Abstract
Rapid and sensitive methods for accurate strain delineation are essential for monitoring and preventing transmission of methicillin-resistant Staphylococcus aureus (MRSA). Pulsed-field gel electrophoresis (PFGE) has been the standard technique for strain typing most bacterial species including MRSA. The goal of this study was to compare the performance of the DiversiLab microbial typing system (Bacterial BarCodes, Inc., Houston, TX) (rep-PCR) to that of PFGE for typing MRSA isolates from five well-defined outbreaks. The DiversiLab rep-PCR assay is a rapid, semiautomated method based on PCR amplification of specific regions between noncoding repetitive sequences in the bacterial genome. rep-PCR was performed according to the manufacturer's recommendations, and the results were analyzed and dendrograms were generated using the DiversiLab analysis software (version 2.1.66a). PFGE was performed and interpreted according to published procedures. rep-PCR results using similarity indices (SI) of 80%, 85%, and 90% were compared to PFGE analysis. In addition, intra- and interrun reproducibility was determined for rep-PCR. Overall, correct assignment to outbreak versus nonoutbreak clusters occurred for 91 of 109 isolates (85% agreement) when using a SI of 85%. For each specific outbreak, concordance between rep-PCR and PFGE ranged from 73% to 100%. There were 18 discrepant results (17%). Fourteen isolates were unique by PFGE, but they were placed in clusters by rep-PCR; the other 4 were placed in clusters different from those assigned by PFGE. Intra- and interrun reproducibility was excellent. Times to results were 12 to 24 h for rep-PCR compared to 2 to 4 days for PFGE. Rapid, standardized results and excellent reproducibility make rep-PCR a valuable tool for use in MRSA investigations. However, since rep-PCR was less discriminatory than PFGE, we recommend that it be used to screen isolates, followed by testing isolates which share the same rep-PCR pattern with a more sensitive method, such as PFGE or multilocus sequence typing.
Methicillin-resistant Staphylococcus aureus (MRSA) is a major cause of nosocomial and community-acquired infections. MRSA is now responsible for 60% of nosocomial infections among intensive care unit patients (15). Therefore, controlling the spread of this pathogen in the hospital environment remains a priority among hospital infection control programs (14). Active surveillance, contact precautions, and adherence to hand hygiene are among the recommended strategies to decrease nosocomial spread (14). In addition, at least one university center has demonstrated the benefits of routine incorporation of molecular strain typing into its comprehensive infection control program (7). In that evaluation, implementation of restriction endonuclease analysis (REA) as the molecular strain typing method in the clinical microbiology laboratory resulted in a 23% decrease in the number of patients with nosocomial infections (7).
Pulsed-field gel electrophoresis (PFGE) has been accepted as the “gold standard” for molecular strain typing of MRSA (1, 2, 17). Advantages are that PFGE can be used to type most bacterial species, it has excellent discriminatory power, and it is also easier to perform than some methods such as chromosomal REA (1, 17, 22, 24). A major advantage is that PFGE results have been correlated with clinical information and criteria for interpretation have been published (2, 23). In addition, with reproducibility and interlaboratory studies, standardization of protocols and interpretation of PFGE has made it possible to create extensive databases for MRSA (5, 11, 13).
PFGE also has disadvantages. The major disadvantage is the time needed for final results. Depending on the specific protocol it may require 2 to 4 days (17, 24). Other disadvantages are variations in analysis and interpretation of the data and the specialized equipment required. Because of the limitations of PFGE, more-rapid yet reproducible methods of strain typing have been sought by clinical laboratories. PCR-based methods are attractive because many laboratories with molecular capability already have the necessary equipment, trained personnel, and reagents available. In general these methods are also more rapid, simpler to perform, and less costly than PFGE (17, 24). Repetitive-sequence-based PCR methods (rep-PCR) are rapid typing procedures that amplify the regions between the noncoding repetitive sequences in bacterial genomes (25). The sizes of these sequences are specific to each bacterial strain, and therefore the sizes of the amplified fragments may vary among different strains (25). Size fractionation of the amplicons is accomplished by standard agarose gel electrophoresis. The resulting band patterns can be used for DNA fingerprinting of a large variety of prokaryotic and eukaryotic organisms (9, 25). The original, nonautomated techniques have been successfully applied to the strain typing of MRSA (6), where, in a multicenter study in Europe, the intracenter reproducibility and discriminatory power compared to PFGE were excellent. However, the authors found poor interlaboratory reproducibility (6). Similar to other PCR-based assays rep-PCR has other disadvantages including the need for the PCR equipment, if not available, potential for contamination and false results, and the requirement for multiple controls. The rep-PCR method has recently been standardized and partially automated by Bacterial BarCodes, Inc. (Houston, TX) in a kit format complete with quality control reagents. The DiversiLab system separates the amplicons and generates patterns by use of microfluidics chips (LabChip device; CaliperTechnologies, Inc.), and it has a bioanalyzer (Agilent Technologies, Inc., Palo Alto, Calif.) for data analysis. Patterns are automatically downloaded onto a laboratory-specific DiversiLab website for analysis and interpretation. Both gel-like images and dendrograms are created for comparative analysis. Recently, a description of this automated rep-PCR technology and its performance characteristics has been published (9).
