Abstract
The aim of this study was to determine antimicrobial resistance, to evaluate and compare the use of two genotyping methods for molecular epidemiology purposes, and to determine the genotypic diversity of Campylobacter coli of porcine origin. A total of 100 C. coli isolates from swine were tested for susceptibility to six antimicrobials using the agar dilution method and genotyped using two high-resolution fingerprinting approaches: multilocus sequence typing (MLST) and pulsed-field gel electrophoresis (PFGE). Evaluation of the methods was based on their resistance patterns, discriminatory indexes (DI), high test throughputs, costs, and turnaround times. Resistance to erythromycin and tetracycline was the most common. Both genotypic methods were found to have high discriminatory power, although MLST had a higher DI (0.936) than PFGE (DI = 0.889). It also had a higher throughput than PFGE. Isolates were clustered into 27 groups by MLST compared to 11 by PFGE. MLST was able to further discriminate the isolates grouped under the same cluster by PFGE. Out of the 65 MLST sequence types (STs) identified among the total isolates, 50 were reported for the first time. Most STs were found to be specific to the farm (n = 38) and to slaughter (n = 22). Resistance against tetracycline and erythromycin was encoded by the tet(O) gene and a A2075G point mutation in the 23S rRNA gene, respectively. A high ciprofloxacin MIC (>64 μg/liter) was conferred by a point mutation in the gyrA gene. The weak clonal structure of the C. coli population among swine was further highlighted by the index of association value of 0.293. The findings of this study indicate that multidrug-resistant diverse C. coli strains exhibiting resistance to ciprofloxacin and erythromycin are concerning, since these are the drugs of choice for treating invasive campylobacteriosis cases in humans.
Food-borne pathogens result in approximately 76 million illnesses in the United States every year (27). Campylobacter is the most common cause of bacterial gastroenteritis with an estimated 2.5 million cases annually in the United States and in 54,000 cases in the United Kingdom (1, 27). The preliminary data, as estimated by FoodNet, for diseases caused by enteric pathogens for the year 2004 show Campylobacter to have an incidence of 12.9 per 100,000 persons (2). Campylobacter jejuni in humans is considered to be the most important Campylobacter species, causing 95% of the food-borne infections. Data from the United Kingdom implicated C. jejuni as being responsible for 91.9% of the cases, followed by Campylobacter coli with 7.9% (6).
Poultry has been recognized as the primary reservoir of C. jejuni, while pigs are mostly implicated as reservoirs of C. coli (19, 20). Recent studies conducted in Spain and the United Kingdom have highlighted the importance of C. coli as an important human pathogen due to its ability to show increased resistance to greater number of antimicrobials and because it causes more indigenously acquired food-borne diseases than Salmonella enterica serovar Typhimurium (40, 44). C. coli has been suggested to be particularly suited to the swine production environment and has been isolated from pigs on farms in up to 100% of the samples collected (40). Epidemiological evidence has suggested the zoonotic transmission of this pathogen from animals to humans (3, 25). Although most of the human cases are sporadic in nature, outbreaks caused by Campylobacter spp. have been reported in the past, caused by the consumption of raw milk and contaminated water (30, 39).
It is important to use typing methods that have high discriminatory power to identify and differentiate sources of this pathogen in animals, humans, and the environment. Many phenotypic and genotypic methods have been developed for Campylobacter (32). Multilocus sequence typing (MLST) is one such genotypic method that is based on indexing the genetic variation in housekeeping genes (7). This technique has been successfully employed for studying the longitudinal epidemiology of C. jejuni in human, animal, and environmental samples (5). Recently, the standardization of the MLST typing method was extended to C. coli (8). However, no molecular epidemiological study has been reported using MLST for C. coli. Pulsed-field gel electrophoresis (PFGE) is another genotyping method that has been used for investigating C. jejuni outbreaks and genotyping C. coli (21, 28, 34, 42). Although this method is highly discriminatory, interlaboratory comparisons could be difficult due to complex protocols and accessibility of equipment and software for analyzing the patterns in multiple laboratories.
Prompted by the importance of C. coli as a food-borne pathogen and the paucity of molecular epidemiological information on this species, we conducted this study. The aim of this study was to evaluate and compare the use of MLST and PFGE for genotyping C. coli isolates from swine based on discriminatory power, throughput, cost, and time. In this study, we also determined the resistance mechanisms coding for predominant antimicrobial resistance phenotypes and show evidence of the clonal structure of C. coli by measuring the index of association (IA).
MATERIALS AND METHODS
Origin of C. coli strains.
A total of 100 isolates were randomly selected from 1,459 C. coli isolates that were isolated as a part of a cross-sectional study conducted on swine farms and slaughter plants (46). The selected strains were from the conventional and antimicrobial-free (ABF) production systems representative of the processing stages at farm and slaughter, and the resistance patterns were observed during the entire study. Briefly, a total of 21 groups of pigs that belong to two distinct production systems (11 conventional and 10 ABF farms) were sampled at the nursery farms (6 weeks of age) and finishing farms (within 48 h of slaughter) followed by carcass swab sampling at the slaughter plant. Collection of swab samples at slaughter was done at three stages, including preevisceration, postevisceration, and postchill. Prior to testing, the isolates were recovered from storage at −80°C and streaked on Mueller-Hinton agar supplemented with 5% sheep blood. All the incubations were done under microaerobic conditions at 42°C for 48 h. Antimicrobial susceptibility testing was done using the agar dilution method as described below. Genotyping of the C. coli isolates was done by MLST and PFGE as described later in this section.
Antimicrobial susceptibility testing.
The agar dilution method was used as recommended by the National Committee for Clinical Laboratory Standards (NCCLS) subcommittee on Veterinary Antimicrobial Susceptibility Testing for determining the susceptibility of Campylobacter isolates to different antimicrobials (31). We tested the isolates for their susceptibility against a panel of six antimicrobials. The following is a list of antimicrobials with their abbreviations and ranges of concentrations used: chloramphenicol (CHL; 0.25 to 128 μg/liter), ciprofloxacin (CIP; 0.008 to 4 μg/liter), erythromycin (ERY; 0.06 to 32 μg/liter), gentamicin (GEN; 0.06 to 32 μg/liter), nalidixic acid (NAL; 0.25 to 128 μg/liter), and tetracycline (TET; 0.06 to 32 μg/liter) (14). All the antimicrobials were procured from Sigma (Sigma, MO) except ciprofloxacin (Serologicals Proteins, Inc., IL). The NCCLS breakpoint interpretative criteria for the Enterobacteriaceae family were used for all the antimicrobials except erythromycin as recommended by NCCLS, as the interpretive standard breakpoint levels for the Campylobacteriaceae family are not yet available (14). For erythromycin (8 μg/liter), the breakpoint level used by the National Antimicrobial Resistance Monitoring System was adopted (12). C. jejuni ATCC 33560 was used as the quality control organism for this test (14). The MIC50 breakpoints used for each antimicrobial were as follows: chloramphenicol (32 μg/liter), ciprofloxacin (4 μg/liter), erythromycin (8 μg/liter), gentamicin (16 μg/liter), nalidixic acid (32 μg/liter), and tetracycline (16 μg/liter). Campylobacter isolates showing resistance against ciprofloxacin at the concentration of 4 μg/liter (the highest on the original panel) were further tested at higher concentrations of the antimicrobial up to 64 μg/liter. Multidrug resistance here is defined as isolates exhibiting resistance against three or more antimicrobials simultaneously.
MLST.
