Abstract
We have evaluated the utility of antineutrophil cytoplasmic antibodies and anti-Saccharomyces cerevisiae mannan antibodies for distinguishing Crohn's disease from ulcerative colitis and other diarrheal illnesses by evaluating sera from 396 patients. Sensitivity, specificity, and phenotypic correlations were investigated. The implications of our findings for implementing these tests in routine clinical testing are discussed.
Differentiation between ulcerative colitis (UC), Crohn's disease (CD), and other causes of diarrhea is not always straightforward. Antineutrophil cytoplasmic antibodies (ANCAs) and anti-Saccharomyces cerevisiae mannan antibodies (ASCAs) are of interest as distinguishing serological markers.
ASCAs in humans recognize a 200-kDa glycoprotein present on Saccharomyces cerevisiae. The presence of ASCAs is associated with Crohn's disease (1, 5-7, 9, 11). Studies performed with small numbers of patients imply that ASCAs may have a role in routine diagnosis. Diagnostic laboratories are frequently asked to add this to their repertoire of tests.
In previous studies ANCAs were present in 50 to 85% of patients with UC but also in 10 to 20% of patients with CD (10). Conversely, ASCAs were present not only in 61% of patients with CD but also in 12% of those with UC (9).
There is racial variation in the prevalence of ASCAs in patients with CD (8), but no genetic concordance was found in studies with twins (4).
The demonstration of a disease association does not always correlate with a good discriminatory diagnostic test. Our study evaluates the utility of routine implementation of analysis for ASCA and ANCA for the diagnosis of chronic diarrheal illness in patients. To achieve this, we first determined the operational characteristics of the tests in patients with inflammatory bowel disease (IBD) and compared them with C-reactive protein (CRP) levels as a nonspecific marker of inflammation (2). We then examined the ability of these tests to distinguish between CD, UC, and other chronic diarrheal states. The etiological significance of ASCAs was explored by analyzing the disease behavior of patients with CD by determination of their ASCA status.
Serum was obtained from 409 consecutive patients with chronic diarrhea attending the gastroenterology (tertiary referral) clinic at the Royal London Hospital. Diagnoses were made according to standard criteria, without knowledge of the ASCA or the ANCA results. The cases of CD and UC seen in the clinic represent the spectrum (mild to severe) of disease seen in a specialist outpatient clinic. Not all patients had active diarrheal symptoms due to concurrent therapy at the time of serological testing. Patients who had neither CD (146 patients) nor UC (127 patients) were classified as “other” (123 patients) and were the disease controls. Thirteen patients were excluded because a definitive diagnosis remained uncertain by the end of the study, 18 months after sample collection. Sixty-nine patients were new referrals with no diagnosis at the time of ASCA and ANCA analysis; of these, 4 patients were subsequently diagnosed with CD, 4 were diagnosed with UC, and 61 were diagnosed with other diarrheal illnesses. The disease behavior in patients with CD was assessed by use of the modified Vienna classification criterion (3). Serum samples from 44 blood donors as healthy controls were analyzed for the ASCA assay. For 241 patients, CRP results, determined by turbidimetry (Olympus 640), were available and used as the comparator (2). Ethical approval was obtained from the East London and City Health Authority Ethics Committee.
The samples were analyzed for ANCAs by indirect immunofluoresence at a dilution of 1 in 20 on ethanol-fixed slides (A. Menarini Diagnostics, Berkshire, United Kingdom) by using rabbit anti-human immunoglobulin G (IgG) fluorescein conjugate (DakoCytomation, Ely, United Kingdom). The fluorescein isothiocyanate/protein ratio (emission wavelength at 495 nm/excitation wavelength at 278 nm) was 0.65 ± 0.05, which corresponded to a molar fluorescein isothiocyanate/protein ratio of 2.5. Samples were double screened by biomedical scientists blinded to any diagnostic information alongside routine clinical samples by using the standard criteria. Briefly, all ANCAs were reported as classical, perinuclear, or atypical. Concurrent HEp-2 cell screening for antinuclear antibodies (ANAs) was conducted, and samples with ANA titers equivalent to or higher than the ANCA titers were reported as ANCA negative (a sample was reported to be ANA positive if the titer was >1/80). The laboratory consistently obtains a misclassification index score of 0 in the United Kingdom National External Quality Assessment Service scheme for ANCA determination. Determination of IgG and IgA ASCA titers was performed by enzyme-linked immunosorbent assay (ELISA; Genesis, Cambridgeshire, United Kingdom), according to the manufacturer's instructions. Receiver operating characteristic (ROC) analysis was carried out to determine the optimal optical density (OD) cutoff for determination of a positive ASCA result in both the IgA and IgG kits in the study population. Statistical analysis was performed by using Graphpad Prism (version 4.0b) and SPSS statistical software.
A total of 453 samples were analyzed; 146 were from patients with Crohn's disease, 127 were from patients with UC, and 123 were from patients with neither Crohn's disease nor UC (“other”). Forty-four blood bank controls were included.
