Abstract
Specific identification of Entamoeba spp. in clinical specimens is an important confirmatory diagnostic step in the management of patients who may be infected with Entamoeba histolytica, the species that causes clinical amebiasis. Distinct real-time PCR protocols have recently been published for identification of E. histolytica and differentiation from the morphologically identical nonpathogenic Entamoeba dispar. In this study, we compared three E. histolytica real-time PCR techniques published by December 2004. The limits of detection and efficiency of each real-time PCR assay were determined using DNA extracted from stool samples spiked with serially diluted cultured E. histolytica trophozoites. The ability of each assay to correctly distinguish E. histolytica from E. dispar was evaluated with DNA extracted from patients' stools and liver aspirates submitted for confirmatory diagnosis. Real-time PCR allowed quantitative analysis of the spiked stool samples, but major differences in detection limits and assay performance were observed among the evaluated tests. These results illustrate the usefulness of comparative evaluations of diagnostic assays.
Clinical features of amebiasis, caused by the protozoan parasite Entamoeba histolytica, range from asymptomatic colonization to amebic dysentery and invasive extraintestinal amebiasis, most commonly in the form of liver abscesses (24). The World Health Organization estimates that amebiasis is one of the three most common causes of death from parasitic disease, responsible for up to 100,000 deaths annually (30). The disease is spread primarily by food or water contaminated with cysts but may also be transmitted from person to person. It is highly prevalent in regions of the world where personal hygiene and/or sanitation are insufficient.
Examination of stained stool smears is used routinely in clinical laboratories to differentiate E. histolytica from nonpathogenic intestinal amebas, such as Entamoeba coli, Entamoeba polecki, and Entamoeba hartmani. However, this gold standard method cannot differentiate E. histolytica from the morphologically identical E. dispar, which occurs worldwide (8). E. dispar is a harmless commensal protozoan, and its presence in clinical specimens does not justify treatment (30). Thus, misidentification of E. histolytica-associated disease may occur if the diagnosis is based solely on examination of smears (29). For final confirmatory identification of intestinal amebiasis, molecular methods or immunologic assays for detection of E. histolytica antigens are needed (21). Currently, the only commercially available antigen test for specific detection of E. histolytica (the histolytica II test from TechLab) is recommended for use exclusively with fresh stool samples, since storage or use of preservatives destroys the antigen.
For diagnosis of extraintestinal amebiasis, the laboratory methods are even more limited. Detection of amebas by microscopy is often unsuccessful (32). Although acceptable results with extraintestinal specimens have been obtained with the TechLab II antigen test (14, 22), this test is designed and marketed for examination of stool specimens only.
PCR, including real-time PCR, has provided means to identify E. histolytica in a variety of clinical specimens, including stools, tissues, and liver abscess aspirates (24). Several PCR assays designed for differential detection of E. histolytica and E. dispar have been developed. Most of them target either the small-subunit rRNA (18S rRNA) gene (5, 10, 15, 18) or species-specific episomal repeats (1, 19, 21). These targets are present on multicopy, extrachromosomal plasmids in the amebas (3). The sensitivity and specificity of PCR assays exceed what can be accomplished with microscopy and are comparable to those of the antigen test (13, 16, 17, 20, 23).
Real-time PCR is a very attractive methodology for laboratory diagnosis of infectious diseases because of its features that eliminate post-PCR analysis, leading to shorter turn-around times and minimized risk of amplicon contamination of laboratory environments. This represents obvious advantages in diagnostics, as amplicon contamination has been reported to be the most frequent cause of false-positive results in PCR amplification (31). In addition, real-time PCR is a quantitative method and may allow the determination of the number of parasites in various samples (2). Although not relevant for estimating the parasite burden in amebiasis patients (the parasite content can vary tremendously between, or even within, specimens from the same patient), quantitative measures can be useful for food, water, and distinct classes of environmental samples.
