Abstract
IS6110 restriction fragment length polymorphism (RFLP) analysis is the most widely applied method for strain differentiation of Mycobacterium tuberculosis complex. We have previously described mixed-linker PCR, an IS6110-based PCR method that favorably compared with other typing methods for M. tuberculosis complex according to reproducibility and ability to differentiate between strains. Here we report the further development of this method, called fast ligation-mediated PCR (FLiP), which allows analysis of strains within one working day and starting from less than 1 ng of mycobacterial DNA or a crude cell lysate. Blinded analysis of a standard set of 131 M. tuberculosis complex and nontuberculous isolates showed the ability to differentiate 81 types among 90 M. tuberculosis complex isolates with 84 different IS6110 RFLP fingerprint patterns and detected 97% of the 31 duplicate samples. We suggest that FLiP can serve to rapidly detect chains of transmission prior to starting high-throughput analysis or standard IS6110 RFLP. It may as well serve as a secondary typing technique for other, non-IS6110-based methods.
Within the last 15 years, molecular epidemiological analysis of tuberculosis has provided remarkable new insights into the natural course and mechanisms of the spread of this disease. In spite of the development of a multitude of methods for typing strains of Mycobacterium tuberculosis complex, however, IS6110 restriction fragment length polymorphism (RFLP) analysis is the most widely applied method and serves a as referral method for novel typing approaches (1, 3, 11, 13, 21). The number and distribution of IS6110 elements within the genome of M. tuberculosis can serve as a marker for the diversity of strains encountered in outbreak investigations and epidemiological studies.
IS6110 RFLP typing requires 2 to 4 μg of mycobacterial DNA, corresponding to a well-grown culture or subculture of the original isolate (3, 20). This limitation encouraged us to develop a typing method based on the amplification of RFLP fragments containing the 3′ end of IS6110 by PCR, named mixed-linker PCR (7). This method has been successfully applied for the analysis of outbreaks, mixed infections, and population-based studies (8-10, 19). A comparison of all available typing methods using a reference set of 131 mycobacterial strains of mainly M. tuberculosis complex showed this method to reliably reflect the strain diversity as determined by IS6110 RFLP typing (11). Since the original publication of mixed-linker PCR, the experience gained has resulted in modifications of this method, streamlining the analytical process and further increasing its specificity. Here we describe this modified method, named fast ligation-mediated PCR (FLiP), and present the results of its application to the published reference set in comparison with mixed-linker PCR and IS6110 RFLP typing.
MATERIALS AND METHODS
Strains.
The reference set of 131 purified mycobacterial DNA samples of the study of Kremer et al. (11) was used for the analysis. This set contained 90 strains of M. tuberculosis complex, 10 nontuberculous mycobacteria, and 31 duplicate DNA samples. IS6110 typing of strains had been carried out by the National Institute of Public Health and the Environment (RIVM), Bilthoven, The Netherlands, as published elsewhere (11, 11a). The samples were coded by the RIVM to allow blinded FLiP analysis unbiased by the previous study. Sensitivity was assessed by using serial dilutions of purified DNA of the M. tuberculosis reference strain H37Rv.
Sample preparation.
DNA samples were diluted 1:1,000 to a final concentration of 0.1 to 1.0 ng/μl in sterile H2O (B. Braun Melsungen AG, Melsungen, Germany). Crude cell lysates were prepared by repeated heating and thawing, as described previously (8).
Linker synthesis.
The sequences of the oligonucleotides used for the linker synthesis (designated NLO and BA) are given in Table 1. Equimolar amounts of both oligonucleotides were added into 100 μl 1× GeneAmp PCR buffer (Applied Biosystems GmbH, Weiterstadt, Germany) to a final concentration of 6.7 μM. The synthesis was carried out in a DNA thermal cycler (Applied Biosystems GmbH) using 0.5-ml Hot Start tubes (Molecular BioProducts, San Diego, Calif.) with an initial denaturation step at 94°C for 15 min, followed by cycling the temperature three times between 58°C for 10 min and 70°C for 5 min for precise annealing of the complementary sequences. Finally, the solution was cooled down to 4°C and aliquots were stored at −20°C.
TABLE 1.