Genomic DNA purification of the isolates for sequencing was done using a QIAGEN DNA purification kit (QIAGEN, Valencia, CA). MLST of the seven housekeeping genes (aspA, glnA, gltA, glyA, pgm, tkt, and uncA) for C. coli was done following the method described previously (8). Briefly, all the housekeeping genes were amplified using PCR, and the products were run on agarose gel to confirm the correct amplicon size. Purification of the PCR products was done using a QiaAmp PCR purification kit following the manufacturer's instructions (QIAGEN, Valencia, CA). Sequencing reactions using the forward and reverse primers in separate wells was done using 2 μl of the BigDye Ready Reaction mix (version 3.1; Applied Biosystems, Foster City, California), 0.5 μl of 1:15 diluted primer, 5.5 μl of molecular grade deionized water, and 2 μl of the purified PCR product. The sequencing reaction was performed on an automated 3700 ABI capillary sequencer (Applied Biosystems, Foster City, California) with running conditions of 30 cycles of 96°C for 10 s, 50°C for 5 s, and 60°C for 4 min. We used the ClustalW program for aligning the forward and reverse sequences (www.ebi.ac.uk/clustalw). The allelic profiles and the sequence types (STs) were then generated by blasting the correct sequence size on the MLST website from the Campylobacter database (accessible at http://www.pubmlst.org/campylobacter or http://www.mlst.net). A dendrogram for the MLST data was generated using the START software (23).
PFGE.
PFGE was done following the rapid protocol for Campylobacter (38). Briefly, 400-ml overnight culture cells were lysed, and intact genomic DNA was digested in agarose-embedded plugs with SmaI restriction enzyme. The digested DNA was then separated by using a contour-clamped homogeneous electric field (CHEF)-DRIII (Bio-Rad Laboratories, Hercules, CA) with the following conditions: 0.5× Tris-borate-EDTA, 1% SeaKem Gold agarose (FMC BioProducts, Rockland, ME), 14°C, 6 V/cm for 18 h with switch times ranging from 6.75 to 38.35 s with an included angle of 120°. S. enterica serovar Braenderup 17 Universal Marker (kindly provided by Leslie Wolf, North Carolina State Laboratory of Public Health, NC) was used as the reference marker. Gels were stained with ethidium bromide for 30 min, destained three times for 20 min each with distilled water, and photographed using an Alpha imager (Alpha 20 Innotech Corporation, San Leandro, CA). Analysis of PFGE data was performed using Bionumerics software (Applied Maths, Kortrijik, Belgium) using a “different bands” algorithm for clustering and the Ward algorithm of tree building approach with 0.47% optimization and 0.48% position tolerance determined by using procedures recommended by the manufacturer. Visual inspection of the patterns was performed as a final step for analysis.
Characterization of resistance genes.
A total of 21 multidrug-resistant C. coli isolates were selected for further molecular characterization to determine the resistance genes coding for antimicrobial resistance against tetracycline and erythromycin and the class I integron. PCR was used to target the tet(O) and tet(M) genes for tetracycline resistance and a mutation at position 2075 in the 23S rRNA for erythromycin resistance, as explained previously (15, 16, 35). Class I integrons were targeted in these isolates following the method described by O'Halloran et al. (33). Targeting of the threonine-to-isoleucine point mutation in the quinolone resistance-determining region (QRDR) of the gyrA gene conferring resistance to ciprofloxacin resistance was done using the mismatch mutation amplification assay (MAMA) PCR (49). Amplification reactions were carried out with 1 μl of purified DNA (QIAGEN DNAeasy tissue kit; QIAGEN, Valencia, CA), 300 μM deoxynucleoside triphosphate, 2.5 mM MgCl2, 50 pmol of primers, and 0.5 U of Gold Taq polymerase (Perkin-Elmer, Foster City, CA). Distilled water was added to bring the final volume to 20 μl. The PCR cycle included initial denaturation at 95°C for 5 min and 30 cycles of denaturation for 1 min at 95°C, primer annealing for 1 min at 54°C, and extension for 1 min at 72°C.
Data analysis.
Simpson's index of diversity was calculated to compare the discriminatory power of the two genotyping methods used in this study (22). The IA was determined using the START program to assess the clonality of the population (26). An absolute value of zero (IA = 0) indicates that the population is freely recombining and is not clonal. A value of 1.0 indicates the high genetic diversity of isolates.
RESULTS
Diversity of sequence types across the two production systems based on MLST.
Assignment of the allele frequencies and STs was done using algorithms described in the Campylobacter MLST database (accessible at http://www.pubmlst.org/campylobacter or http://www.mlst.net). A total of 65 STs were generated from sequence typing of 100 C. coli isolates with 47 STs occurring singly and ST-1413 being the most common seen in seven isolates from the carcasses of ABF pigs. Based on the allelic profiles of the housekeeping genes, 50 new STs were assigned for the first time after submitting the information to the MLST database. Twenty-four out of the 50 new STs originated from the ABF swine production units. The remaining 26 new STs were from C. coli isolates from the conventional production system. Within individual production systems, we observed STs that were found specific to the processing stages either at the farm or slaughter, with 38 out of the 65 STs found to be specific to the farm and another 22 found only at the slaughter stage (Table 1). The three most predominant STs occurring in the database included ST-1413 (7 isolates), ST-854 (6 isolates), and ST-1123 (5 isolates), representing 18% of the isolates. Multiple STs were generated for individual antimicrobial resistance patterns, with the majority being specific either to the farm or the slaughter stage (Table 1). For instance, ST-1413 (n = 7) was observed only among the C. coli ciprofloxacin-resistant strains (resistance pattern: CIP ERY NAL TET) isolated from the conventional nursery pigs. However, two additional isolates from the same farm and sharing the same resistance pattern as above had different STs (ST-1096, cluster 9; and ST-19, cluster 19).
TABLE 1.
Total number of C. coli isolates under each production system including the MLST sequence types and PFGE clusters
| Production type (isolates tested) | Processing stage | No. of strains | No. of STs (% diversity) | No. of new STs (%)a | No. of unique STs (%)b | PFGE cluster(s) (n)c |
|---|---|---|---|---|---|---|
| ABF (50) | Nursery | 9 | 8 (89) | 4 (50) | 4 (50) | 1, 3, 4, 5, 10, 11 (6) |
| Finishing | 27 | 20 (74) | 11 (55) | 11 (55) | 1, 2, 3, 4, 5, 7, 8, 9, 11 (9) | |
| Preevisceration | 5 | 5 (100) | 5 (100) | 3 (60) | 1, 8, 9 (3) | |
| Postevisceration | 7 | 6 (86) | 3 (50) | 3 (50) | 1, 5, 8 (3) | |
| Postchill | 2 | 2 (100) | 1 (50) | 1 (50) | 8 (1) | |
| Conventional (50) | Nursery | 14 | 8 (57) | 7 (88) | 6 (75) | 1, 5, 8, 9, 10 (5) |
| Finishing | 21 | 18 (86) | 12 (67) | 13 (72) | 1, 2, 3, 4, 9, 11 (6) | |
| Preevisceration | 5 | 5 (100) | 4 (80) | 4 (80) | 2, 3 (2) | |
| Postevisceration | 9 | 9 (100) | 2 (22) | 7 (78) | 3, 6, 8, 9 (4) | |
| Postchill | 1 | 1 (100) | 1 (100) | 1 (100) | 2 (1) |
Sequence types that have been reported for the first time.
Sequence types that were found only in the specific processing stage.
Total PFGE clusters and the cluster numbers where the sequence types were grouped.
Based on the MLST dendrogram generated by the START program (data not shown), we observed a total of 27 clusters, with cluster 9 being the largest (n = 18) (Table 2). Seven isolates were represented by single branches and occurred independently without being a part of any group. A majority of the isolates (n = 16) in cluster 9 were from the nursery and finishing farms and included 8 out of the 15 ciprofloxacin-resistant C. coli isolates. Clusters 2, 5, 6, 19, and 21 were comprised of isolates from the farm, while clusters 7, 8, and 24 included slaughter isolates only. Multiple STs were found among isolates from a single farm at different stages of processing both at farm and slaughter. For example, isolates 2003 and 2032 with the tetracycline resistance pattern (TET) were isolated from the same ABF farm but were associated with ST-890 and -825, respectively. Similarly, isolates 3490 and 3491 were from the same pig (TET resistant) reared in the conventional system but were associated with two different STs, ST-1130 and ST-854, respectively.
TABLE 2.