Three real-time PCR assays for the specific detection of E. histolytica had been published as of December 2004: a LightCycler assay utilizing hybridization probes to detect amplification of the 18S rRNA gene (4) and two TaqMan assays targeting the 18S rRNA gene (25) and the episomal repeats (28), respectively. Although each of these assays has been evaluated separately, no comparison of the assays has been published so far. In this study, we present a comparative reevaluation of these assays, plus a SYBR Green-based assay using previously published primers (5). Our emphasis was to compare the weaknesses and strengths of each assay, with focus on their usefulness for clinical laboratory diagnosis. Quantification standards produced by spiking stools with different concentrations of E. histolytica trophozoites and a small set of well-characterized clinical samples were used to compare limits of detection, accuracy, efficiency, relative cost, and ease of use for each assay. Such data are crucial to allow reference laboratories to make informed choices for implementation of real-time PCR for amebiasis.
MATERIALS AND METHODS
Cultured Entamoeba histolytica trophozoites.
Entamoeba histolytica ATCC strain HM1 was grown in Diamond's TYIS-33 medium (9) at 37°C with the following modifications: (i) liver extract (Oxoid), 15 g/liter, was substituted for casein digest peptone (BBL), (ii) yeast extract was used at 25 g/liter, and (iii) the vitamin mixture consisted of NCTC 109 with added vitamins B12, D, and l-thioctic acid and Tween 80 solution. Cultured E. histolytica trophozoites were harvested, centrifuged, and resuspended in phosphate-buffered saline to 0.2 × 106 to 3.0 × 106 cells/ml. A 1-μl aliquot of this harvested batch was placed in a counting chamber (Hausser Scientific Company, Horsham, Pa.), and the concentration of trophozoites was calculated. Three 200-μl aliquots of the same harvested batch were used to produce a set of 120 quantification standards: each aliquot was serially diluted to 10−1 cell/ml, creating three identical dilution series with eight diluted samples in each series. This was repeated four more times, resulting in five batches of three dilution series each. A volume of 200 μl of each diluted sample was used to spike 200 μl parasite-free stools, which were then subjected to DNA extraction (see below). A single stool sample, obtained from a volunteer with no symptoms of intestinal infection, was used in all spiking experiments. Prior to the spiking procedure, this stool was evaluated for the presence of parasites by microscopic examination of wet mounts and for E. histolytica and E. dispar using the conventional PCR described below.
Clinical specimens.
A total of 51 clinical specimens (42 stools and 9 liver abscess specimens) were used to evaluate the real-time PCR tests. The specimens had been submitted to CDC from state health departments in the United States for confirmatory diagnosis during the period from December 2003 to February 2005. Thirty-eight specimens were from patients with suspected amebiasis, collected from United States civilians with a travel history outside of the United States, immigrants, and refugees; of these, 29 were stools positive for E. histolytica/E. dispar by microscopy performed before submission to CDC, and nine were liver aspirates from nine patients clinically suspected to have amebic liver abscesses. These 38 specimens were tested for the presence of amebas by conventional PCR, using primers PSP5/PSP3 and NPSP5/NPSP3 (5), upon reception at CDC. The remaining 13 samples were stools containing other intestinal parasites, as confirmed by conventional PCR and DNA sequencing: two samples each of Entamoeba invadens, Encephalitozoon intestinalis, Enterocytozoon bieneusi, and Cyclospora cayetanensis; one sample each of Entamoeba coli, Entamoeba chattoni, Encephalitozoon cuniculi, Cryptosporidium parvum, and Cryptosporidium hominis.
DNA extraction.
Total genomic DNA was extracted from spiked stools and clinical samples using a modification of the FastDNA method (Q-Biogene, Carlsbad, Calif.) as previously described (6). DNA was extracted from 300 μl of each clinical specimen. Samples were disrupted in the FP120 cell disruptor instrument at a speed of 5.5 for 10 seconds. Potential inhibitors were removed by further purification with the QIAquick PCR purification kit (QIAGEN Inc., Valencia, Calif.) according to the manufacturer's instructions. Purified DNA was stored at 4°C until it was used in PCRs.
PCR amplification.