Oligonucleotides used for linker construction and PCR amplification
| Oligonucleotidea | Length (bp) | Sequence (5′→3′) |
|---|---|---|
| NLO | 30 | 5′-GCA TTT GAA TTC CAC GTC AGC GAC TGC ACG |
| BA | 19 | 5′-TGC AGU CGC UGA CGU GGA A |
| Flip1 | 24 | 5′-TTT GAA TTC CAC GTC AGC GAC TGC |
| IS54 | 22 | 5′-TCG ACT GGT TCA ACC ATC GCC G |
| IS55 | 19 | 5′-TCT GAT CTG AGA CCT CAG C |
Synthesis by INTERACTIVA Biotechnologies, Ulm, Germany.
Ligation-mediated amplification.
The first step of ligation-mediated amplification combined digestion of 0.1 to 1.0 ng purified genomic DNA or 1 μl of the crude lysate with 0.5 U/μl HhaI (Gibco Life Technologies GmbH, Eggenstein, Germany) and ligation of the generated fragments to the linker molecule (0.67 nM) using 0.05 U/μl T4 ligase in 20 μl 1× ligation buffer (Gibco Life Technologies GmbH). Both reactions occurred simultaneously during 2 h of incubation at 25°C in a DNA thermal cycler (Applied Biosystems GmbH). Of this restriction-ligation mix, 5 μl was submitted to PCR using a 9600 GeneAmp PCR system (Applied Biosystems GmbH). The PCR mixture consisted of 12.5 pmol of each primer, IS54 and Flip1 (Table 1), 1.25 mM MgCl2, 0.8 mM nucleoside triphosphates, 0.5 U uracil DNA glycosylase (Gibco Life Technologies GmbH), and 2.5 U AmpliTaq Gold DNA polymerase in 50 μl of GeneAmp PCR buffer II (Applied Biosystems GmbH). The reaction was started by an incubation for 10 min at 50°C—to split the uracil-carrying oligonucleotide of the linker molecule with uracil DNA glycosidase (Gibco Life Technologies GmbH)—followed by 10 min at 95°C for activation of AmpliTaq Gold DNA polymerase (Applied Biosystems GmbH). The amplification profile consisted of 30 rounds of a two-step cycle with 30 s at 69°C and 1 min at 72°C and a final 7-min extension step at 72°C.
Carryover prevention and controls.
To avoid cross-contamination, all reactions were set up in a separate room before addition of the respective templates. Template was added using a class II laminar airflow cabinet. In both preamplification areas, possible contamination with DNA fragments was reduced by UV irradiation (253 nm) of the work surface for 30 min prior to setting up the reactions. Separate negative controls for the restriction-ligation step and the PCR amplification were used. The positive amplification control consisted of 0.1 ng purified genomic DNA of H37Rv.
Gel electrophoresis and digital analysis.
Eighteen microliters of each PCR product was run on an 8% polyacrylamide gel. Patterns were visualized by ethidium bromide staining (0.5%) for 5 min and photographed under UV transillumination (253 nm) on Polaroid 667 instant film (Kodak, Frankfurt, Germany). Band sizes were determined by comparison with a 100-bp DNA mass ladder (Gibco Life Technologies GmbH). Photographic images were scanned using an HP ScanJet IIcx (Hewlett-Packard AG, Bad Homburg, Germany) and analyzed with GelCompar 4.0 (Applied Maths BVBA, Kortrijk, Belgium). Only bands between 100 and 500 bp were included in the analysis. The similarity (percentage) of DNA fingerprint patterns was calculated by the unweighted pair group method with arithmetic averages and Dice similarity coefficient. Matching bands were identified by using a position tolerance of 0.8%.
Hybridization.
After transfer of DNA fragments to a Hybond-N+ nylon membrane (Amersham Life Science, Braunschweig, Germany), hybridization with the diagnostic oligonucleotide IS55 (Table 1), containing part of the IS6110 sequence, was performed using enhanced chemiluminescence detection (ECL kit RPN 2130 oligolabeling and detection system; Amersham Life Science), as described previously. The hybridization was carried out at 42°C, and the stringency wash temperature was 48°C.