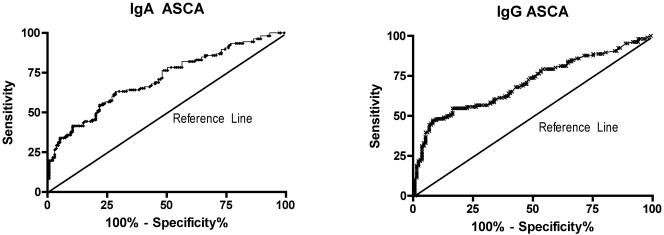
The results of ROC analysis of the IgA and IgG ASCA ELISAs for a diagnosis of Crohn's disease are shown in Fig. 1. The optimal test performance was achieved at ODs that were consistent with the kit manufacturer's recommendations, and these were therefore used for all further data analysis. Similar analysis of the ASCA test performance for the diagnosis of UC was performed.
FIG. 1.
ROC characteristics for IgA and IgG ASCAs for diagnosis of Crohn's disease. P was <0.001 for a significant difference between IgG or IgA ASCAs and the null hypothesis.
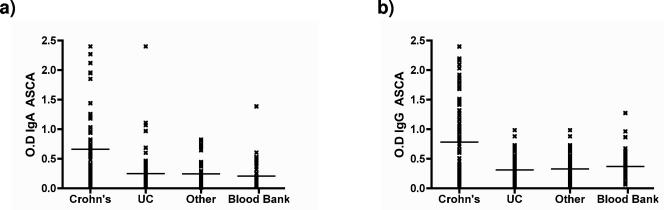
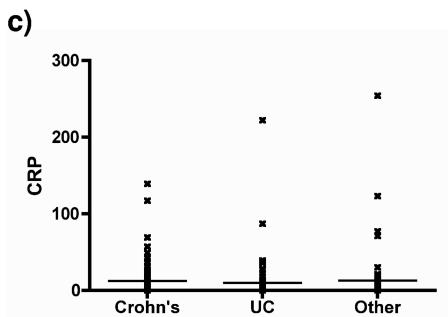
ASCA (IgA or IgG) levels were significantly higher in patients with Crohn's disease than in the other groups (P < 0.001, Dunn's comparison) (Fig. 2). No significant difference between the other diagnostic groups was detected. ANCAs were significantly more frequent in patients with UC than in patients with Crohn's disease or nonspecific diarrhea. The pattern of ANCA staining did not correlate with either the diagnosis for or the subset of patients with UC. Comparison with CRP levels suggests that ANCAs and ASCAs are not simply nonspecific inflammatory markers but are disease specific (Fig. 2).
FIG. 2.
Distribution of (a) IgA ASCAs and (b) IgG ASCAs in patient groups. The results for the samples from healthy individuals (blood bank), patients with other diarrheal illnesses, and UC are not statistically different. For Crohn's disease versus the other groups, P was <0.001 by Dunn's multiple-comparison test. (c) The distribution of CRP does not differ significantly between groups. Horizontal lines denote group arithmetic mean.
IgG ASCAs are more sensitive than IgA ASCAs (0.52 versus 0.45) for the diagnosis of Crohn's disease, with similar specificities (0.86 versus 0.89). IgG ANCAs have a sensitivity (0.56) and a specificity (0.83) similar to those of IgA ANCAs for the diagnosis of UC.
Use of a combination of ANCAs and ASCAs results in a small increase in specificity (0.89 for UC, 0.93 for Crohn's disease), but at the cost of sensitivity (0.43 and 0.41, respectively).
When disease behavior, demography, and risk factors were compared with ASCA status, a positive association with internal penetrating disease and positivity for both IgA and IgG ASCAs was observed (χ2 P < 0.01 compared with other disease behaviors). CD patients who were ASCA positive had longer disease durations (P = 0.03), but there was no association with age of onset. No other disease subtype (fibrostenotic, penetrating, perianal, or penetrating inflammatory disease), immunological treatment, long-term corticosteroid therapy, surgery, number of operations, age at first surgery, surgery after diagnosis, extraintestinal manifestations, smoking, or family history correlated with ASCA and/or ANCA status, nor did the test utility improve when these groups were separated out in isolation. The previous descriptions of ASCA-positive and ANCA-negative classical CD and ANCA-positive and ANCA-negative Crohn's colitis were not borne out by multivariate analysis of our data (9).
This study does confirm the association of ASCAs with Crohn's disease and ANCAs with UC.