All PCRs were performed using commercially available reagents that included a thermostable DNA polymerase, deoxynucleoside triphosphates, MgCl2, and other salts and buffering agents necessary for optimum performance. One microliter of template DNA was added to each reaction mixture, and the total volume was 20 μl in all assays. Conventional PCR, using previously published primers for E. histolytica detection (5), was employed to define the detection limit of the assay. Cycling was carried out in a GeneAmp 9700 PCR thermal cycler (Applied Biosciences, Foster City, Calif.). The LightCycler assay was performed on LightCycler 2.0 (Roche Diagnostics, Indianapolis, Ind.), whereas all other real-time PCR assays were performed on Mx3000P (Stratagene, La Jolla, Calif.). Details about the assays are outlined in Table 1 (target genes and sequences of primers and probes) and Table 2 (cycling structures, reagents, and detection methods).
TABLE 1.
Primers and probes used in the real-time PCR assays compared
| Assay | Gene target | Amplicon size (bp) | Primer or probe | Sequence (5′ to 3′)a | Nucleotide positionsb | Reference |
|---|---|---|---|---|---|---|
| Conventional PCR adapted for SYBR Green real-time PCR | 18S rRNA | 877 | PSP5c | GGCCAATTCATTCAATGAATTGAG | 200-223 | 5 |
| PSP3c | CTCAGATCTAGAAACAATGCTTCTC | 1076-1052 | ||||
| 878 | NPSP5d | GGCCAATTTATGTAAGTAAATTGAG | 200-224 | |||
| NPSP3d | CTTGGATTTAGAAACAATGTTTCTTC | 1077-1052 | ||||
| LightCycler | 18S rRNA | 307 | Eh-S26Cc | GTACAAAATGGCCAATTCATTCAACG | 190-216 | 4 |
| Ed-27Cd | GTACAAAGTGGCCAATTTATGTAAGCA | 191-217 | ||||
| Eh-Ed-AS25e | GAATTGATTTTACTCAACTCTAGAG | 497-473 | ||||
| Eh-Ed-24-Re | LC640-TCGAACCCCAATTCCTCGTTATCCp | 373-350 | ||||
| Eh-Ed-25-De | GCCATCTGTAAAGCTCCCTCTCCGA-FAM | 400-376 | ||||
| TaqMan 1 | 18S rRNA | 231 | Ehd-239Fe | ATTGTCGTGGCATCCTAACTCA | 260-239 | 28 |
| Ehd-88Re | GCGGACGGCTCATTATAACA | 88-107 | ||||
| histolytica-96Tc | FAM-UCAUUGAAUGAAUUGGCCAUUU-BHQ1 | 217-197 | ||||
| dispar-96Td | HEX-UUACUUACAUAAAUUGGCCACUUUG-BHQ1 | 218-194 | ||||
| TaqMan 2 | Episomal repeats | 83 | histolytica-50Fc | CATTAAAAATGGTGAGGTTCTTAGGAA | 50-76 | 28 |
| histolytica-132Rc | TGGTCGTCGTCTAGGCAAAATATT | 132-109 | ||||
| histolytica-78Tc | FAM-TTGACCAATTTACACCGTTGATTTTCGGA-BHQ1 | 106-78 | ||||
| 137 | dispar-1Fd | GGATCCTCCAAAAAATAAAGTTTTATCA | 1-28 | |||
| dispar-137Rd | ATCCACAGAACGATATTGGATACCTAGTA | 137-109 | ||||
| dispar-33d | HEX-UGGUGAGGUUGUAGCAGAGAUAUUAAUU-BHQ1 | 33-60 |
U, 5-propyne-2′-deoxyuridine. This chemistry mimics the effect on hybridization of the minor-groove binding protein in the so-called MGB probes. LC640, LightCycler Red 640; FAM, 6-carboxyFluorescein; BHQ1, Black Hole Quencher 1; HEX, hexachlorofluorescein; p, phosphate.
The 18S rRNA sequences are filed under GenBank accession number X64142 (E. histolytica) or Z49256 (E. dispar). For episomal repeats, see reference 12.
Specific for E. histolytica.
Specific for E. dispar.
Generic for E. histolytica/E. dispar.
TABLE 2.