The goal of this study was to assess the DiversiLab rep-PCR system as a rapid method for MRSA strain delineation. Since the manufacturer does not provide definitive interpretative criteria, we initially determined the similarity indices (SI) that provided the best concordance with PFGE results for 109 strains from five epidemiologically well-characterized MRSA outbreaks. The rep-PCR results were then analyzed in comparison to the PFGE method for accuracy, strain interpretation, reproducibility, ease of use, and cost.
MATERIALS AND METHODS
Study design.
This study was a retrospective comparison and validation of the automated rep-PCR method to PFGE for rapid strain typing using a collection of well-characterized MRSA isolates. PFGE and rep-PCR were performed on all isolates. rep-PCR results were analyzed using the DiversiLab analysis software (version 2.1.66a), and the PFGE results were analyzed by Molecular Analyst software (version 1.6.3) (Bio-Rad Laboratories, Inc., Hercules, Calif.) and were interpreted by using the Tenover criteria (23). Dendrograms were created for both methods, and the agreement between the two methodologies was determined. Agreement was defined as correct assignment by rep-PCR of each isolate to the same PFGE designation, e.g., as identical or epidemiologically related within the outbreak or unique. Additionally, reproducibility studies were performed to assess inter- and intrarun variability.
Bacterial strains and outbreaks.
One hundred nine isolates of MRSA from five epidemiologically well-defined hospital outbreaks were used. For the purposes of this study, the following definitions were used. All isolates were confirmed to be MRSA by agar dilution antimicrobial susceptibility testing according to CLSI (formerly NCCLS) recommendations (16). Outbreaks were defined as nosocomial infections above a threshold for a particular unit or institution over a time interval as monitored by hospital epidemiology and infection control. MRSA surveillance protocols, infection control education, and intervention as well as PFGE studies were performed for outbreaks 1 through 4 as they were occurring and as presented below. PFGE analysis from each institution included strains implicated in the outbreak (clusters) and unique isolates. The first outbreak included 44 MRSA isolates recovered over a 6-month period from 44 adult patients with nosocomial MRSA bacteremia in a Maryland urban hospital (hospital A). Outbreak 2 consisted of 28 isolates of MRSA recovered from 28 infants over a 2-year period from the neonatal intensive care unit from hospital A. Outbreak 3 took place over a 3-month period and included 12 MRSA isolates recovered from five patients and seven health care workers from an urban Maryland hospital (hospital B). Outbreak 4 included 10 MRSA isolates recovered from nine patients and one health care worker over a 6-month period from a suburban Maryland hospital (hospital C). Outbreak 5 included 15 isolates from 15 patients over a 7-month period from a non-U.S. hospital (hospital D).
In addition, U.S. clones 100 to 800 were obtained through the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA), supported under NIAID, NIH, contract no. N01-AI-95359, and were tested by rep-PCR and the reference method, PFGE.
Test method: rep-PCR DiversiLab microbial typing system.
All organisms were cultured to Trypticase soy agar II with 5% sheep blood (Becton Dickinson Diagnostics, Sparks, MD) for 24 h at 37°C. Genomic DNA was extracted using the UltraClean microbial DNA isolation kit (MO BIO Laboratories, Inc., Solana Beach, CA) according to the manufacturer's recommendations. The DNA was quantitated using a DU 640 spectrophotometer (Beckman Coulter, Inc., Fullerton, CA) at 260-nm and 280-nm wavelengths and was diluted to a working concentration of 25 to 50 ng/μl. rep-PCR was performed using the DiversiLab Staphylococcus kit (Bacterial BarCodes, Inc., Houston, TX) according to the manufacturer's recommendations. In brief, each sample PCR mixture consisted of a master mixture and the Staphylococcus primer provided in the PCR kit, as well as GeneAmp 10× PCR buffer, AmpliTaq DNA polymerase (Applied Biosystems, Foster City, CA), and template DNA provided by the user. The Staphylococcus primers used are specific for all Staphylococcus spp., including Staphylococcus aureus. PCR was performed on a Perkin-Elmer 9600 thermocycler (Applied Biosystems, Foster City, Calif.) using the following parameters: initial denaturation at 94°C for 2 min and then 35 cycles of PCR (94°C for 30 s, 45°C for 30 s, and 70°C for 90 s), with a final extension of 70°C for 3 min. The model 2100 bioanalyzer and LabChip reagents (Agilent Technologies, Inc., Palo Alto, CA) were used for DNA separation and visualization according to the manufacturer's recommendations. The repetitive elements of the genome are “surveyed,” and PCR products from 150 to about 5,000 bp are detected and visualized. The DNA fingerprint patterns were automatically downloaded onto a secure laboratory designated DiversiLab website, where each chip went through a quality control step in which all patterns produced were visually analyzed and accepted by the user. The patterns were analyzed by the DiversiLab software, version 2.1.66a, which uses the Pearson correlation coefficient to determine distance matrices and the unweighted pair group method with arithmetic mean (UPGMA) to create dendrograms, scatter plots, and electropherograms for data interpretation (9). In brief, the main task in the analysis is to create a distance matrix for the samples requested in a report. The software calculates pairwise distances between normalized amplicon patterns to create the distance matrix. Each amplicon pattern pair is aligned along the x axis, and the correlation coefficient is calculated for the aligned pair. Based on the resulting distance matrix, the software creates a dendrogram using UPGMA and a scatter plot using multidimensional scaling. The software normalizes the time axis (x axis) of each amplicon pattern individually based on the marker positions at each end of the pattern. The normalized patterns are then stored in the database and can be compared to others at anytime the user requests it. The Pearson correlation is a measure of the degree to which two variables vary together. It is used to measure the degree to which the intensity values of two amplicon patterns vary at each time point. A high correlation (coefficient close to 1) indicates that both amplicon patterns share similar intensities along the full length of the pattern (21).