MLST and PFGE data for the 100 C. coli isolates
| Production system | Processing stage | Resistance patternb | Isolate ID | Allelic profile
|
STc | MLST cluster | PFGE cluster | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aspA | glnA | gltA | glyA | pgm | tkt | uncA | |||||||
| ABF | Nursery | NAL | 53 | 33 | 39 | 30 | 78 | 104 | 43 | 17 | 1068 | 4 | 5 |
| ABF | Nursery | Pansusceptible | 183 | 33 | 39 | 30 | 82 | 104 | 43 | 68 | 1112 | 22 | 11 |
| ABF | Nursery | Pansusceptible | 752 | 33 | 38 | 30 | 82 | 104 | 173 | 68 | 1134 | 18 | 11 |
| ABF | Nursery | ERY TET | 26 | 33 | 39 | 37 | 82 | 104 | 43 | 68 | 1414 | 21 | 11 |
| ABF | Nursery | Pansusceptible | 252 | 33 | 39 | 37 | 82 | 104 | 43 | 68 | 1414 | 21 | 3 |
| ABF | Nursery | TET | 762 | 33 | 39 | 30 | 174 | 118 | 35 | 17 | 1431 | 6 | 1 |
| ABF | Nursery | ERY NAL TET | 7 | 33 | 39 | 46 | 82 | 104 | 43 | 68 | 1437 | 21 | 1 |
| ABF | Nursery | CIP NAL TET | 260 | 33 | 153 | 30 | 82 | 104 | 44 | 17 | 1438 | 15 | 10 |
| ABF | Nursery | TET | 206 | 33 | 38 | 30 | 82 | 104 | 43 | 17 | 854 | 4 | 4 |
| ABF | Finishing | TET | 4010 | 33 | 38 | 30 | 82 | 104 | 43 | 17 | 854 | 4 | 4 |
| ABF | Finishing | TET | 4082 | 33 | 38 | 30 | 82 | 104 | 43 | 17 | 854 | 4 | 4 |
| ABF | Finishing | TET | 2003 | 33 | 38 | 30 | 82 | 104 | 35 | 36 | 890 | 9 | 3 |
| ABF | Finishing | ERY NAL TET | 2112 | 33 | 38 | 30 | 82 | 104 | 35 | 17 | 1096 | 9 | 3 |
| ABF | Finishing | ERY | 793 | 33 | 38 | 30 | 82 | 152 | 173 | 68 | 1102 | 18 | 11 |
| ABF | Finishing | ERY TET | 786 | 33 | 39 | 30 | 82 | 104 | 43 | 68 | 1112 | 22 | 2 |
| ABF | Finishing | Pansusceptible | 3518 | 53 | 38 | 44 | 82 | 118 | 35 | 36 | 1123 | 13 | 2 |
| ABF | Finishing | Pansusceptible | 3978 | 53 | 38 | 44 | 82 | 118 | 35 | 36 | 1123 | 13 | 8 |
| ABF | Finishing | NAL TET | 953 | 33 | 38 | 30 | 82 | 104 | 173 | 68 | 1134 | 18 | 11 |
| ABF | Finishing | Pansusceptible | 2059 | 33 | 38 | 30 | 82 | 104 | 173 | 68 | 1134 | 18 | 11 |
| ABF | Finishing | Pansusceptible | 3819 | 33 | 38 | 30 | 82 | 104 | 173 | 68 | 1134 | 18 | 11 |
| ABF | Finishing | TET | 4115 | 53 | 38 | 30 | 82 | 118 | 35 | 36 | 1200 | 13 | 8 |
| ABF | Finishing | CIP NAL TET | 4076 | 33 | 39 | 132 | 82 | 104 | 44 | 17 | 1424 | 3 | 7 |
| ABF | Finishing | Pansusceptible | 3969 | 53 | 38 | 30 | 81 | 118 | 85 | 36 | 1426 | 1 | 11 |
| ABF | Finishing | ERY TET | 3995 | 33 | 39 | 30 | 79 | 104 | 56 | 17 | 1427 | 5 | 1 |
| ABF | Finishing | ERY | 1987 | 33 | 39 | 30 | 82 | 118 | 35 | 36 | 1432 | 12 | 5 |
| ABF | Finishing | ERY NAL TET | 952 | 33 | 39 | 134 | 82 | 104 | 56 | 17 | 1433 | 5 | 1 |
| ABF | Finishing | TET | 4077 | 33 | 39 | 44 | 82 | 104 | 44 | 17 | 1436 | 3 | 7 |
| ABF | Finishing | ERY TET | 1010 | 33 | 38 | 30 | 82 | 118 | 35 | 17 | 1446 | 9 | 3 |
| ABF | Finishing | ERY TET | 4017 | 33 | 38 | 30 | 82 | 118 | 35 | 17 | 1446 | 9 | 3 |
| ABF | Finishing | Pansusceptible | 1038 | 33 | 38 | 32 | 82 | 104 | 35 | 68 | 1447 | 18 | 11 |
| ABF | Finishing | ERY TET | 2164 | 53 | 39 | 44 | 82 | 118 | 35 | 17 | 1448 | 11 | 8 |
| ABF | Finishing | ERY TET | 2191 | 53 | 39 | 44 | 82 | 104 | 35 | 36 | 1450 | 10 | 1 |
| ABF | Finishing | NAL | 3552 | 53 | 39 | 44 | 82 | 104 | 35 | 36 | 1450 | 10 | 8 |
| ABF | Finishing | CIP NAL TET | 4075 | 33 | 39 | 46 | 82 | 104 | 44 | 17 | 1452 | 3 | 7 |
| ABF | Finishing | Pansusceptible | 4100 | 33 | 39 | 46 | 82 | 104 | 44 | 17 | 1452 | 3 | 7 |
| ABF | Finishing | TET | 2032 | 33 | 39 | 30 | 82 | 113 | 47 | 17 | 825 | 2 | 9 |
| ABF | Preevisceration | ERY | 794 | 33 | 39 | 47 | 82 | 104 | 43 | 36 | 1415 | 22 | 11 |
| ABF | Preevisceration | Pansusceptible | 4029 | 33 | 39 | 30 | 82 | 118 | 35 | 36 | 1432 | 12 | 8 |
| ABF | Preevisceration | Pansusceptible | 843 | 33 | 39 | 30 | 82 | 104 | 85 | 17 | 1445 | 4 | 1 |
| ABF | Preevisceration | Pansusceptible | 3580 | 53 | 39 | 44 | 82 | 104 | 35 | 36 | 1450 | 10 | 8 |
| ABF | Preevisceration | Pansusceptible | 3600 | 53 | 39 | 37 | 82 | 104 | 85 | 17 | 1451 | 25 | 1 |
| ABF | Postevisceration | ERY TET | 4037 | 33 | 38 | 30 | 82 | 104 | 35 | 36 | 890 | 9 | 1 |
| ABF | Postevisceration | TET | 2205 | 33 | 38 | 30 | 78 | 104 | 35 | 17 | 1113 | 8 | 5 |
| ABF | Postevisceration | TET | 4042 | 53 | 38 | 44 | 82 | 118 | 35 | 36 | 1123 | 13 | 8 |
| ABF | Postevisceration | TET | 4049 | 53 | 38 | 44 | 82 | 118 | 35 | 36 | 1123 | 13 | 8 |
| ABF | Postevisceration | ERY | 817 | 33 | 153 | 30 | 82 | 118 | 43 | 17 | 1416 | 15 | 1 |
| ABF | Postevisceration | ERY | 4055 | 33 | 39 | 30 | 82 | 118 | 35 | 36 | 1432 | 12 | 8 |
| ABF | Postevisceration | TET | 2207 | 33 | 38 | 37 | 78 | 104 | 35 | 17 | 1449 | 8 | 5 |
| ABF | Postchill | Pansusceptible | 3635 | 33 | 39 | 30 | 82 | 104 | 43 | 17 | 828 | 4 | 8 |