Details about the compared real-time PCR assays
| Assay | Cycling structure | Enzyme/buffer (source) | Primer/probe concn. | Detection |
|---|---|---|---|---|
| Conventional PCR | 95°C for 5 min, 45 cycles of 95°C for 15 s, 65°C for 15 s, 72°C for 1 min, 72°C for 10 min | AmpliTaq Gold/AmpliTaq Gold PCR Master Mix (Applied Biosciences, Foster City, Calif.) | 0.4 μM of each primer | 2% agarose gel stained with ethidium bromide |
| SYBR Green | 95°C for 15 min, 50 cycles of 95°C for 15 s, 60°C for 1 min, 72°C for 1.5 min, 80°C for 30 s + melting curve | HotStar Taq polymerase/QuantiTect SYBR Green PCR Master Mix (QIAGEN, Valencia, Calif.) | 0.1 μM of each primer | Fluorescence at the end of the 80°C incubation plateau and during the melting curve |
| LightCycler | 95°C for 15 min, 50 cycles of 95°C, 58°C for 10 s,a 72°C for 20 s | HotStarTaq polymerase/ QuantiTect Probe PCR Master Mix (QIAGEN, Valencia, Calif.) | 0.5 μM of each primer/0.1 μM of each probe | Fluorescence at the end of each annealing plateau |
| TaqMan1 and TaqMan 2 | 95°C for 3 min, 40 cycles of 95°C for 15 s, 60°C for 30 s, 72°C for 30 s | iTaq DNA polymerase/IQ Supermix (BioRad, Hercules, Calif.) | 0.5 μM of each primer/0.1 μM of each probe | Fluorescence at the end of each extension plateau |
A touch-down PCR mode was incorporated to stepwise decrease the annealing temperature from 62°C to 58°C during the first eight cycles.
Data analysis.
Except for results from the LightCycler, fluorescence thresholds were manually adjusted to the same numerical value for each run to facilitate comparison of run-to-run variation of threshold cycle (Ct) values. Microsoft Excel (Office 2000; Microsoft Corp., Seattle, Wash.) was used for statistical analysis. Student's t test (two-tailed distribution; unpaired) was performed to determine the quality of quantification standard curves: P values less than 0.05 were considered significant.
DNA sequencing analysis.
Primers Ehd-88R and Eh-Ed-AS25 were used to amplify 410- or 411-bp amplicons, which were purified with the StrataPrep PCR Purification kit (Stratagene, La Jolla, Calif.) and sequenced by cycle sequencing using BigDye version 3.1 chemistry (Applied Biosystems, Foster City, Calif.). Sequencing reactions were purified using MultiScreen-HV plates (Millipore, Billerica, Mass.). Sequencing data were obtained using the ABI Prism 3100 sequence analyzer with data collection software version 2.0 and DNA Sequence Analysis Software version 5.1 (Applied Biosystems, Foster City, Calif.). Sequences were assembled, edited, and aligned in DNASTAR SeqMan (DNASTAR Inc., Madison, Wis.), as well as in the GeneStudio suite (GeneStudio Inc., Suwanee, Ga.).
RESULTS
Quantification standards.
Evaluating the quantitative aspects of the real-time PCR protocols required samples with known concentrations of Entamoeba histolytica, so a set of standard samples was produced based on cultivated E. histolytica trophozoites. E. histolytica cysts were not used, since encystations of E. histolytica cannot be reliably achieved in the laboratory.
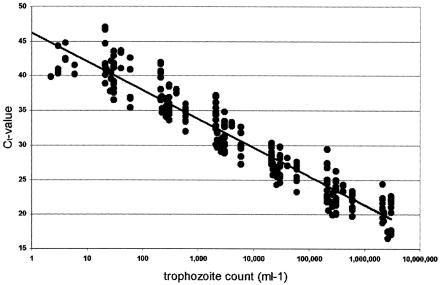
The quantification standards consisted of parasite-free stools spiked with known numbers of trophozoites from cultures. To ensure that the detection limit could be reached even for very sensitive assays, we included samples with a calculated content of less than one cell. DNA extracted from these spiked stool samples was first analyzed in the SYBR Green assay. Figure 1 illustrates the spread of the resulting Ct values, and Table 3 lists the outcome of statistical analysis of the results. The variation in Ct values shown in Fig. 1 and Table 3 was mainly due to differences in DNA concentrations between samples, as each individual sample produced highly reproducible results in independent runs (run-to-run variability for the same sample was normally on the order of less than 1 cycle unit) (data not shown). Counting of trophozoites was as accurate as possible; however, “stickiness” of the parasites could have made it difficult to obtain a homogenous solution before making aliquots and dilutions, resulting in the observed Ct differences between samples. The statistical analysis of the Ct variation indicated that quantification could be determined within 1 logarithmic order of magnitude.