Agreement between methods was assessed at different rep-PCR SI cutoffs, including 80%, 85%, and 90%, as generated by the DiversiLab software. Results at these SI were then correlated to the PFGE data from all 109 isolates to determine the SI that provided the best agreement between the two methods.
Reproducibility assays.
Interrun reproducibility and intrarun reproducibility were assessed with Staphylococcus aureus strains NCTC 8325 and ATCC 43300. Both strains were extracted in duplicate for 10 consecutive runs. Each individual run was then subjected to separate rep-PCR and pattern detection and analysis using the Agilent 2100 bioanalyzer and DiversiLab software, yielding a total of 20 patterns for each organism. Using the DiversiLab software, dendrograms were created which included the images of the samples produced by the analyzer showing the SI of the isolates. Person-to-person variability in test performance was assessed by evaluating the results obtained by three technologists who had various degrees of experience with the DiversiLab procedure. Each technologist performed the entire assay including DNA extraction, PCR, and running the chip on the bioanalyzer with S. aureus ATCC 43300, in triplicate. A dendrogram was created which included the three isolates run by each technologist for a total of nine isolates for comparison.
Reference method: PFGE.
Organisms were grown overnight in brain heart infusion broth, suspended in a 1.6% In-Cert agarose, and pipetted into molds. DNA was extracted by standard PFGE methods (12) and digested with SmaI (New England Biolabs, Beverly, MA). Electrophoresis was performed using a GenePath system (Bio-Rad Laboratories, Hercules, CA) with 1% Bio-Rad PFGE-certified agarose-0.5× Tris-boric acid-EDTA running buffer at 6 V for 23.5 h at 14°C and involved ramping from 5 to 50 s as the initial and final switch times. The gel was stained with ethidium bromide, visualized using the Gel Doc 1000 (Bio-Rad) using Quantity One image capture software, version 4.2.2 (Bio-Rad), and analyzed with the Molecular Analyst fingerprinting software, version 1.61 (Bio-Rad). PFGE results were analyzed using Tenover criteria (23). Isolates were considered to be identical if there were no band differences; they were considered to be epidemiologically related strains if they differed in one to three bands. Unique strains were defined as having four or more band differences. Since four of the five outbreaks occurred over a relatively short time interval, we assume that related strains would still be limited.
RESULTS
Determination of the optimum SI for rep-PCR interpretation.
The purpose of these experiments was to determine the SI that yielded the best rep-PCR agreement with the designations provided by PFGE. Correct assignment of the 109 strains by rep-PCR based upon epidemiological information and the PFGE designations of identical, epidemiologically related, or unique strains was dependent on the SI (Table 1). The percentages of agreement for SI cutoffs at 80%, 85%, and 90% were 79%, 85%, and 81%, respectively. Based upon these results, an SI of 85% was used for final analysis and interpretation of all subsequent experiments.
TABLE 1.
Overall comparison of rep-PCR similarity indices for interpretative agreement with PFGE
| PFGE result | Total no. of isolates | No. (%) of isolates with matching rep-PCR result at SI of:
|
||
|---|---|---|---|---|
| 80% | 85% | 90% | ||
| Identical | 61 | 60 | 60 | 59 |
| Epidemiologically related | 27 | 24 | 24 | 21 |
| Unique | 21 | 2 | 7 | 8 |
| Total | 109 | 86 (79) | 91 (85) | 88 (81) |
Performance of rep-PCR with each of the five outbreaks compared to PFGE.