Because of their relatively high specificities, the presence of IgG or IgA ASCAs could be considered evidence for the diagnosis of Crohn's disease and the presence of IgG ANCAs could be considered evidence for the diagnosis of UC in settings where the prior likelihood of IBD is high; i.e., in a gastroenterology clinic they could be employed to help distinguish IBD patients from non-IBD patients, but patients with a negative serology would still require further assessment (see the comment on false-negative results below). ROC analysis does not support a significant enhancement of the test characteristics when they are used in combination (data not shown). Although ASCA negativity and ANCA positivity for UC and ASCA positivity and ANCA negativity for Crohn's disease had a high positive predictive value, the sensitivity was still unacceptably low. The low sensitivity implies that tests for ASCAs and ANCAs are unsuitable as screening tests because of the frequent occurrence of false-negative results. Conversely, where the likelihood of IBD is low, these tests will not be useful because of unacceptably high false-positive rates. The phenotypic correlations observed may warrant limited use of these tests in tertiary referral settings, but their impact on clinical behavior should be determined by the use of a prospective study.
Acknowledgments
We thank Sarah Gibbs for helping to start up this project.
None of the authors have any competing interests to declare.
REFERENCES
- 1.Bartunkova, J., I. Kolarova, A. Sediva, and E. Holzelova. 2002. Antineutrophil cytoplasmic antibodies, anti-Saccharomyces cerevisiae antibodies, and specific IgE to food allergens in children with inflammatory bowel diseases. Clin. Immunol. 102:162-168. [DOI] [PubMed] [Google Scholar]
- 2.Boirivant, M., M. Leoni, D. Tariciotti, S. Fais, O. Squarcia, and F. Pallone. 1988. The clinical significance of serum C reactive protein levels in Crohn's disease. Results of a prospective longitudinal study. J. Clin. Gastroenterol. 10:401-405. [DOI] [PubMed] [Google Scholar]
- 3.Gasche, C., J. Scholmerich, J. Brynskov, G. D'Haens, S. B. Hanauer, E. J. Irvine, D. P. Jewell, D. Rachmilewitz, D. B. Sachar, W. J. Sandborn, and L. R. Sutherland. 2000. A simple classification of Crohn's disease: report of the Working Party for the World Congresses of Gastroenterology, Vienna 1998. Inflamm. Bowel Dis. 6:8-15. [DOI] [PubMed] [Google Scholar]
- 4.Halfvarson, J., A. Standaert-Vitse, G. Jarnerot, B. Sendid, T. Jouault, L. Bodin, A. Duhamel, J. F. Colombel, C. Tysk, and D. Poulain. 2005. Anti-Saccharomyces cerevisiae antibodies in twins with inflammatory bowel disease. Gut 54:1237-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hisabe, T., T. Matsui, T. Sakurai, Y. Murakami, H. Tanabe, H. Matake, T. Yao, S. Kamachi, and A. Iwashita. 2003. Anti-Saccharomyces cerevisiae antibodies in Japanese patients with inflammatory bowel disease: diagnostic accuracy and clinical value. J. Gastroenterol. 38:121-126. [DOI] [PubMed] [Google Scholar]
- 6.Montanelli, A., E. Mainardi, A. Vagni, V. Villanacci, C. Zambelli, R. Cestari, P. Cengia, L. Minelli, and G. Missale. 2005. Immunological markers anti-Saccharomyces cerevisiae antibodies (ASCA) and anti-neutrophil cytoplasmic antibodies (ANCA) in inflammatory bowel disease: a helpful diagnostic tool. Minerva Gastroenterol. Dietol. 51:201-207. [PubMed] [Google Scholar]
- 7.Oudkerk Pool, M., G. Bouma, S. G. Meuwissen, B. M. von Blomberg, J. P. van de Merwe, W. L. Deville, J. C. Fonk, and A. S. Pena. 1995. Serological markers to differentiate between ulcerative colitis and Crohn's disease. J. Clin. Pathol. 48:346-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Preda, C. M., M. Diculescu, V. Mirea, C. Marica, S. Vermeire, P. Rutgeerts, and A. Oproiu. 2005. Significance of anti-Saccharomyces cerevisiae antibodies (ASCA) in patients with inflammatory bowel diseases in Romania. Rom. J. Gastroenterol. 14:23-26. [PubMed] [Google Scholar]
- 9.Quinton, J. F., B. Sendid, D. Reumaux, P. Duthilleul, A. Cortot, B. Grandbastien, G. Charrier, S. R. Targan, J. F. Colombel, and D. Poulain. 1998. Anti-Saccharomyces cerevisiae mannan antibodies combined with antineutrophil cytoplasmic autoantibodies in inflammatory bowel disease: prevalence and diagnostic role. Gut 42:788-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roozendaal, C., and C. G. Kallenberg. 1999. Are anti-neutrophil cytoplasmic antibodies (ANCA) clinically useful in inflammatory bowel disease (IBD)? Clin. Exp. Immunol. 116:206-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutton, C. L., H. Yang, Z. Li, J. I. Rotter, S. R. Targan, and J. Braun. 2000. Familial expression of anti-Saccharomyces cerevisiae mannan antibodies in affected and unaffected relatives of patients with Crohn's disease. Gut 46:58-63. [DOI] [PMC free article] [PubMed] [Google Scholar]