FIG. 1.
Variation in Ct values of the standard DNA samples. SYBR Green assay Ct values obtained with DNA extracted from stools containing 106 to 10−1 E. histolytica trophozoites per ml. Each sample was tested three times, and the resulting Ct values were plotted against the trophozoite concentration. The line corresponds to a linear regression of all values. The variation in Ct values between samples containing the same parasite concentration was mainly due to variable DNA concentrations in the samples, as explained in Results.
TABLE 3.
Statistical analysis of the quantification standards as evaluated in the SYBR Green assay
| Parameter | Value at indicated cell count/ml
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 106 | 105 | 104 | 103 | 102 | 101 | 100 | 10−1 | |
| Avg Ct | 20.4 | 23.7 | 28.0 | 32.6 | 37.0 | 40.7 | 41.7 | |
| SD | 2.0 | 2.3 | 2.0 | 2.2 | 2.8 | 2.2 | 1.8 | |
| No. of Ct valuesa | 36 | 45 | 45 | 45 | 40 | 30 | 7 | 0 |
| Ct intervals | 16.5-24.3 | 20.0-29.4 | 24.3-32.7 | 28.9-37.2 | 33.6-47.0 | 36.5-46.7 | 39.9-44.8 | |
| Significance | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P = 0.18 | ||
The maximum number of individual Ct values is 45 (3 tests at 15 samples each) for all concentrations except for the most concentrated (106/ml), which had 12 samples and thus a maximum of 36 individual Ct values.
Detection limits and efficiency measures of evaluated real-time PCR tests.
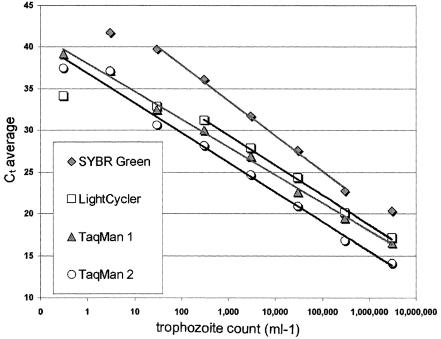
Limits of detection for all four real-time PCR tests were estimated in quantification experiments using the DNA standard samples described above. The probe-based assays (i.e., the LightCycler and the two TaqMan assays) were very sensitive, with detection limits of less than 10 cells per ml of spiked stool (Table 4). The SYBR Green assay was the least sensitive of the real-time PCR assays, but it still had approximately 10-fold-higher sensitivity than conventional PCR (Table 4).
TABLE 4.
Performance characteristics of assays
| Assay | Limit of detection (cells/ml [±SD]) | Linear range | Slope (efficiency) | Relative costa | Timeb (h) |
|---|---|---|---|---|---|
| Conventional PCR | 119 (±890) | NAc | NA | + | 7 |
| SYBR Green | 17 (±57) | 105-101 | −4.2 (72%) | ++ | 7 |
| LightCycler | 4 (±14) | 106-102 | −3.5 (90%) | ++++ | 4 |
| TaqMan 1 | 1 (±4) | 106-10−1 | −3.3 (100%) | +++ | 4 |
| TaqMan 2 | 0.5 (±1.2) | 106-10−1 | −3.5 (91%) | +++ | 4 |
Cost for equipment not included. The LightCycler assay requires a LightCycler thermocycler. The other real-time assays can be performed on less expensive real-time thermocyclers.
Estimated time from reception of specimen to final result (2 h for DNA extraction is included).
NA, not applicable.