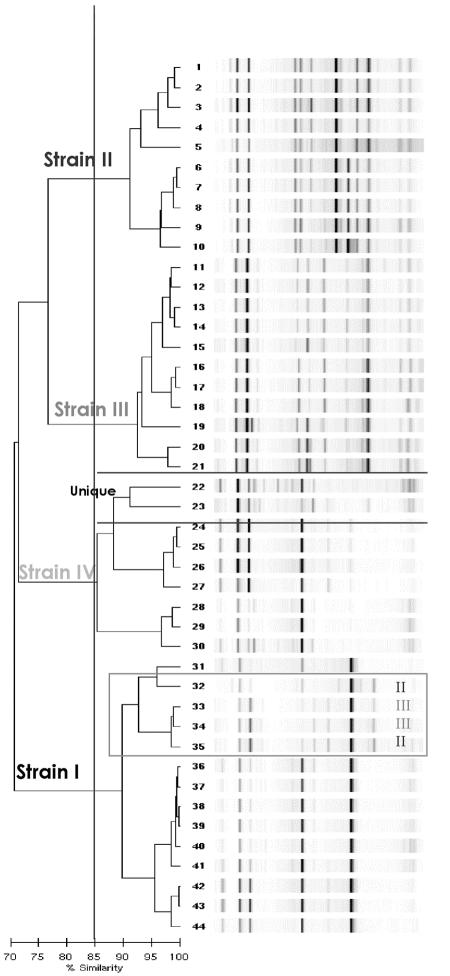
Analysis of each individual outbreak by the rep-PCR method using an SI of 85% for each outbreak is presented in Table 2. Outbreak 1 from hospital A had four distinct clusters (I to IV) by PFGE analysis. There was an agreement of 86% by rep-PCR, that is, 38 of the 44 strains were correctly assigned compared to PFGE (Fig. 1). Six strains were incorrectly assigned by rep-PCR. Two unique isolates by PFGE clustered with PFGE strain IV on the rep-PCR dendrogram (Fig. 1). Four other strains assigned to two distinct PFGE clones (two strains each) were misaligned with PFGE strain I. The dendrogram generated by the DiversiLab software for the rep-PCR results for these 44 isolates from outbreak 1 is illustrated in Fig. 1 as an example of the visual comparisons that can be made using the DiversiLab analytical software. As can be seen in Fig. 1 for outbreak 1, all four major strains can be visualized using an SI of 85%. Outbreak 2 from the neonatal intensive care unit of hospital A consisted of two distinct PFGE clusters. There was a 77% agreement for the rep-PCR results. For this group of isolates, identical and epidemiologically related strains were correctly assigned, but two isolates unique by PFGE were clustered by rep-PCR with PFGE strain I, two isolates were misaligned with PFGE strain II, and two unique isolates by PFGE were identified as the same strain by rep-PCR (dendrogram not shown). Outbreak 3 consisted of three distinct clusters. There was 83% agreement. Similar to the results for outbreak 2, two unique isolates by PFGE clustered with PFGE strain III (dendrogram not shown). There was 100% agreement between rep-PCR and PFGE for outbreak 4. For outbreak 5, four unique strains clustered with the two PFGE clones, resulting in an agreement of only 73% (dendrogram not shown). In addition, we compared all 109 isolates that were used in the five outbreaks to the published NARSA U.S. MRSA isolates in one large database (data not shown). As expected, due to the small number of known MRSA clones, several of the outbreak strains in the five individual outbreaks matched each other as well as U.S. strains 100 to 700 by both rep-PCR and PFGE. U.S. strain 800 did not match any of the isolates from the five outbreaks.
TABLE 2.
MRSA strain typing results by rep-PCR compared to PFGE-defined strains from five MRSA outbreaks using an SI of 85%
| Outbreak/hospital (n) | No. of strains for which rep-PCR result matched PFGE result/no. of strains with PFGE result ofa:
|
Discrepant-isolate explanation (n) | Agreement at 85% SI | ||
|---|---|---|---|---|---|
| Identical | Epidemiologically related | Unique | |||
| 1/A (44) | 25/26 (strain I, 10/10; strain II, 10/11; strain III, 3/3; strain IV 2/2) | 13/16 | 0/2 | 4/6 isolates assigned to two distinct PFGE strains (2 strains each) were misaligned by rep-PCR with PFGE strain I; 2/6 unique isolates by PFGE clustered with rep-PCR strain IV (6) | 38/44 (86%) |
| 2/A (28) | 16/16 (strain I, 12/12; strain II, 4/4) | 2/2 | 4/10 | 2/6 unique PFGE isolates clustered by rep-PCR with PFGE strain I; 2/6 unique PFGE isolates clustered by rep-PCR with PFGE strain II; 2/6 unique PFGE isolates clustered as the same strain by rep-PCR (6) | 22/28 (77%) |
| 3/B (12) | 5/5 (strain I, 3/3; strain II, 1/1; strain III, 1/1) | 5/5 | 0/2 | 2/2 unique isolates by PFGE clustered with PFGE strain III (2) | 10/12 (83%) |
| 4/C (10) | 9/9 (strain I) | 1/1 | No discrepant isolates | 10/10 (100%) | |
| 5/D (15) | 5/5 (strain I, 3/3; strain II, 2/2) | 4/4 | 2/6 | 2/4 unique isolates by PFGE clustered by rep-PCR with PFGE strain I; 2/4 unique isolates by PFGE clustered by rep-PCR with PFGE strain II (4) | 11/15 (73%) |
| Total | 60/61 | 24/27 | 7/21 | 91/109 (85%) | |
Strain designations for each outbreak do not correspond to the same rep-PCR or PFGE strain type. Each outbreak has its own separate interpretation, e.g., strain type I in outbreak 1 is not the same as strain type I in outbreak 2.