| ABF | Postchill | ERY | 3630 | 53 | 38 | 30 | 81 | 118 | 35 | 36 | 1469 | 1 | 8 |
| Conva | Nursery | CIP ERY NAL TET | 475 | 33 | 38 | 30 | 82 | 104 | 35 | 17 | 1096 | 9 | 5 |
| Conv | Nursery | CIP ERY NAL TET | 524 | 33 | 38 | 30 | 82 | 104 | 117 | 17 | 1413 | 9 | 10 |
| Conv | Nursery | CIP ERY NAL TET | 525 | 33 | 38 | 30 | 82 | 104 | 117 | 17 | 1413 | 9 | 1 |
| Conv | Nursery | CIP ERY NAL TET | 549 | 33 | 38 | 30 | 82 | 104 | 117 | 17 | 1413 | 9 | 9 |
| Conv | Nursery | CIP ERY NAL TET | 552 | 33 | 38 | 30 | 82 | 104 | 117 | 17 | 1413 | 9 | 9 |
| Conv | Nursery | CIP ERY NAL TET | 554 | 33 | 38 | 30 | 82 | 104 | 117 | 17 | 1413 | 9 | 10 |
| Conv | Nursery | CIP ERY NAL TET | 555 | 33 | 38 | 30 | 82 | 104 | 117 | 17 | 1413 | 9 | 10 |
| Conv | Nursery | CIP ERY NAL TET | 556 | 33 | 38 | 30 | 82 | 104 | 117 | 17 | 1413 | 9 | 10 |
| Conv | Nursery | ERY | 84 | 33 | 39 | 44 | 82 | 104 | 35 | 36 | 1417 | 10 | 8 |
| Conv | Nursery | ERY TET | 503 | 33 | 39 | 46 | 174 | 104 | 35 | 17 | 1418 | 6 | 1 |
| Conv | Nursery | CIP ERY GEN NAL TET | 548 | 33 | 38 | 46 | 82 | 104 | 117 | 17 | 1419 | 19 | 9 |
| Conv | Nursery | CIP ERY GEN NAL TET | 461 | 33 | 39 | 46 | 82 | 113 | 35 | 17 | 1440 | 2 | 1 |
| Conv | Nursery | CIP ERY NAL TET | 526 | 33 | 38 | 37 | 82 | 104 | 117 | 17 | 1441 | 19 | 11 |
| Conv | Nursery | ERY GEN TET | 533 | 33 | 39 | 30 | 82 | 113 | 117 | 17 | 1465 | 2 | 1 |
| Conv | Finishing | ERY TET | 3441 | 33 | 38 | 30 | 82 | 104 | 43 | 17 | 854 | 4 | 2 |
| Conv | Finishing | TET | 3736 | 33 | 38 | 30 | 82 | 104 | 43 | 17 | 854 | 4 | 4 |
| Conv | Finishing | ERY TET | 4246 | 33 | 39 | 30 | 82 | 104 | 43 | 36 | 1056 | 22 | 2 |
| Conv | Finishing | ERY TET | 827 | 33 | 153 | 30 | 82 | 104 | 35 | 17 | 1059 | 15 | 3 |
| Conv | Finishing | CHL ERY TET | 4511 | 33 | 38 | 30 | 82 | 104 | 35 | 17 | 1096 | 9 | 3 |
| Conv | Finishing | ERY | 872 | 33 | 38 | 30 | 82 | 104 | 85 | 17 | 1177 | 9 | 3 |
| Conv | Finishing | ERY TET | 4489 | 33 | 39 | 44 | 82 | 104 | 35 | 36 | 1417 | 10 | 2 |
| Conv | Finishing | TET | 648 | 32 | 39 | 115 | 115 | 104 | 85 | 17 | 1420 | 26 | 1 |
| Conv | Finishing | ERY TET | 423 | 33 | 38 | 46 | 82 | 104 | 173 | 17 | 1422 | 19 | 11 |
| Conv | Finishing | ERY TET | 429 | 33 | 39 | 46 | 82 | 104 | 47 | 17 | 1423 | 3 | 1 |
| Conv | Finishing | CHL CIP ERY NAL TET | 4516 | 33 | 39 | 134 | 174 | 104 | 43 | 68 | 1425 | 21 | 1 |
| Conv | Finishing | CHL CIP ERY NAL TET | 4517 | 33 | 39 | 134 | 174 | 104 | 43 | 68 | 1425 | 21 | 1 |
| Conv | Finishing | ERY TET | 841 | 33 | 39 | 44 | 82 | 189 | 35 | 36 | 1429 | 10 | 2 |
| Conv | Finishing | TET | 4396 | 32 | 153 | 30 | 82 | 104 | 44 | 36 | 1430 | 16 | 2 |
| Conv | Finishing | TET | 448 | 32 | 38 | 44 | 82 | 104 | 43 | 17 | 1434 | 23 | 2 |
| Conv | Finishing | ERY TET | 4490 | 33 | 153 | 30 | 82 | 104 | 44 | 17 | 1438 | 15 | 9 |
| Conv | Finishing | ERY NAL TET | 458 | 33 | 38 | 30 | 82 | 104 | 173 | 17 | 1439 | 9 | 11 |
| Conv | Finishing | ERY NAL TET | 459 | 33 | 38 | 30 | 82 | 104 | 173 | 17 | 1439 | 9 | 11 |
| Conv | Finishing | ERY TET | 623 | 33 | 38 | 132 | 82 | 104 | 85 | 17 | 1442 | 19 | 3 |
| Conv | Finishing | TET | 653 | 33 | 153 | 30 | 173 | 217 | 44 | 68 | 1466 | 14 | 11 |
| Conv | Finishing | ERY | 3451 | 33 | 39 | 30 | 82 | 113 | 47 | 17 | 825 | 2 | 9 |
| Conv | Preevisceration | ERY TET | 3480 | 33 | 38 | 30 | 82 | 104 | 35 | 36 | 890 | 18 | 3 |
| Conv | Preevisceration | ERY | 690 | 33 | 38 | 44 | 82 | 104 | 117 | 36 | 1421 | 20 | 2 |
| Conv | Preevisceration | ERY | 694 | 33 | 39 | 134 | 161 | 118 | 43 | 17 | 1435 | 24 | 3 |
| Conv | Preevisceration | ERY | 699 | 32 | 38 | 44 | 82 | 113 | 43 | 36 | 1443 | 23 | 2 |
| Conv | Preevisceration | ERY TET | 4324 | 32 | 38 | 30 | 167 | 104 | 35 | 17 | 1453 | 7 | 3 |
| Conv | Postevisceration | TET | 3491 | 33 | 38 | 30 | 82 | 104 | 43 | 17 | 854 | 4 | 11 |
| Conv | Postevisceration | TET | 4559 | 33 | 38 | 30 | 82 | 104 | 85 | 68 | 887 | 18 | 6 |
| Conv | Postevisceration | ERY TET | 3507 | 53 | 38 | 30 | 81 | 104 | 44 | 36 | 1097 | 1 | 3 |
| Conv | Postevisceration | ERY TET | 4276 | 53 | 38 | 44 | 82 | 118 | 35 | 36 | 1123 | 13 | 8 |
| Conv | Postevisceration | ERY TET | 892 | 33 | 38 | 134 | 161 | 104 | 43 | 17 | 1125 | 24 | 3 |
| Conv | Postevisceration | TET | 3490 | 33 | 38 | 30 | 82 | 104 | 43 | 68 | 1130 | 18 | 11 |
| Conv | Postevisceration | TET | 4549 | 33 | 153 | 30 | 82 | 104 | 43 | 36 | 1142 | 22 | 6 |
| Conv | Postevisceration | ERY TET | 4338 | 58 | 38 | 30 | 167 | 118 | 35 | 17 | 1454 | 7 | 3 |
| Conv | Postevisceration | TET | 4560 | 33 | 38 | 37 | 82 | 104 | 85 | 68 | 1455 | 17 | 6 |
| Conv | Postchill | ERY TET | 4445 | 53 | 38 | 30 | 81 | 118 | 43 | 36 | 1428 | 1 | 2 |
Conventional farms.
