, J. P., J. Spink, P. F. Cannon, M. J. Daniels, and A. E. Osbourn. 1999. Isolation, characterization, and avenacin sensitivity of a diverse collection of cereal-root-colonizing fungi. Appl. Environ. Microbiol. 65:3364-3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, C. H., T. Soino, and K. H. Lee. 1971. Total synthesis of (−)-bisnorargemonine. J. Pharm. Sci. 60:1634-1638. [DOI] [PubMed] [Google Scholar]
- 5.Chopra, I., and M. Roberts. 2001. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 65:232-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colombo, M. L., and E. Bosisio. 1996. Pharmacological activities of Chelidonium majus L. (Papaveraceae). Pharmacol. Res. 33:127-134. [DOI] [PubMed] [Google Scholar]
- 7.Cyong, J., T. Matsumoto, K. Arakawa, H. Kiyohara, H. Yamada, and Y. Otsuka. 1987. Anti-Bacteroides fragilis substance from rhubarb. J. Ethnopharmacol. 19:279-283. [DOI] [PubMed] [Google Scholar]
- 8.Didry, N., L. Dubreuil, and M. Pinkas. 1994. Activity of anthraquinonic and naphthoquinonic compounds on oral bacteria. Pharmazie 49:681-683. [PubMed] [Google Scholar]
- 9.Didry, N., L. Dubreuil, F. Trotin, and M. Pinkas. 1998. Antimicrobial activity of aerial parts of Drosera peltata Smith on oral bacteria. J. Ethnopharmacol. 60:91-96. [DOI] [PubMed] [Google Scholar]
- 10.Dixon, R. A. 2001. Natural products and plant disease resistance. Nature 411:843-847. [DOI] [PubMed] [Google Scholar]
- 11.Duncan, S. H., H. J. Flint, and C. S. Stewart. 1998. Inhibitory activity of gut bacteria against Escherichia coli O157 mediated by dietary plant metabolites. FEMS Microbiol. Lett. 164:283-288. [DOI] [PubMed] [Google Scholar]
- 12.Ferrer, J. L., J. M. Jez, M. E. Bowman, R. A. Dixon, and J. P. Noel. 1999. Structure of chalcone synthase and the molecular basis of plant polyketide biosynthesis. Nat. Struct. Biol. 6:775-784. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Pasayo, R., and E. Martinez-Romero. 2000. Multiresistance genes of Rhizobium etli CFN42. Mol. Plant. Microbe Interact. 13:572-577. [DOI] [PubMed] [Google Scholar]
- 14.Han, G.-Y., B.-X. Xu, X.-P. Wang, M.-Z. Liu, X.-Y. Xu, L.-N. Meng, Z.-L. Chen, and D.-Y. Zhu. 1981. Study on the active principle of Polyalthia nemoralis. Acta Chim. Sinica 39:433-437. (In Chinese.) [Google Scholar]
- 15.Hatano, T., H. Uebayashi, H. Ito, S. Shiota, T. Tsuchiya, and T. Yoshida. 1999. Phenolic constituents of Cassia seeds and antibacterial effect of some naphthalenes and anthraquinones on methicillin-resistant Staphylococcus aureus. Chem. Pharm. Bull. (Tokyo) 47:1121-1127. [DOI] [PubMed] [Google Scholar]
- 16.Hauben, L., E. R. Moore, L. Vauterin, M. Steenackers, J. Mergaert, L. Verdonck, and J. Swings. 1998. Phylogenetic position of phytopathogens within the Enterobacteriaceae. Syst. Appl. Microbiol. 21:384-397. [DOI] [PubMed] [Google Scholar]
- 17.Hirai, M. Y., H. Suzuki, M. Yamazaki, and K. Saito. 2000. Biochemical and partial molecular characterization of bitter and sweet forms of Lupinus angustifolius, an experimental model for study of molecular regulation of quinolizidine alkaloid biosynthesis. Chem. Pharm. Bull. (Tokyo) 48:1458-1461. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh, P. C., S. A. Siegel, B. Rogers, D. Davis, and K. Lewis. 1998. Bacteria lacking a multidrug pump: a sensitive tool for drug discovery. Proc. Natl. Acad. Sci. USA 95:6602-6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwasa, K., D. U. Lee, S. I. Kang, and W. Wiegrebe. 1998. Antimicrobial activity of 8-alkyl- and 8-phenyl-substituted berberines and their 12-bromo derivatives. J. Nat. Prod. 61:1150-1153. [DOI] [PubMed] [Google Scholar]