FIG. 1.
rep-PCR-generated dendrogram for the 44 MRSA isolates from outbreak 1, hospital A. An SI cutoff of 85% was used for interpretation of relatedness as depicted by the vertical line. The four clusters are designated strains I to IV.
Overall, using an 85% SI, 91 of 109 (85%) of the isolates were in agreement by both methods. When each category of relatedness is examined separately, rep-PCR and PFGE were in agreement for 60 of 61 (98%) of the total number of identical strains, 24 of 27 (89%) of the epidemiologically related strains, and 7 of 21 (33%) of the unique strains.
Reproducibility studies.
The reproducibility studies were performed to assess whether a given strain would yield the identical rep-PCR pattern and definitely fall within a much tighter range than that resulting from comparing epidemiologically related strains. For the interrun and intrarun reproducibility using the Staphylococcus aureus NCTC 8325 isolate, all 20 samples clustered tightly (SI, 96.5%). Similarly, with the ATCC 43300 isolate, the 20 samples likewise tightly clustered at an SI of 95.6%. Therefore, we assume that all PFGE isolates with identical patterns would be within an SI of 96%. There was also agreement (96% similarity) when the results for the three laboratory technologists were compared.
DISCUSSION
Large, university-affiliated and reference laboratories have incorporated molecular strain typing into the clinical microbiology laboratory as an adjunct to infection control activities. In order to be useful for determining clonal dissemination, the typing method should fulfill the following criteria in the following areas: ease of use and interpretation, applicability to a broad range of organisms (typeability), reproducibility, cost-effectiveness, and rapidity of turnaround (18). PFGE as a tool for strain typing MRSA fulfills most of the criteria. Its major limitations include the cost of the special equipment needed and the length of time required for results.
The rep-PCR technique has been shown to be useful for typing prokaryotic and eukaryotic organisms, including those with epidemiological significance such as Acinetobacter spp. (20), Streptococcus pneumoniae (26), Mycobacterium tuberculosis (3), and Aspergillus (8). The advantage of the rep-PCR system validated in our study is that it is commercially available, adapted into a kit-based, semiautomated format in which a microfluidics chip is used for fractionation and detection of the DNA amplicons (8). In addition, the DiversiLab system provides an Internet-based computer-assisted data analysis and user-specific data storage and retrieval system (9).
There are relatively few publications on the performance of the DiversiLab system (3, 8, 9, 19). Cangelosi et al. (3) evaluated the utility of the DiversiLab system as a typing method for Mycobacterium tuberculosis and the Mycobacterium avium complex. The authors found that this method was able to generate fingerprints for mycobacteria not typeable by other methods (3). In addition, results were generated rapidly in real time, and the discriminatory power was equal to that of IS6110 restriction fragment length polymorphism (RFLP) (3). A drawback of the method in that study was that, at the authors' chosen interpretive SI cutoff of 93%, there were some “false linkages” relative to the gold standard, RFLP, such that results had to be interpreted in combination with another method, i.e., spoligotyping (3).
The DiversiLab system has also been used to identify Aspergillus strains to species level and to assess the ability of rep-PCR to determine strain relatedness among fungi (8). That study was performed in a single institution with a limited number of species. However, there was a 98% concordance with identification using morphology-based criteria and 100% concordance with internal transcribed spacer region sequenced-based identification (8). In terms of strain level differentiation, too few strains, and no strains that were clonal, were incorporated for comparison; therefore no conclusions can be drawn about its utility for strain typing fungi (8). At least two studies report the utility of the DiversiLab system for typing Candida albicans strains that have developed resistance to azoles, but the studies were not designed to compare rep-PCR to other techniques (4, 10). Finally, in an analysis of both outbreak- and non-outbreak-associated Neisseria meningitidis isolates of various serogroups, the DiversiLab system had excellent serogroup and strain level discrimination (9).
The results of the present study confirm those of a recent publication (19) and extend our knowledge of rep-PCR technology as performed by the DiversiLab analysis system. The present study increases the number of isolates tested by the DiversiLab system through the evaluation of well-characterized strains (both microbiologically and epidemiologically). Determination of reproducibility and analysis of the data with various SI cutoffs to maximize the concordance with PFGE-defined strains were also performed in this evaluation. It was important in this verification study to challenge the discriminatory capability of the DiversiLab system as a strain typing method. This was done by comparing it to a gold standard reference method, PFGE. In addition, isolates for comparison were chosen from four diverse institutions including a hospital located outside of the United States and included a variety of epidemiologically related clinical strains and unique isolates.