CHL, chloramphenicol; CIP, ciprofloxacin; ERY, erythromycin; GEN, gentamicin; NAL, nalidixic acid; TET, tetracycline.
Sequence type indicates the unique number assigned on the basis of the allelic profile generated based on the allele nucleotide sequence number in the MLST database (www.pubmlst.org/campylobacter).
The IA for the whole population was 0.293, indicating a weak clonal structure. However, the IA values for the ABF and the conventional populations were 0.279 and 0.535, respectively, indicating a higher clonality of C. coli isolates from the conventional system. Simpson's index of diversity calculated for the MLST method was found to be 0.936. Such a high DI value indicates that MLST has a very high discriminatory power.
Genotypic diversity based on PFGE fingerprinting.
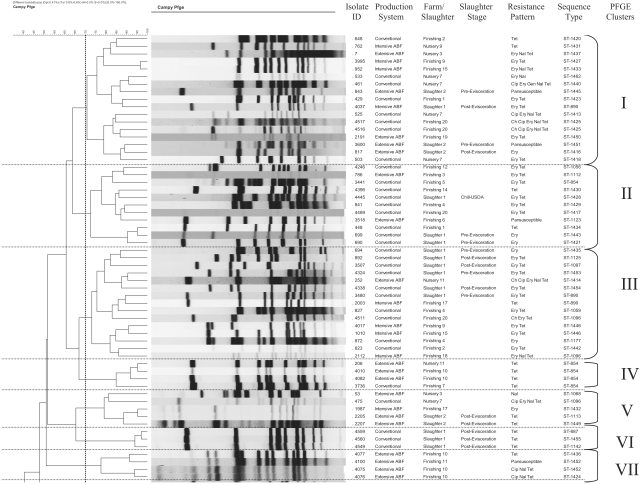
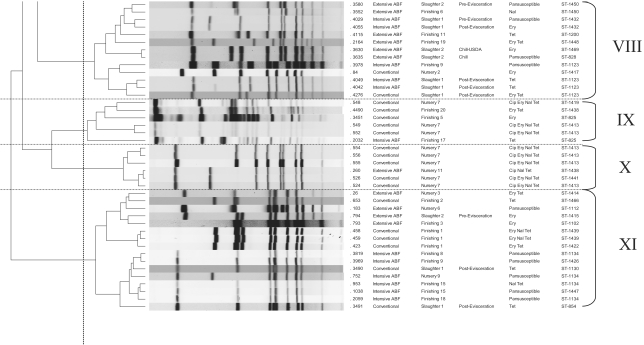
The SmaI-digested genome of C. coli resulted in the generation of an average of 6 to 10 bands. Using 70% genetic similarity as the cutoff, a total of 11 clusters were observed, with clusters 1 (17 isolates), 11 (16 isolates), and 3 (15 isolates) being the predominant ones comprising 48% of the total isolates (Fig. 1). We followed the recommended criteria for interpreting PFGE banding patterns, with isolates differing by one to three bands being most likely clonal (45).
FIG. 1.
PFGE dendrogram for the 100 C. coli isolates showing the 11 clusters at 70% genetic similarity among the band profiles.
Three clusters had isolates grouped together based on their antimicrobial resistance patterns. These included cluster 4 (n = 4; TET), cluster 6 (n = 3; TET), and cluster 10 (n = 6; CIP ERY NAL TET). All the isolates from cluster 6 and five out of six isolates in cluster 10 were epidemiologically related, being isolated from the same slaughter and farm groups. One group of isolates each in clusters 1 (isolates 762, 3995, and 4037) and 11 (isolates 26, 793, and 794) were epidemiologically related, representing the nursery, finishing, and slaughter stages of two different ABF farms. However, other isolates from these farms and slaughter stages were found to be unrelated and clustered separately. Overall, the population exhibited considerable genotypic diversity. The remaining clusters were very diverse based on the location and time of isolation and the resistance patterns of these isolates. This indicates the diverse nature of the C. coli isolates. The PFGE method had a discriminatory index of 0.889.
Comparison of clustering by MLST and PFGE.
We identified a total of 27 and 11 clusters for the 100 C. coli isolates genotyped using MLST and PFGE, respectively. The association of MLST STs with PFGE clusters is shown in Fig. 1 and Table 2. Out of the 65 MLST STs observed for the complete data set, 53 STs (81.5%) representing 62 C. coli isolates were represented by a single PFGE cluster, indicating that these isolates were not distinguished by the PFGE method. The remaining 12 STs were clustered in two to three PFGE clusters. A single PFGE cluster (cluster 4) was found to be associated with a single ST (ST-854). The remaining 10 PFGE clusters were associated with multiple STs. PFGE type 1 was the most heterogeneous cluster and included 16 STs representing the farm and slaughter stages of both production systems. This indicates the poor correlation in polymorphism between MLST and PFGE findings.
Both of the methods were able to further differentiate the isolates clustered together in a single group by either MLST or PFGE. For example, PFGE cluster types 1 (n = 17 isolates) and 11 (n = 16 isolates) were differentiated into 10 and 8 MLST clusters, respectively. Similarly, MLST cluster 9 (n = 18 isolates) was represented by six PFGE clusters when typed by PFGE. However, we found that MLST had a better discriminatory power of 0.936 compared to 0.889 for PFGE. Visual comparison of the two genotyping approaches as shown in Table 2 reflected the ability of MLST to discriminate better between isolates that were clustered in the same group by PFGE. Overall, MLST was able to discriminate better between the C. coli isolates from the conventional and the ABF systems. MLST had a higher test throughput, as reactions can be carried out in 96-well plates. However, PFGE was more cost-effective, costing approximately $7.00 per reaction compared to $42 forMLST.
Identification of antimicrobial resistance genes.
All of the 21 C. coli isolates were positive for the tet(O) gene as shown in Table 3. These isolates were resistant at the highest concentration of tetracycline tested (32 μg/liter). A point mutation from adenine to guanine at position A2075G in the peptidyl transferase region of 23S rRNA was detected in all C. coli isolates that were resistant to erythromycin. Sixteen of the 21 isolates were resistant at the highest concentration of erythromycin tested (32 μg/liter). A single isolate for which the MIC was 16 μg/liter also carried the point mutation in the 23S rRNA gene. Ten of the 17 ciprofloxacin-resistant C. coli isolates were positive by MAMA PCR, indicating the point mutation at amino acid position 86 with threonine (Thr) replaced by isoleucine (Ile) (Table 3).
TABLE 3.