RESULTS
The construction of the new linker results in specific amplification of RFLP fragments carrying IS6110 after a single amplification step. Together with the optimization of the work flow, this allows gel electrophoretic analysis of the DNA fingerprints after 6.5 h, starting from purified mycobacterial DNA or crude cell lysates (Fig. 1).
FIG. 1.
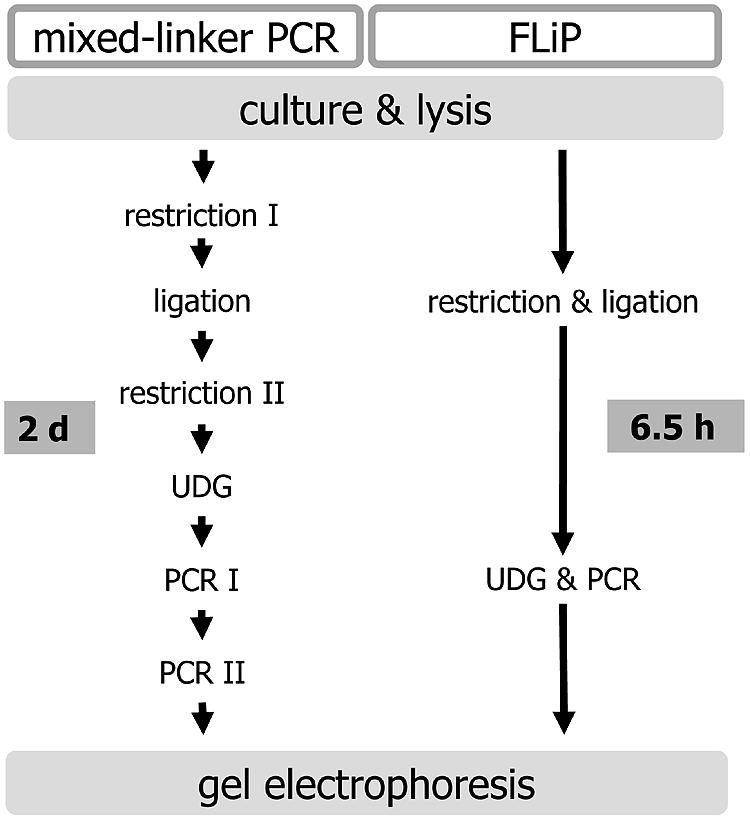
Schematic comparison of mixed-linker PCR and FLiP workflow.
The sensitivity of FLiP was determined by stepwise dilutions (1:10) of genomic DNA of M. tuberculosis H37Rv between 10 ng/μl and 10 fg/μl. A complete fingerprint pattern, suitable for computer analysis, was obtained when as little as 10 pg of purified DNA was used as template (Fig. 2).
FIG. 2.

Sensitivity analysis of FLiP using purified DNA. Lane M, 100-bp ladder; lanes 1 to 7, dilution series of H37Rv DNA at 10 ng/μl, 1 ng/μl, 100 pg/μl, 10 pg/μl, 1 pg/μl, 100 fg/μl, and 10 fg/μl; lane-K, negative control; lane +K, positive control (M. tuberculosis crude lysate).
Diversity and reproducibility of the FLiP DNA fingerprints were tested using a reference set of 131 purified DNA samples representing 90 M. tuberculosis complex strains and 10 isolates of nontuberculous mycobacteria analyzed at the RIVM (11, 11a). The analysis using the novel FLiP method was carried out in a blinded fashion. The number of bands in the FLiP patterns ranged from 0 to 16, with an average of 8 bands.
Figure 3A shows the FLiP patterns of the duplicate samples. Of 31 duplicate samples, including 2 isolates of M. tuberculosis without IS6110, 30 were correctly recognized, corresponding to a reproducibility of 97% (not statistically significant different from 100%). Repeated analysis of the two discordant samples (strains 103 and 108) after decoding of the set resulted in identical patterns, thus suggesting a labeling error during the initial analysis (Fig. 3A).
FIG.3.
A. Dendrogram of FliP duplicate DNA fingerprints among the set of 131 strains. Boxed strain numbers indicate the duplicate identified by reanalysis of the two discordant samples. B. Dendrogram of DNA fingerprints of individual M. tuberculosis complex strains. Boxed strain numbers within the M. bovis-like cluster designate two isolates that showed a single band of different size by IS6110 standard RFLP and the additional duplicate found.