The DiversiLab system compared favorably to PFGE for strain typing MRSA, with an overall agreement of 85% based on analysis of a reasonable sample size of 109 isolates including isolates from different geographic locations as well as epidemiologically related and unrelated strains. Additionally, we confirmed that several of the strains identified by both rep-PCR and PFGE belonged to the NARSA U.S. clones 100 to 700. The lengths of the outbreaks were short enough that great variability should not have occurred. The agreement for each individual outbreak, however, ranged from 73% to 100%. The discrepancies were mainly with the analysis of strains unique by PFGE. The most plausible explanation is that rep-PCR is less discriminatory compared to PFGE, and 14 of 21 PFGE-defined unique strains were falsely assigned to outbreak clusters.
The above findings are similar to those of Cangelosi et al. (3) in their evaluation of DiversiLab strain typing of Mycobacterium spp. At the 93% SI cutoff used by that group the majority of the PFGE-defined unique isolates in our study still would not have been correctly assigned. It will be important to see whether rep-PCR is less discriminatory than PFGE with other bacterial species, especially those with more diversity than MRSA.
In contrast, when identical and epidemiologically related strains were compared independently, the agreements were 98% and 89%, respectively. Adjustment of the SI cutoff did not improve the discriminatory power of the rep-PCR method. Identical isolates were unaffected by the different SI evaluated. Correct assignment of the related, but not identical, isolates, however, decreased as the SI increased, and the agreement among the unique isolates increased only slightly as the SI increased. In our study, the SI cutoff which provided the best discrimination was 85%. Using this SI, we were unlikely to miss a member of an outbreak strain, although some of the unique isolates were incorrectly assigned. Since the manufacturer does not provide definitive interpretation for the rep-PCR, this is a limitation of the method.
Another potential limitation is the inability of the rep-PCR assay to identify the subtypes of an outbreak strain with consistency compared to PFGE. Four of the five outbreaks in our investigation included PFGE-defined subtypes. rep-PCR was not able to reliably identify and cluster the PFGE-defined subtypes. The majority of the time rep-PCR was able to identify all members of the outbreak, including identical and related subtypes, but unable to correctly cluster, with any consistency, the related subtypes into separate sections of the dendrogram.
The rep-PCR patterns for the inter- and intrarun experiments for the ATCC 43300 and NCTC 8325 strains were reproducible, giving 95.6% and 96.5% SI, respectively. These patterns were generated using four separate lot numbers of LabChips run on the analyzer to generate the results. This demonstrates the analyzer's ability to generate reproducible rep-PCR patterns from several lots of LabChips. The person-to-person reproducibility also gave favorable results at a 96% SI. A dendrogram was created which included the rep-PCR patterns of the technologist reproducibility data (data not shown). Two of the isolate patterns that were generated by the technologist with the least amount of experience yielded additional bands. This was likely not a result of the rep-PCR assay itself, but rather most likely due to the extraction procedure done prior to the rep-PCR step. However, the additional bands did not significantly alter the results.
The DiversiLab rep-PCR is a relatively simple procedure to perform. The cost of rep-PCR, including capital equipment, reagents, and personnel expenses, is significantly more than PFGE (Table 3). However, if a laboratory is already performing molecular testing and has access to a thermocycler, the costs of rep-PCR and PFGE are comparable. The time, however, to obtain rep-PCR results is a definite advantage over PFGE and makes rep-PCR very appealing (Table 4). Bench medical technologists do not require extensive training to reliably perform this assay, as indicated by our laboratory personnel reproducibility studies. This may appeal to laboratories that would like to bring a molecular typing method into the laboratory.
TABLE 3.
Overall cost results for PFGE and the DiversiLab system
| Item | Cost (U.S. $)
|
|
|---|---|---|
| PFGEa | DiversiLab systemb | |
| Instrument | 25,000 | 35,000 |
| Thermocycler | NAd | 7,000 |
| Software | 7,000 | — |
| Reagents (/batch) | 20.00 | 350.00 |
| Personnel | 114.00 | 114.00 |
| Total | 32,134.00 | 42,114.00 (35,114.00c) |
Johns Hopkins Hospital reagent costs.
Manufacturer's list price. —, included.
If thermocycler is available.
NA, not applicable.
TABLE 4.
Overall time results for PFGE and the DiversiLab system
| Time | Duration for:
|
|
|---|---|---|
| PFGE | DiversiLab system | |
| Instrument run time | 24 h | 5 h |
| Technologist time/batch | 3.5 h | 3.5 h |
| Total time to results | 3-4 days | 9 h |
In summary, our data indicate that the semiautomated DiversiLab rep-PCR assay would be valuable for strain typing of MRSA during outbreak investigations. The obvious strength is the generation of results in real time. This rapidity coupled with the ability of the rep-PCR to reliably identify identical clones and epidemiologically related strains supports its potential as an initial screen during epidemiologic investigations. The finding that the rep-PCR is less discriminatory than PFGE in identifying unique strains in outbreak clusters, however, requires that isolates sharing the same pattern be tested with a more discriminatory method, e.g., PFGE or multilocus sequence typing when appropriate.