Regression analysis identified variable linear ranges for the assays, with the TaqMan assays having the largest possible linear range of 8 orders of magnitude (Fig. 2 and Table 4). The slopes of the linear parts of the standard curves in Fig. 2 were used to estimate the amplification efficiency. A slope of −3.3 translates into 100% efficiency, which in practice means that the number of amplicon copies doubles for each amplification cycle. By using this criterion, only the TaqMan assay targeting the 18S rRNA demonstrated 100% efficiency (Table 4).
FIG. 2.
Detection limits and linearity of the real-time PCR assay. Shown are average Ct values by the four real-time PCR assays evaluated in this study, obtained with DNA extracted from stools containing 106 to 10−1 E. histolytica trophozoites per ml. The average Ct values for each concentration range were calculated and plotted against the trophozoite concentration. The linear parts of the resulting curves were determined using regression analysis and are displayed as lines.
The last two columns in Table 4 provide a rough estimate of the time and cost involved in running each evaluated real-time PCR assay. The SYBR Green assay and the LightCycler assay used species-specific primers to distinguish E. histolytica from E. dispar. Thus, two separate reactions had to be run in parallel for each sample, resulting in slightly longer set-up times than for the TaqMan assays. In addition, the LightCycler requires the reactions to be run in special glass capillaries, which are expensive and fragile, requiring extra care to work with. On the other hand, the LightCycler thermocycler has very fast ramping of the temperature, leading to shorter cycle times. Due to the high cost of fluorescent probes, the probe-free SYBR Green assay was the least expensive. However, because it was based on amplification of a large amplicon size, it required long cycling times, making it more time-consuming than the other real-time assays.
Specificity of evaluated real-time PCR tests.
The abilities of the tests to accurately differentiate E. histolytica from E. dispar were determined using 29 stool and 9 liver aspirate specimens submitted for confirmatory diagnosis at CDC. These samples had been previously tested for amebiasis by conventional PCR with primers PSP3/PSP5 and NPSP3/NPSP5 (i.e., the same primers used in the SYBR Green assay). This had confirmed 10 of these samples as positive for E. histolytica (4 of the liver aspirates and 6 of the stool specimens), 16 of the stools as positive for E. dispar, and 1 stool as positive for both species. All 38 samples were reevaluated in this study, using the four real-time PCR assays, and the results are displayed in Tables 5 and 6. To ensure the accuracy of species determination, all samples that were positive for amebas in any of the evaluated tests were also amplified with primers Ehd-88R and Eh-Ed-AS25, and the amplicons were sequenced (Tables 5 and 6). The SYBR Green assay reported seven of the initially positive samples as negative for amebas. This could have been associated with long-term storage and subsequent degradation of nucleic acids, bringing the concentration of the targets below the detection limit of the SYBR Green assay before real-time PCR was performed. On the other hand, the probe-based assays (i.e., the LightCycler and the two TaqMan assays) confirmed the initial PCR results in all 27 positive samples (23 stools and 4 liver aspirates), and in addition detected amebas in four samples (3 stools and 1 liver aspirate) that had been negative in the initial PCR (Tables 5 and 6). One of the latter stool samples had been stored for some time before arriving at the CDC. The other two stools were from patients with suspected amebiasis based on microscopic findings in previous samples. The liver aspirate came from a person with a history of travel to countries where E. histolytica infections are endemic. Although confirmed to contain E. histolytica by sequencing, these four samples had very low parasite content, as judged by consistently high Ct values in the real-time PCR (Ct = 34 or above, depending on sample and assay). This corresponds to around 10 trophozoites or less per ml, which can explain the negative results in the conventional PCR and the SYBR Green assay.
TABLE 5.
Conventional and real-time PCR results for stool specimens submitted to CDC for confirmatory diagnosis
| Methodology | No. of stool specimens identified as:
|
Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
E. histolytica
|
E. dispar
|
Mixed
|
Negative | |||||||
| E. histolytica | Mixed | Negative | E. dispar | Mixed | Negative | Mixed | E. dispar | |||
| Sequencinga | 9 | 0 | 0 | 16 | 0 | 0 | 1 | 0 | 16c | 42 |
| Conventional PCRb | 6 | 0 | 3 | 16 | 0 | 0 | 1 | 0 | 3 | 29 |
| SYBR Green | 4 | 0 | 5 | 12 | 0 | 4 | 0 | 1 | 16c | 42 |
| LightCycler | 8 | 1 | 0 | 11 | 5 | 0 | 1 | 0 | 16c | 42 |
| TaqMan 1 | 9 | 0 | 0 | 16 | 0 | 0 | 0 | 1 | 16c | 42 |
| TaqMan 2 | 9 | 0 | 0 | 13 | 3 | 0 | 1 | 0 | 16c | 42 |
DNA sequencing analysis was performed only to assure the results and not for comparison purposes.