FLiP typing differentiated 81 patterns among the 90 M. tuberculosis complex strains (Fig. 3A and B). Previously, IS6110 RFLP typing differentiated 84 patterns among this strain collection (11). The discriminative ability of FLiP typing was slightly lower than that of IS6110 RFLP, because one M. tuberculosis strain from The Netherlands (number 47) and one M. tuberculosis strain from India (number 36) exhibited a single band of identical size to those of the M. bovis and M. bovis BCG strains in the study (Fig. 3B), while these strains each showed a band at another position in IS6110 RFLP. FLiP typing identified one additional cluster of two strains consisting of two M. pinnipedii strains from Argentina (81 and 101) (Fig. 3B). The IS6110 RFLP patterns of these strains differed only by a single band (11).
As by standard IS6110 RFLP typing, all but two M. tuberculosis complex strains were typeable by FLiP PCR, and none of the 10 nontuberculous mycobacterial isolates yielded a DNA fingerprint pattern. A comparison of the FLiP results with those obtained by IS6110 RFLP typing and mixed-linker PCR is presented in Table 2.
TABLE 2.
Types obtained and reproducibility of three IS6110-based typing methods for differentiation of 90 M. tuberculosis complex strains and 10 non-M. tuberculosis complex strains
| Characteristic | Value for typing method (reference)
|
||
|---|---|---|---|
| IS6110 RFLP (11) | Mixed-linker PCR (11) | FLiP | |
| Reproducibility (%)a | 100 | 100 | 97 |
| No. of types obtained | 84 | 81 | 81 |
| No. of nontypeable strains | 2 | 2 | 2 |
| No. of non-M. tuberculosis complex strains | 0 | 3 | 0 |
Fraction of 31 duplicate samples.
To investigate the specificity of the patterns amplified by FLiP analysis, the typing of a subset of 20 strains was repeated, and the FLiP fragments were blotted onto a membrane. After hybridization of the membrane under stringent conditions, all fragments were recognized by the diagnostic oligonucleotide IS55, directed to the IS6110 sequence, demonstrating the specificity of the amplification (data not shown).
DISCUSSION
The mixed-linker method originally published in 1993 has been successfully applied in several outbreak investigations and population-based studies (6-9, 15, 19, 22). However, in spite of its potential to reliably differentiate between strains of M. tuberculosis complex, it has only been used by a limited number of research groups (4, 5, 12, 17). This might be due to the need for reamplification, increasing the risk of cross-contamination and multiple hands-on steps (Fig. 1). In this study, we present FLiP, a simplified and faster method based on the mixed-linker approach, which allows IS6110 strain typing within 6.5 h from less than 1 ng of mycobacterial DNA. The cost per sample is likely to be less than that of IS6110 RFLP typing, mainly depending on the number of samples analyzed in one batch. In contrast to mixed-linker PCR, no nonspecific amplification from mycobacteria other than M. tuberculosis complex was observed (11). The limitations of FLiP are similar to all IS6110-based methods: comparison between different strains is based on pattern recognition, and the method is not informative for low-copy-number strains (containing less than five IS6110 elements) or strains lacking IS6110.
The ability to differentiate 81 out of 84 different IS6110 RFLP patterns and its high reproducibility is only reached by the newly developed variable-number tandem repeat (VNTR) typing methods (11, 11a, 14, 18). However, a standardized set of VNTR loci best suitable for outbreak investigations has not been published so far. Also, strain resolution by VNTR typing differs according to the loci analyzed and between different strain families (11, 11a, 18). In addition, VNTR methods require multiple PCR amplifications per sample that have to be analyzed separately. This only can be overcome by a high degree of apparative resources. For achieving a similar degree of differentiation, such as by using FLiP, 12 or more genomic loci are analyzed by automated high-throughput mycobacterial interspersed repetitive unit genotyping. Therefore, a method with an intermediate differentiation ability by focusing on only six loci has been suggested recently to allow analysis also in areas with fewer resources (16). Spoligotyping, which has been widely applied for strain typing, has a much lower resolution capacity for different strains (11). FLiP uses technology that is available in most mycobacteriology laboratories today, where amplification-based techniques for diagnostic purposes are established. It is relatively inexpensive and seems therefore well suited for rapid analysis of presumed outbreaks or laboratory cross-contaminations. Using fluorescent primers, it also could be adapted for automated analysis, as has been demonstrated for mixed-linker PCR (2). It may as well serve as a secondary typing technique for other, non-IS6110-based methods.