The cost and ease of performance of the rep-PCR also make it a reasonable option for those microbiology laboratories where typing volume is sufficient. The rep-PCR technology, as well as PFGE, has initial expenditures for the purchase of specialized equipment, but extensive training does not seem to be required in order to perform the former assay accurately. One downside is that, in addition to validation studies, interpretive guidelines may need to be defined by the user for use with each bacterial species evaluated. Further studies must be completed in order to realize the maximum potential of this technology. This fact that this method is commercially available and the potential for use with a wide range of pathogenic microorganisms make it a very attractive alternative to more labor-intensive and time-consuming technologies.
Acknowledgments
We thank Mimi Healy, from Bacterial BarCodes, Inc., for providing information on the DiversiLab data analysis software. Additionally, we thank Anna Boyer for her help in performing the reproducibility studies described in the paper.
This work was supported in part from NIH/NIAID MARCE grant AI-02-031.
REFERENCES
- 1.Bannerman, T. L., G. A. Hancock, F. C. Tenover, and J. M. Miller. 1995. Pulsed-field gel electrophoresis as a replacement for bacteriophage typing of Staphylococcus aureus. J. Clin. Microbiol. 33:551-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blanc, D. S., M. J. Struelens, A. Deplano, R. DeRyck, P. M. Hauser, C. Petignat, and P. Francioli. 2001. Epidemiological validation of pulsed-field gel electrophoresis patterns for methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 39:3442-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cangelosi, G. A., R. J. Freeman, K. N. Lewis, D. Livingston-Rosanoff, K. S. Shah, S. J. Milan, and S. V. Goldberg. 2004. Evaluation of a high-throughput repetitive-sequence-based PCR system for DNA fingerprinting of Mycobacterium tuberculosis and Mycobacterium avium complex strains. J. Clin. Microbiol. 42:2685-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chau, A. S., C. A. Mendrick, F. J. Sabatelli, D. Loebenberg, and P. M. McNicholas. 2004. Application of real-time quantitative PCR to molecular analysis of Candida albicans strains exhibiting reduced susceptibility to azoles. Antimicrob. Agents Chemother. 48:2124-2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chung, M., H. de Lencastre, P. Matthews, A. Tomasz, I. Adamsson, M. Aires de Sousa, T. Camou, C. Cocuzza, A. Corso, I. Couto, A. Dominguez, M. Gniadkowski, R. Goering, A. Gomes, K. Kikuchi, A. Marchese, R. Mato, O. Melter, D. Oliveira, R. Palacio, R. Sa-Leao, I. Santos Sanches, J. H. Song, P. T. Tassios, P. Villari, and the Multilaboratory Project Collaborators. 2000. Molecular typing of methicillin-resistant Staphylococcus aureus by pulsed-field gel electrophoresis: comparison of results obtained in a multilaboratory effort using identical protocols and MRSA strains. Microb. Drug Resist. 6:189-198. [DOI] [PubMed] [Google Scholar]
- 6.Deplano, A., A. Schuermans, J. Van Eldere, W. Witte, H. Meugnier, J. Etienne, H. Grundmann, D. Jonas, G. T. Noordhoek, J. Dijkstra, A. van Belkum, W. van Leeuwen, P. T. Tassios, N. J. Legakis, A. van der Zee, A. Bergmans, D. S. Blanc, F. C. Tenover, B. C. Cookson, G. O'Neil, M. J. Struelens, and the European Study Group on Epidemiological Markers of the ESCMID. 2000. Multicenter evaluation of epidemiological typing of methicillin-resistant Staphylococcus aureus strains by repetitive-element PCR analysis. J. Clin. Microbiol. 38:3527-3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hacek, D. M., T. Suriano, G. A. Noskin, J. Kruszynski, B. Reisberg, and L. R. Peterson. 1999. Medical and economic benefit of a comprehensive infection control program that includes routine determination of microbial clonality. Am. J. Clin. Pathol. 111:647-654. [DOI] [PubMed] [Google Scholar]
- 8.Healy, M., K. Reece, D. Walton, J. Huong, K. Shah, and D. P. Kontoyiannis. 2004. Identification to the species level and differentiation between strains of Aspergillus clinical isolates by automated repetitive-sequence-based PCR. J. Clin. Microbiol. 42:4016-4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Healy, M., J. Huong, T. Bittner, M. Lising, S. Frye, S. Raza, R. Schrock, J. Manry, A. Renwick, R. Nieto, C. Woods, J. Versalovic, and J. R. Lupski. 2005. Microbial DNA typing by automated repetitive-sequence-based PCR. J. Clin. Microbiol. 43:199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li, X., N. Brown, A. S. Chau, J. L. Lopez-Ribot, M. T. Ruesga, G. Quindos, C. A. Mendrick, R. S. Hare, D. Loebenberg, B. DiDomenico, and P. M. McNicholas. 2004. Changes in susceptibility to posaconazole in clinical isolates of Candida albicans. J. Antimicrob. Chemother. 53:74-80. [DOI] [PubMed] [Google Scholar]