Results from confirmatory diagnosis, performed directly upon reception of the specimen at CDC.
13 of these were clinical specimens containing other parasites than E. histolytica or E. dispar; see “Clinical specimens” for details. They were included in this study as negative controls for the real-time PCR assays. Conventional PCR for ameba detection was not performed on these samples, as they were not from suspected amebiasis cases.
TABLE 6.
Conventional and real-time PCR results for specimens from suspected extraintestinal amebiasis (liver abscesses)
| Methodology | No. of liver aspirates specimens identified as:
|
Total | ||
|---|---|---|---|---|
|
E. histolytica
|
Negative | |||
| E. histolytica | Negative | |||
| Sequencinga | 5 | 0 | 4c | 9 |
| Conventional PCRb | 4 | 1 | 4 | 9 |
| SYBR Green | 3 | 2 | 4 | 9 |
| LightCycler | 5 | 0 | 4 | 9 |
| TaqMan 1 | 5 | 0 | 4 | 9 |
| TaqMan 2 | 5 | 0 | 4 | 9 |
DNA sequencing analysis was performed only to assure the results and not for comparison purposes.
Results from confirmatory diagnosis, performed directly upon reception of the specimen at CDC.
Negative liver aspirates were positive for bacterial liver abscess by culture methods.
The TaqMan targeting episomal repeats (TaqMan 2) and the LightCycler assay gave mixed results for three and five stool samples, respectively, that contained only E. dispar as judged by the other methods (Table 5). In addition, the LightCycler assay had problems with the standard DNA samples from cultured E. histolytica, which occasionally were detected as positive for E. dispar as well (data not shown). Attempts to reduce this problem by altering the PCR conditions were mostly unsuccessful, although a slight improvement in the LightCycler assay was seen when the concentration of oligonucleotides was decreased compared to the published protocol (data not shown). The sequencing analysis verified the results obtained with the 18S rRNA-targeting TaqMan assay (TaqMan 1) and did not support the mixed results produced by TaqMan 2 or the LightCycler assay.
DISCUSSION
E. histolytica is the agent of human intestinal and extraintestinal amebiasis, a parasitic infectious disease responsible for significant morbidity and mortality, mainly in developing countries. Accurate differentiation of the invasive E. histolytica from the morphologically identical commensal E. dispar is crucial to clinical management of patients and to epidemiologic investigation of outbreaks of amebiasis. Molecularly based differentiation of these two amebas has proven to be adequate for this purpose, and the use of real-time PCR should enhance it even further as a diagnostic application, as it allows fast and sensitive detection of E. histolytica in clinical specimens. This study provides a comparison of all real-time PCR procedures for laboratory diagnosis of amebiasis published through 2004 (4, 25, 27, 28). In addition, we evaluated a SYBR Green assay adapted from a conventional PCR technique published previously (5).
Compared to conventional PCR, real-time PCR has several advantages: not requiring postamplification analysis, which minimizes the risks for laboratory contamination (7); ability to differentiate between E. histolytica and E. dispar infections in a duplex profile (27, 28); and numerical results, which are easier to interpret than the visual examination of a stained gel from a conventional PCR. Nevertheless, real-time PCR is a costly procedure compared with morphological stool exams and antigen-based detection tests. Thus, poor regions of the world, where E. histolytica is most prevalent, will unfortunately be less likely to benefit from real-time PCR. Instead, this technique will be feasible primarily in clinical laboratories in developed countries that need to diagnose amebiasis in travelers and immigrants from regions of the world where E. histolytica is endemic.
An important aspect of real-time PCR is its enhanced sensitivity compared to conventional PCR. As expected, all real-time PCR assays in this study were more sensitive than the conventional PCR, a result that is in agreement with a recent study comparing a novel real-time PCR assay with conventional PCR for amebiasis (22).