Acknowledgments
Sources for M. tuberculosis H37Rv were the Centers for Disease Control and Prevention (Atlanta, Ga.) and the Hygiene Institut (University of Heidelberg, Heidelberg, Germany). The positive control was a M. tuberculosis strain obtained from M. Burger (Corichiba, Brazil).
This study was carried out within the framework of the Concerted Action project “Next generation genetic markers and techniques to study the epidemiology and control of tuberculosis,” supported by European Union grant QLK2-CT-2000-00630. This work was also supported by research grant HA 1921 3-1 and 3-2 from the Deutsche Forschungsgemeinschaft.
REFERENCES
- 1.Barnes, P. F., and M. D. Cave. 2003. Molecular epidemiology of tuberculosis. N. Engl. J. Med. 349:1149-1156. [DOI] [PubMed] [Google Scholar]
- 2.Butler, W. R., W. H. Haas, and J. T. Crawford. 1996. Automated DNA fingerprinting analysis of Mycobacterium tuberculosis using fluorescent detection of PCR products. J. Clin. Microbiol. 34:1801-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crawford, J. T. 2003. Genotyping in contact investigations: a CDC perspective. Int. J. Tuberc. Lung Dis. 7:S453-S457. [PubMed] [Google Scholar]
- 4.Fomukong, N., M. Beggs, H. El-Hajj, G. Templeton, K. Eisenach, and M. D. Cave. 1998. Differences in the prevalence of IS6110 insertion sites in Mycobacterium tuberculosis strains: low and high copy number of IS6110. Tuber. Lung Dis. 78:109-116. [DOI] [PubMed] [Google Scholar]
- 5.Gascoyne-Binzi, D. M., R. E. Barlow, A. Essex, R. Gelletlie, M. A. Khan, S. Hafiz, T. A. Collyns, R. Frizzell, and P. M. Hawkey. 2002. Predominant VNTR family of strains of Mycobacterium tuberculosis isolated from South Asian patients. Int. J. Tuberc. Lung Dis. 6:492-496. [DOI] [PubMed] [Google Scholar]
- 6.Haas, W. H., G. Bretzel, B. Amthor, K. Schilke, G. Krommes, S. Rusch-Gerdes, V. Sticht-Groh, and H. J. Bremer. 1997. Comparison of DNA fingerprint patterns of isolates of Mycobacterium africanum from east and west Africa. J. Clin. Microbiol. 35:663-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haas, W. H., W. R. Butler, C. L. Woodley, and J. T. Crawford. 1993. Mixed-linker polymerase chain reaction: a new method for rapid fingerprinting of isolates of the Mycobacterium tuberculosis complex. J. Clin. Microbiol. 31:1293-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haas, W. H., G. Engelmann, B. Amthor, S. Shyamba, F. Mugala, M. Felten, M. Rabbow, M. Leichsenring, O. J. Oosthuizen, and H. J. Bremer. 1999. Transmission dynamics of tuberculosis in a high-incidence country: prospective analysis by PCR DNA fingerprinting. J. Clin. Microbiol. 37:3975-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Horn, D. L., D. Hewlett, Jr., W. H. Haas, W. R. Butler, C. Alfalla, E. Tan, A. Levine, A. Nayak, and S. M. Opal. 1994. Superinfection with rifampicin-isonoazid-streptomycin-ethambutol (RISE)-resistant tuberculosis in three patients with AIDS: confirmation by polymerase chain reaction fingerprinting. Ann. Intern. Med. 121:115-116. [DOI] [PubMed] [Google Scholar]
- 10.Jereb, J. A., D. R. Burwen, S. W. Dooley, W. H. Haas, J. T. Crawford, L. J. Geiter, M. B. Edmond, J. N. Dowling, R. Shapiro, and A. W. Pasculle. 1993. Nosocomial outbreak of tuberculosis in a renal transplant unit: application of a new technique for restriction fragment length polymorphism analysis of Mycobacterium tuberculosis isolates. J. Infect. Dis. 168:1219-1224. [DOI] [PubMed] [Google Scholar]
