Abstract
Recent studies have shown that the human fecal microbiota is composed of a consortium of species specific to the host and resistant to modifications over time. Antibiotics are known to affect the intestinal microflora, and ensuing changes may result in antibiotic-associated diarrhea. It is therefore important to characterize the nature and amplitude of these modifications and the ability of this ecosystem to return to its original profile—i.e., its resilience. Six healthy volunteers received oral amoxicillin (1.5 g/day) for 5 days. Fecal samples were collected at day 0 (D0) before antibiotic treatment and at set intervals until 60 days thereafter. Fecal DNA was isolated, and V6-to-V8 regions of the 16S rRNA genes were amplified by PCR with general primers and analyzed by temporal temperature gradient gel electrophoresis. Dominant species profiles were compared on the basis of similarity (Pearson correlation coefficient). Dominant species profiles at D0 were used as a reference. The fecal microbiota showed a major shift in dominant species upon antibiotic treatment, starting 24 h after treatment initiation and reaching an average similarity of only 74% after 4 days. Within 30 days following antibiotic treatment, the fecal microbiota tended to reach an average similarity of 88% to the D0 value; within 60 days, the average similarity to the D0 value was 89%. However, in one subject, important modifications persisted for at least 2 months, with similarity to the D0 value remaining below 70%. We demonstrated the resilience of the dominant human fecal microbiota upon short-course antibiotic challenge. Yet the persistence of long-term alterations in some subjects may explain susceptibilities to antibiotic-associated diarrhea. Furthermore, these findings suggest that strategies reinforcing the ability of the fecal microbiota to resist modifications would be of clinical relevance.
The human intestinal tract harbors a large, active, and complex community of microbes (22). The intestinal microbiota plays several significant roles in the digestion of food, metabolism of endogenous and exogenous compounds, immunopotentiation, and prevention of colonization by pathogens in the gastrointestinal tract and hence is involved in maintaining human health (12). Recent culture-independent molecular studies on healthy individuals have shown that the intestinal microbiota is specific to the host and resistant to modifications over time (26).
Molecular analysis of the bacterial microbiota based on the 16S rRNA genes have attracted attention as reliable methods for detection and identification of bacterial species (9, 13, 14). It is now impossible to adequately describe microbial communities without small-subunit rRNA gene data. Molecular technologies, typically based on comparative nucleic acid sequence information, provide data to identify specific microorganisms in a particular environment, such as human gut, to assign functional roles to these microorganisms and to assess their significance or contribution to environmental processes (27). They obviate the need for culture and can be used to characterize approximately 90% of the dominant fecal microflora in healthy subjects (21). Thus, techniques such as temporal temperature gradient gel electrophoresis (TTGE) are high-throughput methods for monitoring communities and population shifts and for rapid comparative analysis (26). It successfully differentiates bacterial gene fragments of the same size but different thermal stabilities. Such an approach has recently been used to assess dominant intestinal species in Crohn's disease (20).
Oral antibiotic administration profoundly affects the intestinal microbiota (1, 11). These changes may result in antibiotic-associated diarrhea and sometimes in severe intestinal complications such as Clostridium difficile-related colitis (2, 25). Obviously, the human gastrointestinal tract is one of the most complex ecosystems known in microbial ecology, containing over 1011 bacteria per gram of stool (23). It is therefore important to characterize the nature and amplitude of these antibiotic-related modifications and the ability of the fecal microbiota to resist modification but also to return to its original balanced composition.
The aim of our work was to assess the ability of the human fecal microbiota to return to its original dominant species profile after a 5-day course of amoxicillin, one of the most prescribed antibiotics in Europe.
MATERIALS AND METHODS
Patients.
Six healthy volunteers (2 females and 4 males, aged 18 to 55) received a 5-day course of antimicrobial chemotherapy. The criteria for enrolment were as follows: age of at least 18 years, no antibiotic treatment or hospitalization during the previous 6 months, no known human immunodeficiency virus infection and allergy, and no diarrhea (more than two loose stools per day) the day before enrolment. All volunteers gave informed consent to the protocol that was approved by the ethics committee of Nantes hospital.
Study protocol.
The antibiotic prescribed was amoxicillin (Clamoxyl) at a dose of 500 mg, three times a day, for 5 days. Volunteers were asked to give their last stool before enrolment (D0), the first stool passed at the beginning of the antibiotic treatment (D1), and a stool 30 days (D30) and 60 days (D60) after enrolment. Stool analyses were also done for four volunteers on day 2 (D2) and day 3 (D3) and for three volunteers on day 4 (D4) after enrolment.