- 11.McDougal, L. K., C. D. Steward, G. E. Killgore, J. M. Chaitram, S. K. McAllister, and F. C. Tenover. 2003. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: establishing a national database. J. Clin. Microbiol. 41:5113-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miranda, A. G., K. V. Singh, and B. E. Murray. 1991. DNA fingerprinting by pulsed-field gel electrophoresis may be a useful epidemiologic tool. J. Clin. Microbiol. 29:2752-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murchan, S., M. E. Kaufmann, A. Deplano, R. de Ryck, M. Struelens, C. E. Zinn, V. Fussing, S. Salmenlinna, J. Vuopio-Varkila, N. El Solh, C. Cuny, W. Witte, P. T. Tassios, N. Legakis, W. van Leeuwen, A. van Belkum, A. Vindel, I. Laconcha, J. Garaizar, S. Haeggman, B. Olsson-Liljequist, U. Ransjo, G. Coombes, and B. Cookson. 2003. Harmonization of pulsed-field gel electrophoresis protocols for epidemiological typing of strains of methicillin-resistant Staphylococcus aureus: a single approach developed by consensus in 10 European laboratories and its application for tracing the spread of related strains. J. Clin. Microbiol. 41:1574-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muto, C. A., J. A. Jernigan, B. E. Ostrowsky, H. M. Richet, W. R. Jarvis, J. M. Boyce, and B. M. Farr. 2003. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect. Control Hosp. Epidemiol. 24:362-386. [DOI] [PubMed] [Google Scholar]
- 15.National Nosocomial Infections Surveillance System. 2004. National Nosocomial Infections Surveillance (NNIS) System report, data summary from January 1992 through June 2004, issued October 2004. Am. J. Infect. Control 32:470-485. [DOI] [PubMed] [Google Scholar]
- 16.NCCLS. 2004. Performance standards for antimicrobial susceptibility testing. 14th informational supplement. NCCLS document M100-S14. NCCLS, Wayne, Pa.
- 17.Olive, D. M., and P. Bean. 1999. Principles and applications of methods for DNA-based typing of microbial organisms. J. Clin. Microbiol. 37:1661-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sader, H. S., R. J. Hollis, and M. A. Pfaller. 1995. The use of molecular techniques in the epidemiology and control of infectious diseases. Contemp. Issues Clin. Microbiol. 15:407-431. [PubMed] [Google Scholar]
- 19.Shutt, C. K., J. I. Pounder, S. R. Page, B. J. Schaecher, and G. L. Woods. 2005. Clinical evaluation of the DiversiLab microbial typing system using repetitive-sequence-based PCR for characterization of Staphylococcus aureus strains. J. Clin. Microbiol. 43:1187-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snelling, A. M., P. Gerner-Smidt, P. M. Hawkey, J. Heritage, P. Parnell, C. Porter, A. R. Bodenham, and T. Inglis. 1996. Validation of use of whole-cell repetitive extragenic palindromic sequence-based PCR (REP-PCR) for typing strains belonging to the Acinetobacter calcoaceticus-Acinetobacter baumannii complex and application of the method to the investigation of a hospital outbreak. J. Clin. Microbiol. 34:1193-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sokal, R. R., and F. J. Rohlf. 1995. Biometry: the principles and practice of statistics in biological research, 3rd ed. W. H. Freeman and Co., New York, N.Y.
- 22.Tenover, F. C., R. Arbeit, G. Archer, J. Biddle, S. Byrne, R. Goering, G. Hancock, G. Ann Hébert, B. Hill, R. Hollis, W. R. Jarvis, B. Kreiswirth, W. Eisner, J. Maslow, L. K. McDougal, J. M. Miller, M. Mulligan, and M. A. Pfaller. 1994. Comparison of traditional and molecular methods of typing isolates of Staphylococcus aureus. J. Clin. Microbiol. 32:407-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. A. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Belkum, A., M. Struelens, A. De Visser, H. Verbrugh, and M. Tibayrenc. 2001. Role of genomic typing in taxonomy, evolutionary genetics, and microbial epidemiology. Clin. Microbiol. Rev. 14:547-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Versalovic, J., T. Koeuth, and J. R. Lupski. 1991. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 19:6823-6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Versalovic, J., V. Kapur, E. O. Mason, Jr., U. Shah, T. Koeuth, J. R. Lupski, and J. M. Musser. 1993. Penicillin-resistant Streptococcus pneumoniae strains recovered in Houston: identification and molecular characterization of multiple clones. J. Infect. Dis. 167:850-856. [DOI] [PubMed] [Google Scholar]