Antimicrobial resistance and molecular characterization of C. coli isolates isolated from swine in this study
| Isolate ID | Production system | Processing stage | MIC (μg/ml)
|
Resistance patternb | Mutationc
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CHL (32)a | CIP (4) | ERY (8) | GEN (16) | NAL (32) | TET (16) | Thr 86 Ile | 23S rRNA gene | ||||
| 49 | ABF | Nursery | 4 | 0.25 | 8 | 2 | 32 | >32 | ERY NAL TET | − | − |
| 260 | ABF | Nursery | 8 | 8 | 4 | 8 | >128 | >32 | CIP NAL TET | − | − |
| 952 | ABF | Finishing | 16 | 2 | >32 | 2 | >128 | >32 | ERY NAL TET | − | − |
| 966 | ABF | Finishing | 8 | 2 | 16 | 2 | >128 | >32 | ERY NAL TET | − | + |
| 1005 | ABF | Finishing | 4 | 1 | >32 | 1 | >128 | >32 | ERY NAL TET | − | + |
| 4075 | ABF | Finishing | 8 | 8 | 4 | 8 | >128 | >32 | CIP NAL TET | − | − |
| 4076 | ABF | Finishing | 8 | 8 | 4 | 8 | >128 | >32 | CIP NAL TET | − | − |
| 3804 | ABF | Finishing | 8 | 4 | >32 | 2 | 64 | >32 | CIP ERY NAL TET | − | − |
| 461 | Conventional | Nursery | 4 | 4 | >32 | 16 | >128 | >32 | CIP ERY GEN NAL TET | − | + |
| 475 | Conventional | Nursery | 8 | >4 | >32 | 2 | 128 | >32 | CIP ERY NAL TET | + | + |
| 524 | Conventional | Nursery | 8 | >4 | >32 | 8 | >128 | >32 | CIP ERY NAL TET | + | + |
| 525 | Conventional | Nursery | 8 | 64 | >32 | 1 | 128 | >32 | CIP ERY NAL TET | + | + |
| 526 | Conventional | Nursery | 8 | 64 | >32 | 1 | 128 | >32 | CIP ERY NAL TET | + | + |
| 548 | Conventional | Nursery | 2 | >64 | >32 | 1 | 128 | >32 | CIP ERY NAL TET | + | + |
| 549 | Conventional | Nursery | 2 | >64 | >32 | 2 | 128 | >32 | CIP ERY NAL TET | + | + |
| 552 | Conventional | Nursery | 4 | >64 | >32 | 2 | 128 | >32 | CIP ERY NAL TET | + | + |
| 554 | Conventional | Nursery | 4 | >64 | >32 | 2 | 128 | >32 | CIP ERY NAL TET | + | + |
| 555 | Conventional | Nursery | 4 | >64 | >32 | 2 | 128 | >32 | CIP ERY NAL TET | + | + |
| 459 | Conventional | Finishing | 8 | 0.5 | >32 | 1 | 32 | >32 | ERY NAL TET | − | + |
| 4516 | Conventional | Finishing | 32 | 4 | >32 | 1 | 128 | >32 | CHL CIP ERY NAL TET | − | + |
| 4517 | Conventional | Finishing | 32 | 4 | >32 | 1 | 128 | >32 | CHL CIP ERY NAL TET | − | + |
Breakpoint level based on C. jejuni ATCC 33560 in μg/ml antimicrobial concentration.
CHL, chloramphenicol; CIP, ciprofloxacin; ERY, erythromycin; GEN, gentamicin; NAL, nalidixic acid; TET, tetracycline.
Point mutations in gyrA and the 23S rRNA genes coding for ciprofloxacin and erythromycin resistance, respectively. All isolates were positive for the tet(O) gene.
Ciprofloxacin-resistant C. coli isolates that were resistant at the concentration of 4 μg/liter were further tested by the same method at concentrations up to 64 μg/liter to determine the MIC. Five out of the 10 C. coli isolates were found resistant at the highest concentration tested, while an MIC of 64 μg/liter was found for two isolates. Overall, MICs for ciprofloxacin were higher for isolates from the conventional system than for those from the ABF system. Sequencing of the QRDR of the gyrA region for all of the 17 isolates revealed a point mutation at amino acid position 147 with glutamic acid replaced by aspartic acid (GAA replaced by GAC) and two silent mutations in isolates 554 and 556 at positions 116 (alanine; GCA replaced by GCT) and 108 (glycine; GGA replaced by GGC), respectively (Table 3). The remaining isolates, including the MAMA PCR-negative isolates, did not reveal any mutation in their QRDRs.
DISCUSSION
There are a multitude of studies that have reported the weak clonal population structure and the hypervariable genome of Campylobacter (7, 8, 34, 47). This makes the choice of using a genotyping method for determining the source of an outbreak or comparing isolates from different sources more complex and difficult to interpret. To circumvent this problem, an MLST scheme was developed for C. jejuni and has been shown to be a reliable method for typing human, animal, and environmental strains of this pathogen (5, 7, 24, 25, 42). Recently, the MLST scheme was extended for typing C. coli, another important species of Campylobacter besides C. jejuni that causes food-borne gastroenteritis (6, 8, 44). Another technique used routinely for typing both C. jejuni and C. coli is PFGE (3, 4, 13, 22, 41, 42). This method is being used by PulseNet within the United States for the nationwide surveillance of this pathogen along with other food-borne pathogens like Salmonella and Shigella (43). It has been used both for typing this pathogen and for discriminating between C. jejuni isolates responsible for 12 outbreaks in the United States (42). These two methods have not been used together to investigate the genetic diversity of C. coli strains isolated from swine. The utility of both the methods as tools for understanding the epidemiology of this pathogen in the swine production environment is important. Therefore, in this study, we used these two methods for genotyping 100 phenotypically diverse C. coli isolated from swine reared under conventional and antimicrobial-free production systems and compared their discriminatory powers, throughputs, and group associations.
MLST and PFGE differentiated the isolates into 26 and 11 clusters, respectively, exhibiting high levels of discrimination depending on the farm type and the antimicrobial resistance pattern. Our results were consistent with other studies where both these methods have been shown to differentiate between closely related strains of Campylobacter (24, 41, 42). The distribution of specific STs among the isolates at different stages of production and with particular resistance patterns indicated that certain STs were adapted to a specific stage of production. Specific STs were found either at the farm or at the slaughter stage (Tables 1 and 2). For example, ST-1413 was seen in C. coli isolates with a CIP ERY NAL TET resistance pattern and only in isolates from pigs at a nursery farm in the conventional production system. Similar results have been reported by other studies where specific clones of C. jejuni have been found associated with particular niches (5). A study in the United Kingdom reported a C. coli strain that may have become adapted to persist in water and act as a source of infection to humans (24).
The results observed in our study of specific STs being associated with a specific production or processing stage such as slaughter may imply that not all strains detected at slaughter originated from the farm, and other factors such as cross-contamination during trucking and in holding pens remain a concern. The genotyping results provided evidence of multiple Campylobacter genotypes grouped together in different clusters. We observed clusters with isolates that were not related either temporally or spatially, indicating significant genotypic diversity. Hume et al., reported the absence of shared genotype from isolates that were isolated from the sow, its respective piglets and the littermates highlighting the diverse genome of this pathogen (21). Similar observations were made when we analyzed the clusters with respect to the resistance patterns. Barring a few STs that were restricted to specific resistance patterns (ST-1413 was associated with isolates with a CIP ERY NAL TET resistance pattern), most of the STs were found to be associated with multiple resistance patterns. C. jejuni isolates with similar PFGE patterns but with different resistance patterns have been reported before (3).
We found MLST to have a better discriminatory power and test throughput than PFGE. Previous studies have reported the better discriminatory power of PFGE compared to MLST when used for typing C. jejuni (41, 42). However, MLST has been found to be as discriminatory as PFGE for distinguishing between temporally related isolates and the epidemic-causing isolates in different outbreaks caused by C. jejuni (42). The ability of MLST to have the same value for epidemiological typing as that of PFGE, AFLP, and ribotyping has also been demonstrated before (10). In the current study, PFGE resulted in good discrimination, but it did not discriminate as well as MLST. These findings imply that both genotyping methods are very good, and the use of both methods would ideally be a preferred way for tracking clones. It should also be emphasized that the outcome of PFGE typing is susceptible to changes in the chromosome and inter- and intragenomic recombinations, making this method unsuitable for studying the epidemiology of pathogens with hyperplastic genome including Campylobacter (18, 34, 47, 48).
Analysis of clusters generated by the two methods revealed the high level of genotypic diversity present in C. coli. We detected clustering of isolates based on the processing stage or sample type rather than the production system. However, in a study conducted in a dairy cattle environment, no clustering of C. coli isolates based on sample type was observed (24). This difference could be attributed to the different environments for dairy cattle and pigs. Seventeen clonal complexes have been defined so far for C. jejuni, and association of these clonal complexes with the given host has been shown by MLST and PFGE (5, 7). There are only two clonal complexes that have been defined for C. coli so far (29). Our lab is currently involved in defining the clonal complex for this species of Campylobacter in swine. This will help us in understanding its epidemiology in different hosts and environments.