DNA isolation and 16S rRNA gene amplification.
Stool samples were collected in sterile tubes and immediately stored at −80°C until analysis. Total DNA was extracted as described previously (7). Concentration and integrity of the nucleic acids were determined visually after electrophoresis on a 1% agarose gel containing ethidium bromide. Primers U968-GC (5′ CGC CCG GGG CGC GGC CCG GGC GGG GCG GGG GCA CGG GGG GAA CGC GAA GAA CCT TAC) and L1401 (5′ GCG TGT GTA CAA GAC CC) were used to amplify the V6-to-V8 regions of the bacterial 16S rRNA genes. PCR was performed using Hot Star Taq DNA polymerase (QIAGEN, Courtaboeuf, France). PCR mixtures (25 μl) contained the following: 1× PCR buffer, 1.5 mM MgCl2, 0.1 mM of each dNTP, 0.5 μM of primers U968-GC and L1401, 2.5 U of Hot Star Taq polymerase, and approximately 1 ng of DNA. DNAs were amplified in an MJ Research PTC-100 thermal cycler (GMI, Albertville, Minnesota) using the following program: 95°C for 15 min; 30 cycles of 94°C for 1 min, 56°C for 1 min, and 72°C for 1 min 30 s; and finally, 72°C for 15 min. Both negative (without DNA) and positive controls (DNA extracted from a Clostridium perfringens strain; 1.5 ng in Tris-EDTA) were employed in every series of reaction. Aliquots of 4 μl were analyzed by electrophoresis on a 1.5% agarose gel containing ethidium bromide to check the size and concentration of the obtained amplicons.
TTGE analysis of PCR amplicons.
The Dcode universal mutation detection system (Bio-Rad, Paris, France) was used for sequence-specific separation of PCR products. Electrophoresis was performed through a 1-mm-thick, 16- by 16-cm polyacrylamide gel (8% [wt/vol] acrylamide-bisacrylamide, 7 M urea, 1.25× Tris-acetate-EDTA [TAE], 55 μl and 550 μl of Temed, and 10% ammonium persulfate) using 7 liters of 1.25× TAE as electrophoresis buffer. Electrophoresis was run at a fixed voltage of 65 V for 969 min with an initial temperature of 66°C and a ramp rate of 0.2°C/h. For a better resolution, voltage was fixed at 20 V for 5 min at the beginning of electrophoresis. Each well was loaded with 100 to 200 ng of amplified DNA plus an equal volume of 2× gel loading dye (0.05% bromophenol blue, 0.05% xylene cyanol, and 70% glycerol). A marker was used for each gel. It was made as a standard ladder picked from a clone library. It consisted of a PCR amplicon mix of cloned rRNA genes from seven bacterial strains using the same universal primers (in order of migration: Staphylococcus epidermidis 99008139, Clostridium perfringens 99000184, Klebsiella oxytoca 99008113, Klebsiella pneumoniae 99008044, Enterobacter cloacae 99008179 from the Hospital collection center [CHU-Nantes, France], Bifidobacterium longum 56.7T from the collection of Institut Pasteur, and Lactobacillus gasseri 99R083 from ENITIAA-Nantes, France). After completion of electrophoresis, the gel was stained in a SYBR Green solution (SYBR Green I; Sigma-Aldrich, St. Quentin Fallavier, France), destained in 1.25× TAE, and analyzed using Quantity One software of the Gel Doc 2000 system (Bio-Rad, Paris, France). Profiles were scanned and gray intensity recorded along a densitogram, each band giving rise to a peak.
TTGE gel analysis.
TTGE profiles were combined using the Gel Compar II software (Applied-Maths, Sint-Martens-Latem, Belgium). Analysis took into account the number of bands, their position on the gel, and their intensity. The TTGE patterns shown are negative digitized images of TTGE profiles (10). The marker consisting of PCR amplicon mix was used to normalize the profiles. Similarity coefficients (Pearson correlation) were then calculated for each pair of profiles. This generated a similarity matrix between TTGE profiles. Variations due to methodology and sampling were previously assessed (M. Sutren, personal communication). Three aliquots from each of six fecal samples were analyzed after independent DNA extractions and PCR-TTGE. The positive similarity threshold indicative of methodological error and/or reproducibility of the method was defined as 96% (range: 91.3 to 98.8) (20).