- 20.Jennings, B. R., and P. J. Ridler. 1983. Interaction of chromosomal stains with DNA. An electrofluorescence study. Biophys. Struct. Mech. 10:71-79. [DOI] [PubMed] [Google Scholar]
- 21.Jensen, E. C., C. Ogg, and K. W. Nickerson. 1992. Lipoxygenase inhibitors shift the yeast/mycelium dimorphism in Ceratocystis ulmi. Appl. Environ. Microbiol. 58:2505-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaatz, G. W., S. M. Seo, L. O'Brien, M. Wahiduzzaman, and T. J. Foster. 2000. Evidence for the existence of a multidrug efflux transporter distinct from NorA in Staphylococcus aureus. Antimicrob. Agents Chemother. 44:1404-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khattar, M. M., W. G. Salt, and R. J. Stretton. 1988. The influence of pyrithione on the growth of micro-organisms. J. Appl. Bacteriol. 64:265-272. [DOI] [PubMed] [Google Scholar]
- 24.Kibwage, I. O., J. Hoogmartens, E. Roets, H. Vanderhaeghe, L. Verbist, M. Dubost, C. Pascal, P. Petitjean, and G. Levol. 1985. Antibacterial activities of erythromycins A, B, C, and D and some of their derivatives. Antimicrob. Agents Chemother. 28:630-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy, S. B. 1998. The challenge of antibiotic resistance. Sci. Am. 278:46-53. [DOI] [PubMed] [Google Scholar]
- 26.Lewis, K. 2001. In search of natural substrates and inhibitors of MDR pumps. J. Mol. Microbiol. Biotechnol. 3:247-254. [PubMed] [Google Scholar]
- 27.Lewis, K., and O. Lomovskaya. 2001. Drug efflux, p. 61-90. In K. Lewis, A. Salyers, H. Taber, and R. Wax (ed.), Bacterial resistance to antimicrobials: mechanisms, genetics, medical practice and public health. Marcel Dekker, Inc., New York, N.Y.
- 28.Lewis, K., A. Salyers, H. Taber, and R. Wax (ed.). 2001. Bacterial resistance to antimicrobials: mechanisms, genetics, medical practice and public health. Marcel Dekker, Inc., New York, N.Y.
- 29.Li, X. Z., L. Zhang, R. Srikumar, and K. Poole. 1998. β-Lactamase inhibitors are substrates for the multidrug efflux pumps of Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 42:399-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin, J., J. C. Tiller, S. B. Lee, K. Lewis, and A. M. Klibanov. 2002. Insights into bactericidal action of surface-attached poly(vinyl-N-hexylpyridinium) chains. Bio/Technol. Lett. 24:801-805. [Google Scholar]
- 31.Lomovskaya, O., and K. Lewis. 1992. Emr, an Escherichia coli locus for multidrug resistance. Proc. Natl. Acad. Sci. USA 89:8938-8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markham, P. N., E. Westhaus, K. Klyachko, M. E. Johnson, and A. A. Neyfakh. 1999. Multiple novel inhibitors of the NorA multidrug transporter of Staphylococcus aureus. Antimicrob. Agents Chemother. 43:2404-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nikaido, H. 1999. Microdermatology: cell surface in the interaction of microbes with the external world. J. Bacteriol. 181:4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okunade, A. L., C. D. Hufford, M. D. Richardson, J. R. Peterson, and A. M. Clark. 1994. Antimicrobial properties of alkaloids from Xanthorhiza simplicissima. J. Pharm. Sci. 83:404-406. [DOI] [PubMed] [Google Scholar]