The main mechanism of resistance against ciprofloxacin was the threonine-to-isoleucine point mutation at amino acid position 86 as confirmed by both MAMA PCR and sequencing of the QRDR. Mutation at amino acid position 86 has been shown to confer high resistance to this antimicrobial at concentrations ranging from 32 μg/liter up to 128 μg/liter (17, 36). A single isolate carried another mutation at position 147 in addition to the mutation at position 86. We do not know at this stage whether the additional mutation has potentiated the ability of this isolate to become resistant to even higher concentration of ciprofloxacin than isolates that carry a mutation only at position 86. Other mutations in the QRDR that have been linked to ciprofloxacin resistance in Campylobacter include Asp-90, Ala-70, and Pro-104 mutations (11, 36). Recently, high resistance to moxifloxacin, a fluoroquinolone, was shown in Campylobacter isolates that had a double mutation in the QRDR of the gyrA gene.
Resistance against tetracycline was mediated through the tet(O) gene in 100% of the isolates that showed the tetracycline resistance phenotype and tested with PCR. The tet(O) gene, which can be both plasmid and chromosomally located, has been shown to confer high resistance against tetracycline ranging from >256 to 512 μg/liter concentrations in C. coli and C. jejuni, respectively (16, 37). All of the 21 isolates tested were highly resistant to tetracycline (MIC > 32 μg/liter). The ability of this gene to be transferred both intraspecies and interspecies in Campylobacter through conjugation could explain the high frequency of resistance seen against this antimicrobial in isolates in this study. C. coli isolates with an A2075G point mutation in their 23S rRNA gene were responsible for resistance against the macrolide erythromycin. In C. coli, resistance against erythromycin has been shown before to be mediated through the A2075G point mutation (16, 35). Although the highest concentration of erythromycin tested in our study was 32 μg/liter, C. coli isolates with the above point mutation have been shown to exhibit resistance to as high as >1,024 μg/liter of the antimicrobial (16). The simultaneous resistance to important classes of antimicrobials, including fluoroquinolones and macrolides, shown by the 21 characterized C. coli isolates is very concerning.
The overall C. coli population had a weaker clonal structure (IA = 0.293) compared to the IA value of 0.57 for C. jejuni (7). Our findings show that the C. coli population has low clonal structure, and widespread genotypic diversity was seen. In C. jejuni, 17 clonal complexes have been defined so far, and association of these clonal complexes with the given host has been shown by MLST and PFGE (5, 7). Our lab is currently involved in defining a clonal complex for this species of Campylobacter. We found the C. coli population isolated from the conventional production system to be slightly more clonal than that of the ABF system. This could be attributed to the presence of a different lineage of this species circulating in these production systems. Our results differ from other studies that have reported C. coli to be less diverse than C. jejuni by using MLST and AFLP (8, 9). However, it should also be noted that the isolates in the current study are not representative of the C. coli population existing in these systems, and we are cautious that no generalization is deduced from the study. We emphasize that MLST has the potential to be used for studying the molecular epidemiology of Campylobacter due to its high discriminatory power, the simplicity of data handling and analysis, reproducibility of sequence data, high test throughput, and the ease with which data can be exchanged between different laboratories via the internet. This study highlights the high genotypic diversity of antimicrobial-resistant C. coli in the swine production systems.
Acknowledgments
The work was supported by a research grant funded by the U.S. Department of Agriculture to W.A.G. (2002-51110-01508).
REFERENCES
- 1.Anonymous. 2001. Trends in selected gastrointestinal infections—2001. Commun. Dis. Rep. Wkly. 11:1-2. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2005. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food—10 sites, United States, 2004. Morb. Mortal. Wkly. Rep. 54:352-356. [PubMed] [Google Scholar]
- 3.Chu, Y. W., M. Y. Chu, K. Y. Luey, Y. W. Ngan, K. L. Tsang, and K. M. Kam. 2004. Genetic relatedness and quinolone resistance of Campylobacter jejuni strains isolated in 2002 in Hong Kong. J. Clin. Microbiol. 42:3321-3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cloak, O. M., and P. M. Fratamico. 2002. A multiplex polymerase chain reaction for the differentiation of Campylobacter jejuni and Campylobacter coli from a swine processing facility and characterization of isolates by pulsed-field gel electrophoresis and antibiotic resistance profiles. J. Food Prot. 65:266-273. [DOI] [PubMed] [Google Scholar]
- 5.Colles, F. M., K. Jones, R. M. Harding, and M. C. Maiden. 2003. Genetic diversity of Campylobacter jejuni isolates from farm animals and the farm environment. Appl. Environ. Microbiol. 69:7409-7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Communicable Disease Surveillance Centre. 2000. Sentinel surveillance of Campylobacter in England and Wales. Commun. Dis. Rep. Wkly. 10:172. [PubMed] [Google Scholar]
- 7.Dingle, K. E., F. M. Colles, D. R. Wareing, R. Ure, A. J. Fox, F. E. Bolton, H. J. Bootsma, R. J. Willems, R. Urwin, and M. C. Maiden. 2001. Multilocus sequence typing for Campylobacter jejuni. J. Clin. Microbiol. 39:14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dingle, K. E., F. M. Colles, D. Falush, and M. C. Maiden. 2005. Sequence typing and comparison of population biology of Campylobacter coli and Campylobacter jejuni. J. Clin. Microbiol. 43:340-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duim, B., T. M. Wassenaar, A. Rigter, and J. Wagenaar. 1999. High-resolution genotyping of Campylobacter strains isolated from poultry and humans with amplified fragment length polymorphism fingerprinting. Appl. Environ. Microbiol. 65:2369-2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duim, B., P. C. Godschalk, N. van den Braak, K. E. Dingle, J. R. Dijkstra, E. Leyde, J. van der Plas, F. M. Colles, H. P. Endtz, J. A. Wagenaar, M. C. Maiden, and A. van Belkum. 2003. Molecular evidence for dissemination of unique Campylobacter jejuni clones in Curaçao, Netherlands Antilles. J. Clin. Microbiol. 41:5593-5597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Engberg, J., F. M. Aarestrup, D. E. Taylor, P. Gerner-Smidt, and I. Nachamkin. 2001. Quinolone and macrolide resistance in Campylobacter jejuni and C. coli: resistance mechanisms and trends in human isolates. Emerg. Infect. Dis. 7:24-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.FDA/USDA/CDC. 1999. National antimicrobial resistance monitoring system: enteric bacteria (NARMS) 1998 annual report. Centers for Disease Control and Prevention, Atlanta, Ga.