Sequence analysis.
To perform sequence-based phylogenetic identification, specific bands were cut out from polyacrylamide gel. Gel fragments were washed once in 200 μl of PCR water and kept in 100 μl of PCR water overnight at +4°C for diffusion. rRNA gene fragments were then amplified from the dialysate. The PCR was the same as described above. Size and concentration of the amplicons were evaluated on 1.5% agarose gel containing ethidium bromide. The obtained PCR products were sequenced by Genome Express (Meylan, France). Newly determined sequences were compared directly with those in GenBank by BlastN search (NCBI) and using the Ribosomal Database Project RDP II sequence match facility (Michigan State University).
RESULTS
Evolution of dominant fecal microbiota during and after antibiotic treatment.
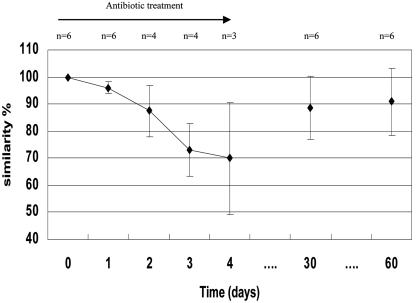
To determine whether fecal communities were significantly altered all along the study, intraindividual similarity indices of the TTGE profiles were calculated, considering D0 as the reference for each volunteer. Table 1 shows the monitoring of fecal samples of the six volunteers over time. On D1, we observed similarity percentages ranging from 93% to 99%. From D2 to D4, similarity percentages decreased to an average 73% (62% to 82%) on D3 and 74% (46% to 94%) on D4. Similarity percentages were as low as 62% on D3 for volunteer 6 and 46% on D4 for volunteer 1. On D30 and D60, similarity percentages raised back to an average of 88% (65% to 95%) and of 89% (66% to 98%), respectively. At these times, two volunteers still showed values below 90% (Fig. 1).
TABLE 1.
Evolution of dominant species diversity of the fecal microbiota of six healthy subjects during and after a 5-day course of antimicrobial chemotherapy
| Subject no. | Similarity (%) ata:
|
|||||
|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 30 | Day 60 | |
| 1 | 97.2 | 76.2 | 67.9 | 46.2 | 65.6 | 66.4 |
| 2 | 93.9 | 88.9 | 80.3 | 81.2 | 86.4 | 90 |
| 3 | 95.9 | 85.9 | 82 | 93.2 | 96.2 | |
| 4 | 93.2 | 94.3 | 98.1 | |||
| 5 | 97.1 | 95.5 | 96.7 | |||
| 6 | 99.1 | 98.1 | 62.1 | 94.8 | 93.8 | 87.4 |
Similarity coefficients (Pearson correlation) were calculated by comparison between each profile and the corresponding day 0 (D0) pattern of dominant microbiota for the same individual (similarity is 100% for all subjects at D0 by definition).
FIG. 1.
Similarity indices (%) of TTGE profiles of volunteers from D1 to D60. n, number of subjects tested; ⧫, mean value; bars indicate standard deviations.
Dynamic analyses of the banding pattern.
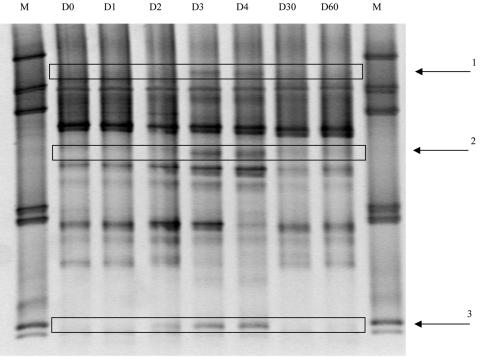
Dynamic analyses of the banding pattern have been done for three volunteers. As an example, Fig. 2 shows TTGE of PCR products of the V6-to-V8 regions for fecal samples from volunteer 1 from D0 to D60: some bands clearly showed variable intensities over time. Three bands, undetectable on D0 and D1, were detected under antibiotic treatment and were again undetectable at D30 and D60. Comigrating bands were detected with the same dynamics in fecal samples of D3 and D4 for the two other volunteers tested (data not shown). These three bands from volunteer 1 were excised from the gel and sequenced.
FIG. 2.