- 35.Palumbo, J. D., C. I. Kado, and D. A. Phillips. 1998. An isoflavonoid-inducible efflux pump in Agrobacterium tumefaciens is involved in competitive colonization of roots. J. Bacteriol. 180:3107-3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papadopoulou, K., R. E. Melton, M. Leggett, M. J. Daniels, and A. E. Osbourn. 1999. Compromised disease resistance in saponin-deficient plants. Proc. Natl. Acad. Sci. USA 96:12923-12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pierce, M. L., E. C. Cover, P. E. Richardson, V. E. Scholes, and M. Essenberg. 1996. Adequacy of cellular phytoalexin concentrations in hypersensitively responding cotton leaves. Physiol. Mol. Plant Pathol. 48:305-324. [Google Scholar]
- 38.Pistelli, L., A. Bertoli, I. I. Giachi, I. I. Morelli, P. Rubiolo, and C. Bicchi. 2001. Quinolizidine alkaloids from Genista ephedroides. Biochem. Syst. Ecol. 29:137-141. [DOI] [PubMed] [Google Scholar]
- 39.Renau, T. E., R. Leger, E. M. Flamme, J. Sangalang, M. W. She, R. Yen, C. L. Gannon, D. Griffith, S. Chamberland, O. Lomovskaya, S. J. Hecker, V. J. Lee, T. Ohta, and K. Nakayama. 1999. Inhibitors of efflux pumps in Pseudomonas aeruginosa potentiate the activity of the fluoroquinolone antibacterial levofloxacin. J. Med. Chem. 42:4928-4931. [DOI] [PubMed] [Google Scholar]
- 40.Rogers, B., A. Decottignies, M. Kolaczkowski, E. Carvajal, E. Balzi, and A. Goffeau. 2001. The pleitropic drug ABC transporters from Saccharomyces cerevisiae. J. Mol. Microbiol. Biotechnol. 3:207-214. [PubMed] [Google Scholar]
- 41.Severina, I. I., M. S. Muntyan, K. Lewis, and V. P. Skulachev. 2001. Transfer of cationic antibacterial agents berberine, palmatine and benzalkonium through bimolecular planar phospholipid film and Staphylococcus aureus membrane. IUBMB-Life Sci. 52:321-324. [DOI] [PubMed] [Google Scholar]
- 42.Spencer, C. M., and M. I. Wilde. 1997. Diacerein. Drugs 53:98-106. [DOI] [PubMed] [Google Scholar]
- 43.Stermitz, F., M. A. Caolo and J. A. Swinehart. 1980. Alkaloids and other constituents of Zanthoxylum williamsii, Z. monophyllum and Z. fagara. Phytochemistry 19:1469-1472. [Google Scholar]
- 44.Stermitz, F. R., R. D. Beeson, P. J. Mueller, J.-F. Hsiang, and K. Lewis. 2001. Staphylococcus aureus MDR efflux pump inhibitors from a Berberis and a Mahonia (sensu strictu) species. Biochem. Syst. Ecol. 29:793-798. [DOI] [PubMed] [Google Scholar]
- 45.Stermitz, F. R., P. Lorenz, J. N. Tawara, L. Zenewicz, and K. Lewis. 2000. Synergy in a medicinal plant: antimicrobial action of berberine potentiated by 5′-methoxyhydnocarpin, a multidrug pump inhibitor. Proc. Natl. Acad. Sci. USA 97:1433-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stermitz, F. R., J. Tawara-Matsuda, P. Lorenz, P. Mueller, L. Zenewicz, and K. Lewis. 2000. 5′-Methoxyhydnocarpin and pheophorbide a: Berberis species components which potentiate berberine growth inhibition of resistant Staphylococcus aureus. J. Nat. Prod. 63:1146-1149. [DOI] [PubMed] [Google Scholar]
- 47.Tiller, J. C., C. J. Liao, K. Lewis, and A. M. Klibanov. 2001. Designing surfaces that kill bacteria on contact. Proc. Natl. Acad. Sci. USA 98:5981-5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verpoorte, R. 1998. Antimicrobially active alkaloids, p. 397-433. In M. F. Roberts and M. Wink (ed.), Alkaloids. Biochemistry, ecology, and medicinal applications. Plenum Press, New York, N.Y.
- 49.Zgurskaya, H. I., and H. Nikaido. 1999. Bypassing the periplasm: reconstitution of the AcrAB multidrug efflux pump of Escherichia coli. Proc. Natl. Acad. Sci. USA 96:7190-7195. [DOI] [PMC free article] [PubMed] [Google Scholar]