- 11.Kremer, K., D. van Soolingen, R. Frothingham, W. H. Haas, P. W. Hermans, C. Martin, P. Palittapongarnpim, B. B. Plikaytis, L. W. Riley, M. A. Yakrus, J. M. Musser, and J. D. van Embden. 1999. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J. Clin. Microbiol. 37:2607-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11a.Kremer, K., C. Arnold, A. Cataldi, M. C. Gutierrez, W. H. Haas, S. Panaiotov, R. A. Skuce, P. Supply, A. G. M. van der Zanden, and D. van Soolingen. 2005. Discriminatory power and reproducibility of novel DNA typing methods for Mycobacterium tuberculosis complex strains. J. Clin. Microbiol. 43:5628-5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller, A. C., W. R. Butler, B. McInnis, J. Boutotte, S. Etkind, S. Sharnprapai, J. Bernardo, J. Driscoll, M. McGarry, J. T. Crawford, and E. Nardell. 2002. Clonal relationships in a shelter-associated outbreak of drug-resistant tuberculosis: 1983-1997. Int. J. Tuberc. Lung Dis. 6:872-878. [PubMed] [Google Scholar]
- 13.Narayanan, S. 2004. Molecular epidemiology of tuberculosis. Indian J. Med. Res. 120:233-247. [PubMed] [Google Scholar]
- 14.Roring, S., A. Scott, D. Brittain, I. Walker, G. Hewinson, S. Neill, and R. Skuce. 2002. Development of variable-number tandem repeat typing of Mycobacterium bovis: comparison of results with those obtained by using existing exact tandem repeats and spoligotyping. J. Clin. Microbiol. 40:2126-2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schilke, K., K. Weyer, G. Bretzel, B. Amthor, J. Brandt, V. Sticht-Groh, P. B. Fourie, and W. H. Haas. 1999. Universal pattern of RpoB gene mutations among multidrug-resistant isolates of Mycobacterium tuberculosis complex from Africa. Int. J. Tuberc. Lung Dis. 3:620-626. [PubMed] [Google Scholar]
- 16.Sola, C., I. Filliol, E. Legrand, S. Lesjean, C. Locht, P. Supply, and N. Rastogi. 2003. Genotyping of the Mycobacterium tuberculosis complex using MIRUs: association with VNTR and spoligotyping for molecular epidemiology and evolutionary genetics. Infect. Genet. Evol. 3:125-133. [DOI] [PubMed] [Google Scholar]
- 17.Steinlein, L. M., and J. T. Crawford. 2001. Reverse dot blot assay (insertion site typing) for precise detection of sites of IS6110 insertion in the Mycobacterium tuberculosis genome. J. Clin. Microbiol. 39:871-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Supply, P., S. Lesjean, E. Savine, K. Kremer, D. van Soolingen, and C. Locht. 2001. Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J. Clin. Microbiol. 39:3563-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Theisen, A., C. Reichel, S. Rüsch-Gerdes, W. H. Haas, J. K. Rockstroh, U. Spengler, and T. Sauerbruch. 1995. Mixed-strain infection with a drug-sensitive and multidrug-resistant strain of Mycobacterium tuberculosis. Lancet 345:1512-1513. [DOI] [PubMed] [Google Scholar]
- 20.van Embden, J. D. A., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martín, R. McAdam, T. M. Shinnick, and P. M. Small. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Soolingen, D. 2001. Molecular epidemiology of tuberculosis and other mycobacterial infections: main methodologies and achievements. J. Intern. Med. 249:1-26. [DOI] [PubMed] [Google Scholar]
- 22.Zaza, S., H. M. Blumberg, C. Beck-Sagué, W. H. Haas, C. L. Woodley, M. Pineda, C. Parrish, J. T. Crawford, J. E. McGowan, Jr., and W. R. Jarvis. 1995. Nosocomial transmission of Mycobacterium tuberculosis: role of health care workers in outbreak propagation. J. Infect. Dis. 172:1542-1549. [DOI] [PubMed] [Google Scholar]