TGGE of 16S rRNA gene amplicons (obtained using primers for the V6-to-V8 regions) extracted from fecal samples of volunteer 1 from D0 to D60. M, marker for TTGE. Bands: 1, C. nexile; 2, R. torques; 3, deep branching line within the β-Proteobacteria (Burkholderia, 86% similarity).
Sequence analyses.
Sequences obtained for the three bands specific for the TTGE patterns under antibiotic treatment were subjected to phylogenetic assignment. Two of them showed more than 99% similarity with known sequences. Band 1 was related to Clostridium nexile and band 2 to Ruminococcus torques. They are members of the commensal microbiota and belong to the Clostridium coccoides phylogenetic group (Clostridium cluster XIVa) (4). As for band 3, it corresponded to a deep branching line within the β-Proteobacteria but was not closely related to any known cultured bacterial species. Its closest relative was Burkholderia (86% similarity).
DISCUSSION
Using molecular profiling of bacterial 16S rRNA genes, we found that dominant species of the human fecal microbiota were markedly modulated within 2 to 3 days of an antibiotic treatment. The dominant fecal microbiota tended to return to its initial profile within 60 days following a 5-day amoxicillin treatment (500 mg per day). Indeed, the microbiota profile for five out of six volunteers came back to near initial composition within 60 days (87% similarity or more). However, individual responses were such that in one subject important modifications persisted for at least 2 months. These results may explain the occasional development of chronic disorders following antibiotic treatment. Furthermore, these findings suggest that strategies reinforcing the ability of the fecal microbiota to resist modifications would be of clinical relevance.
To identify the exact profile at equilibrium would be rather difficult, and homeostasis itself is not likely to correspond to absolute stability, being influenced for instance by external factors such as diet (6). Fingerprinting methods indicated that the predominant intestinal microbiota was stable and host specific in human subjects (24, 26). It was determined that upon natural oscillations of dominant fecal microbiota TTGE profiles would remain within 90% of similarity with the equilibrium state over a period of 2 years in one volunteer (20). The alterations observed here in the structure of the microbiota upon antibiotic treatment were, by the second day onward, much greater than any variation observed over time for an untreated healthy individual. Except for the maturation of the microbiota after birth, known to be a period of major fluctuation (6), the comparison between acute and remission phases of inflammatory bowel diseases was the only situation for which such marked intraindividual alterations of the fecal microbiota were reported (20). Thus, the present report provides evidence for temporal alterations in the structure of the dominant microbiota upon antibiotic treatment. The clear tendency of each fecal microbiota to return, within 1 to 2 months after antibiotic treatment, to a community structure made of many of the same dominant species is indicative of the resilience of the balanced microbiota. This suggests that very robust determinants, possibly mediated by a cross talk between host cells and its gut bacterial community, may play a role in the homeostasis of the intestinal microbiota. Nevertheless, the molecular determinants of this stability and host specificity have yet to be identified.
Identification of two strains stimulated upon antibiotic chemotherapy showed that they belonged to C. nexile and R. torques, two closely related species belonging to the phylogenetically defined cluster C. coccoides. This phylogenetic cluster is a normal component of the dominant gastrointestinal microbiota (4). It is well known that R. torques produces extracellular alpha- and beta-glycosidases that degrade intestinal mucin oligosaccharides and glycosphingolipids (4, 5). Thus, changes in dominant microbiota under antibiotic pressure will most likely induce changes in metabolism. The third sequence was only distantly related to any organism known to date, possibly representing a yet-uncultured microbial species. This is not surprising since novel or yet-uncultured species are most often identified upon characterization of fecal microbiota using cloned 16S rRNA gene libraries (3, 9, 21). These observations would warrant confirmation for a larger cohort of patients. This could be tested using specific hybridization probes designed to recognize the species of interest and their application using, for example, fluorescent in situ hybridization (18).