- 13.Fitzgerald, C., K. Stanley, S. Andrew, and K. Jones. 2001. Use of pulsed-field gel electrophoresis and flagellin gene typing in identifying clonal groups of Campylobacter jejuni and Campylobacter coli in farm and clinical environments. Appl. Environ. Microbiol. 67:1429-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge, B., S. Bodeis, R. D. Walker, D. G. White, S. Zhao, P. F. McDermott, and J. Meng. 2002. Comparison of the Etest and agar dilution for in vitro antimicrobial susceptibility testing of Campylobacter. J. Antimicrob. Chemother. 50:487-494. [DOI] [PubMed] [Google Scholar]
- 15.Gibreel, A., D. M. Tracz, L. Nonaka, T. M. Ngo, S. R. Connell, and D. E. Taylor. 2004. Incidence of antibiotic resistance in Campylobacter jejuni isolated in Alberta, Canada, from 1999 to 2002, with special reference to tet(O)-mediated tetracycline resistance. Antimicrob. Agents Chemother. 48:3442-3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gibreel, A., V. N. Kos, M. Keelan, C. A. Trieber, S. Levesque, S. Michaud, and D. E. Taylor. 2005. Macrolide resistance in Campylobacter jejuni and Campylobacter coli: molecular mechanism and stability of the resistance phenotype. Antimicrob. Agents Chemother. 49:2753-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griggs, D. J., M. M. Johnson, J. A. Frost, T. Humphrey, F. Jorgensen, and L. J. Piddock. 2005. Incidence and mechanism of ciprofloxacin resistance in Campylobacter spp. isolated from commercial poultry flocks in the United Kingdom before, during, and after fluoroquinolone treatment. Antimicrob. Agents Chemother. 49:699-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanninen, M. L., M. Hakkinen, and H. Rautelin. 1999. Stability of related human and chicken Campylobacter jejuni genotypes after passage through chick intestine studied by pulsed-field gel electrophoresis. Appl. Environ. Microbiol. 65:2272-2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harvey, R. B., C. R. Young, R. L. Ziprin, M. E. Hume, K. J. Genovese, R. C. Anderson, R. E. Droleskey, L. H. Stanker, and D. J. Nisbet. 1999. Prevalence of Campylobacter species isolated from the intestinal tract of pigs raised in an integrated swine production system. J. Am. Vet. Med. Assoc. 215:1601-1604. [PubMed] [Google Scholar]
- 20.Heuer, O. E., K. Pedersen, J. S. Andersen, and M. Madsen. 2001. Prevalence and antimicrobial susceptibility of thermophilic Campylobacter in organic and conventional broiler flocks. Lett. Appl. Microbiol. 33:269-274. [DOI] [PubMed] [Google Scholar]
- 21.Hume, M. E., R. E. Droleskey, C. L. Sheffield, and R. B. Harvey. 2002. Campylobacter coli pulsed field gel electrophoresis genotypic diversity among sows and piglets in a farrowing barn. Curr. Microbiol. 45:128-132. [DOI] [PubMed] [Google Scholar]
- 22.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 26:2465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jolley, K. A., E. J. Feil, M. S. Chan, and M. C. Maiden. 2001. Sequence type analysis and recombinational tests (START). Bioinformatics 17:1230-1231. [DOI] [PubMed] [Google Scholar]
- 24.Leatherbarrow, A. J., C. A. Hart, R. Kemp, N. J. Williams, A. Ridley, M. Sharma, P. J. Diggle, E. J. Wright, J. Sutherst, and N. P. French. 2004. Genotypic and antibiotic susceptibility characteristics of a Campylobacter coli population isolated from dairy farmland in the United Kingdom. Appl. Environ. Microbiol. 70:822-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manning, G., C. G. Dowson, M. C. Bagnall, I. H. Ahmad, M. West, and D. G. Newell. 2003. Multilocus sequence typing for comparison of veterinary and human isolates of Campylobacter jejuni. Appl. Environ. Microbiol. 69:6370-6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maynard-Smith, J. M., N. H. Smith, M. O'Rourke, and B. G. Spratt. 1993. How clonal are bacteria? Proc. Natl. Acad. Sci. USA 90:4384-4388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michaud, S., S. Menard, and R. D. Arbeit. 2005. Role of real-time molecular typing in the surveillance of Campylobacter enteritis and comparison of pulsed-field gel electrophoresis profiles from chicken and human isolates. J. Clin. Microbiol. 43:1105-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, W. G., S. L. On, G. Wang, S. Fontanoz, A. J. Lastovica, and R. E. Mandrell. 2005. Extended multilocus sequence typing system for Campylobacter coli, C. lari, C. upsaliensis, and C. helveticus. J. Clin. Microbiol. 43:2315-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan, D., C. Gunneberg, D. Gunnell, T. D. Healing, S. Lamerton, N. Soltanpoor, D. A. Lewis, and D. G. White. 1994. An outbreak of Campylobacter infection associated with the consumption of unpasteurized milk at a large festival in England. Eur. J. Epidemiol. 10:581-585. [DOI] [PubMed] [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards. 1999. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals: approved standard M31-A. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 32.Newell, D. G., J. A. Frost, B. Duim, J. Wagenaar, R. H. Madden, J. van der Plas, and S. L. W. On. 2000. New developments in the subtyping of Campylobacter species, p. 27-44. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. American Society for Microbiology, Washington, D.C.
- 33.O'Halloran, F., B. Lucey, B. Cryan, T. Buckley, and S. Fanning. 2004. Molecular characterization of class 1 integrons from Irish thermophilic Campylobacter species. J. Antimicrob. Chemother. 53:952-957. [DOI] [PubMed] [Google Scholar]
- 34.On, S. L. W. 1998. In vitro genotypic variation of Campylobacter coli documented by pulsed-field gel electrophoretic DNA profiling: implications for epidemiological studies. FEMS Microbiol. Lett. 165:341-346. [DOI] [PubMed] [Google Scholar]
- 35.Payot, S., L. Avrain, C. Magras, K. Praud, A. Cloeckaert, and E. Chaslus-Dancla. 2004. Relative contribution of target gene mutation and efflux to fluoroquinolone and erythromycin resistance, in French poultry and pig isolates of Campylobacter coli. Int. J. Antimicrob. Agents 23:468-472. [DOI] [PubMed] [Google Scholar]
- 36.Piddock, L. J., V. Ricci, L. Pumbwe, M. J. Everett, and D. J. Griggs. 2003. Fluoroquinolone resistance in Campylobacter species from man and animals: detection of mutations in topoisomerase genes. J. Antimicrob. Chemother. 51:19-26. [DOI] [PubMed] [Google Scholar]
- 37.Pratt. A., and V. Korolik. 2005. Tetracycline resistance of Australian Campylobacter jejuni and Campylobacter coli isolates. J. Antimicrob. Chemother. 55:452-460. [DOI] [PubMed] [Google Scholar]
- 38.Ribot, E. M., C. Fitzgerald, K. Kubota, B. Swaminathan, and T. J. Barrett. 2001. Rapid pulsed-field gel electrophoresis protocol for subtyping of Campylobacter jejuni. J. Clin. Microbiol. 39:1889-1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sacks, J. J., S. Lieb, L. M. Baldy, S. Berta, C. M. Patton, M. C. White, W. J. Bigler, and J. J. Witte. 1986. Epidemic campylobacteriosis associated with a community water supply. Am. J. Public Health 76:424-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saenz, Y., M. Zarazaga, M. Lantero, M. J. Gastanares, F. Baquero, and C. Torres. 2000. Antibiotic resistance in Campylobacter strains isolated from animals, foods, and humans in Spain in 1997-1998. Antimicrob. Agents Chemother. 44:267-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sails, A. D., B. Swaminathan, and P. I. Fields. 2003. Clonal complexes of Campylobacter jejuni identified by multilocus sequence typing correlate with strain associations identified by multilocus enzyme electrophoresis. J. Clin. Microbiol. 41:4058-4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sails, A. D., B. Swaminathan, and P. I. Fields. 2003. Utility of multilocus sequence typing as an epidemiological tool for investigation of outbreaks of gastroenteritis caused by Campylobacter jejuni. J. Clin. Microbiol. 41:4733-4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swaminathan, B., T. J. Barrett, S. B. Hunter, R. V. Tauxe, and the CDC PulseNet Task Force. 2001. PulseNet: the molecular subtyping network for foodborne bacterial disease surveillance, United States. Emerg. Infect. Dis. 7:382-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tam, C. C., S. J. O'Brien, G. K. Adak, S. M. Meakins, and J. A. Frost. 2003. Campylobacter coli—an important foodborne pathogen. J. Infect. 47:28-32. [DOI] [PubMed] [Google Scholar]
- 45.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thakur, S., and W. A. Gebreyes. Prevalence and antimicrobial resistance of Campylobacter in antimicrobial-free and conventional pig production systems. J. Food Prot., in press. [DOI] [PubMed]
- 47.Wassenaar, T. M., B. Geilhausen, and D. G. Newell. 1998. Evidence of genomic instability in Campylobacter jejuni isolated from poultry. Appl. Environ. Microbiol. 64:1816-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weijtens, M. J., R. D. Reinders, H. A. Urlings, and J. Van der Plas. 1999. Campylobacter infections in fattening pigs; excretion pattern and genetic diversity. J. Appl. Microbiol. 86:63-70. [DOI] [PubMed] [Google Scholar]
- 49.Zirnstein, G., L. Helsel, Y. Li, B. Swaminathan, and J. Besser. 2000. Characterization of gyrA mutations associated with fluoroquinolone resistance in Campylobacter coli by DNA sequence analysis and MAMA PCR. FEMS Microbiol. Lett. 190:1-7. [DOI] [PubMed] [Google Scholar]