This study assessed, for each individual, the biodiversity modulations upon short-course antibiotic challenges and the resilience of dominant fecal microbiota, but we did not intend to determine the composition of the dominant fecal microbiota in terms of bacterial genera or species. The uses and limits of TTGE in microbial ecology have been previously explored (17). TTGE is a culture-independent molecular method that has proven most appropriate in dynamic studies of dominant species diversity within complex ecosystems like the colon (8). The repeatability of amplifications with the universal primers used, and TTGE runs using the same starting sample, has already been shown (16, 19). Furthermore, in all cases, a clear limit of detection was observed when the minority species accounted for 1:100 or less of the total DNA concentration (15, 17). Thus, any changes in bacterial population within this limit would be observed in the TTGE pattern. Only the dominant fraction of the microbiota is assessed using the PCR-TTGE technique, as applied here, with universal primers. Considering that 1 g of feces contains approximately 1011 cells, bacteria which reach levels below 107 cells or fewer per g of feces will not be visualized by TTGE. This dominant fraction is also the fraction represented in 16S rRNA gene libraries obtained by direct cloning from fecal DNA (3, 8, 21). These contain approximately 20 to 50 different rRNA gene sequences per 100 clones analyzed, many of which are represented by a unique sequence. On that basis, the complexity of the profiles observed by TTGE, containing 15 to 30 bands, will accordingly represent the most prevalent species. It would be highly relevant to analyze alterations and resilience of the subdominant fraction of the fecal microbiota. Furthermore, specificities of the mucosa-associated microbiota have recently been described. It has been found to differ from the fecal microbiota and seems to be specific of the individual (10). Considering that the mucosa-associated microbiota can provide seeds for a postantibiotic restructuring of the colonic microbiota, it would be most appropriate to study it to complete our understanding of the resilience of this ecosystem.
Acknowledgments
T. Durand was supported by a grant from Biocodex, Inc.
REFERENCES
- 1.Bartosch, S., A. Fite, G. T. Macfarlane, and M. E. McMurdo. 2004. Characterization of bacterial communities in feces from healthy elderly volunteers and hospitalized elderly patients by using real-time PCR and effects of antibiotic treatment on the fecal microbiota. Appl. Environ. Microbiol. 70:3575-3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beaugerie, L., and J. C. Petit. 2004. Microbial-gut interactions in health and disease. Antibiotic-associated diarrhoea. Best Pract. Res. Clin. Gastroenterol. 18:337-352. [DOI] [PubMed] [Google Scholar]
- 3.Bonnet, R., A. Suau, J. Dore, G. R. Gibson, and M. D. Collins. 2002. Differences in rDNA libraries of faecal bacteria derived from 10- and 25-cycle PCRs. Int. J. Syst. Evol. Microbiol. 52:757-763. [DOI] [PubMed] [Google Scholar]
- 4.Collins, M. D., P. A. Lawson, A. Willems, J. J. Cordoba, J. Fernandez-Garayzabal, P. Garcia, J. Cai, H. Hippe, and J. A. Farrow. 1994. The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations. Int. J. Syst. Bacteriol. 44:812-826. [DOI] [PubMed] [Google Scholar]
- 5.Falk, P., L. C. Hoskins, and G. Larson. 1990. Bacteria of the human intestinal microbiota produce glycosidases specific for lacto-series glycosphingolipids. J. Biochem. (Tokyo) 108:466-474. [DOI] [PubMed] [Google Scholar]
- 6.Favier, C. F., E. E. Vaughan, W. M. De Vos, and A. D. Akkermans. 2002. Molecular monitoring of succession of bacterial communities in human neonates. Appl. Environ. Microbiol. 68:219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godon, J. J., E. Zumstein, P. Dabert, F. Habouzit, and R. Moletta. 1997. Molecular microbial diversity of an anaerobic digestor as determined by small-subunit rDNA sequence analysis. Appl. Environ. Microbiol. 63:2802-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi, H., M. Sakamoto, and Y. Benno. 2002. Fecal microbial diversity in a strict vegetarian as determined by molecular analysis and cultivation. Microbiol. Immunol. 46:819-831. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi, H., M. Sakamoto, and Y. Benno. 2002. Phylogenetic analysis of the human gut microbiota using 16S rDNA clone libraries and strictly anaerobic culture-based methods. Microbiol. Immunol. 46:535-548. [DOI] [PubMed] [Google Scholar]
- 10.Lepage, P., P. Seksik, M. Sutren, M. F. de la Cochetiere, R. Jian, P. Marteau, and J. Dore. 2005. Biodiversity of the mucosa-associated microbiota is stable along the distal digestive tract in healthy individuals and patients with IBD. Inflamm. Bowel Dis. 11:473-480. [DOI] [PubMed] [Google Scholar]
- 11.Lode, H., N. Von der Hoh, S. Ziege, K. Borner, and C. E. Nord. 2001. Ecological effects of linezolid versus amoxicillin/clavulanic acid on the normal intestinal microflora. Scand. J. Infect. Dis. 33:899-903. [DOI] [PubMed] [Google Scholar]
- 12.Marteau, P., P. Lepage, I. Mangin, A. Suau, J. Dore, P. Pochart, and P. Seksik. 2004. Gut flora and inflammatory bowel disease. Aliment. Pharmacol. Ther. 20(Suppl. 4):18-23. [DOI] [PubMed] [Google Scholar]
- 13.Matsuki, T., K. Watanabe, J. Fujimoto, Y. Miyamoto, T. Takada, K. Matsumoto, H. Oyaizu, and R. Tanaka. 2002. Development of 16S rRNA-gene-targeted group-specific primers for the detection and identification of predominant bacteria in human feces. Appl. Environ. Microbiol. 68:5445-5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuki, T., K. Watanabe, J. Fujimoto, T. Takada, and R. Tanaka. 2004. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl. Environ. Microbiol. 70:7220-7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muyzer, G., E. C. de Waal, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muyzer, G., and K. Smalla. 1998. Application of denaturing gradient gel electrophoresis (DGGE) and temperature gradient gel electrophoresis (TGGE) in microbial ecology. Antonie Leeuwenhoek 73:127-141. [DOI] [PubMed] [Google Scholar]
- 17.Ogier, J. C., O. Son, A. Gruss, P. Tailliez, and A. Delacroix-Buchet. 2002. Identification of the bacterial microflora in dairy products by temporal temperature gradient gel electrophoresis. Appl. Environ. Microbiol. 68:3691-3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rigottier-Gois, L., V. Rochet, N. Garrec, A. Suau, and J. Dore. 2003. Enumeration of Bacteroides species in human faeces by fluorescent in situ hybridisation combined with flow cytometry using 16S rRNA probes. Syst. Appl. Microbiol. 26:110-118. [DOI] [PubMed] [Google Scholar]
- 19.Seksik, P., P. Lepage, M. F. de la Cochetiere, A. Bourreille, M. Sutren, J.-P. Galmiche, J. Doré, and P. Marteau. 2005. Search for localized dysbiosis in Crohn's disease ulcerations by temporal temperature gradient gel electrophoresis of 16S rRNA. J. Clin. Microbiol. 43:4654-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seksik, P., L. Rigottier-Gois, G. Gramet, M. Sutren, P. Pochart, P. Marteau, R. Jian, and J. Dore. 2003. Alterations of the dominant faecal bacterial groups in patients with Crohn's disease of the colon. Gut 52:237-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suau, A., R. Bonnet, M. Sutren, J. J. Godon, G. R. Gibson, M. D. Collins, and J. Dore. 1999. Direct analysis of genes encoding 16S rRNA from complex communities reveals many novel molecular species within the human gut. Appl. Environ. Microbiol. 65:4799-4807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tannock, G. W. 2002. Analysis of the intestinal microflora using molecular methods. Eur. J. Clin. Nutr. 56(Suppl. 4):S44-S49. [DOI] [PubMed] [Google Scholar]
- 23.Vanhoutte, T., G. Huys, E. De Brandt, and J. Swings. 2004. Temporal stability analysis of the microbiota in human feces by denaturing gradient gel electrophoresis using universal and group-specific 16S rRNA gene primers. FEMS Microbiol. Ecol. 48:437. [DOI] [PubMed] [Google Scholar]
- 24.Vaughan, E. E., F. Schut, H. G. Heilig, E. G. Zoetendal, W. M. de Vos, and A. D. Akkermans. 2000. A molecular view of the intestinal ecosystem. Curr. Issues Intest. Microbiol. 1:1-12. [PubMed] [Google Scholar]
- 25.Wilcox, M. H. 2003. Gastrointestinal disorders and the critically ill. Clostridium difficile infection and pseudomembranous colitis. Best Pract. Res. Clin. Gastroenterol. 17:475-493. [DOI] [PubMed] [Google Scholar]
- 26.Zoetendal, E. G., A. D. Akkermans, and W. M. De Vos. 1998. Temperature gradient gel electrophoresis analysis of 16S rRNA from human fecal samples reveals stable and host-specific communities of active bacteria. Appl. Environ. Microbiol. 64:3854-3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zoetendal, E. G., C. T. Collier, S. Koike, R. I. Mackie, and H. R. Gaskins. 2004. Molecular ecological analysis of the gastrointestinal microbiota: a review. J. Nutr. 134:465-472. [DOI] [PubMed] [Google Scholar]