The probe-based real-time PCR assays evaluated in this study were able to identify E. histolytica in four clinical samples with very low parasite concentrations, which the conventional PCR could not detect. The most sensitive real-time PCR assay tested was TaqMan 2, i.e., the one designed to amplify episomal-repeat regions (28). The calculated detection limit for this assay was 0.5 cells per ml of spiked stool, which would mean that samples containing only 0.1 cell (DNA was extracted from 0.2 ml stool) were detectable. One explanation for this very low figure is the fact that the DNA target for this assay is located on extrachromosomal, multicopy plasmids (3). Each disrupted cell would therefore release several hundred copies of the DNA target, resulting in the presence of amplifiable DNA even in samples that should contain no cells according to cell count calculations. This fact applies to the other assays in this work as well, since they all target the same multicopy plasmids. Thus, even though the actual values of the detection limits in Table 4 may be slightly overestimated, they can still be used for comparison between the assays. Among the original descriptions of the real-time PCR assays, the detection limit was addressed only for the LightCycler assay (4). The authors used the same approach with spiked stools as in this work and stated that one single trophozoite per sample could be detected. Based on the results with our quantification samples, we have no reason to believe that the LightCycler assay would be less sensitive in our hands. Thus, it seems reasonable to assume that the LightCycler and the two TaqMan assays were able to detect one trophozoite per extracted stool sample, which in this work corresponds to five trophozoites per ml of stool.
The differences in sensitivity among the assays can be partially explained by amplicon size and amplification efficiency. The recommended amplicon size for real-time PCR assays is less than 200 base pairs, so only TaqMan 2 is well designed by this criterion (28). However, sensitivity is also influenced by amplification efficiency, which in turn is related to the quality of the primers (no mismatches, secondary structures, or primer-dimer formation). The primer pair designed for TaqMan 1 (25) resulted in an amplification efficiency of 100%, thus contributing to high sensitivity despite a larger amplicon size. The comparatively very large amplicon size of the SYBR Green assay explains the lower amplification efficiency and sensitivity of the assay.
The clinical sample containing both E. histolytica and E. dispar produced different results in the different assays. The sample was positive only for E. dispar in the SYBR Green assay, probably because the content of E. histolytica was below the detection limit of the assay. The LightCycler assay and TaqMan 2 were more sensitive and thus detected both species. However, TaqMan 1 was also very sensitive but reported the mixed sample as containing E. dispar only. The explanation for this is associated with the duplex assay profile of the latter TaqMan assay, which used the same primers for simultaneous amplification of both species. The overabundance of one species can mask the ability to detect a second species when the same amplification primers are shared. When presented with these circumstances, such duplex (or multiplex) assays that distinguish between targets only by different probes are not suitable for simultaneous detection of more than one microorganism.
Two of the assays could not reliably distinguish E. histolytica from E. dispar: the LightCycler assay and the TaqMan assay targeting episomal repeats (TaqMan 2). The LightCycler assay occasionally reported false-positive results for both E. dispar- and E. histolytica-containing samples, including pure E. histolytica cultures. Furthermore, the false-positive results were not consistent but varied from run to run. This behavior clearly illustrates a lack of specificity of the primers. Thus, in our hands, the LightCycler assay was not considered specific enough to serve as a diagnostic tool for the main purpose of distinguishing E. histolytica from E. dispar. The TaqMan 2 assay produced false results with a few samples containing E. dispar. Two other publications have reported peculiar results concerning detection of E. dispar in conventional PCRs targeting these episomal-repeat sequences. Verweij and coworkers (26) were unable to detect one sample that was positive for E. dispar in two other PCR assays, concluding that the episomal-repeat region seemed to be absent in that particular E. dispar sample. A recent study (11) detected both E. dispar and E. histolytica in a liver pus sample, which must be a false result for E. dispar, since the species is not invasive. Thus, an explanation for the nonspecific results obtained with TaqMan 2 in this study may be that these target sequences are not as species specific as previously reported. Supporting this explanation is the presence of a sequence from an E. dispar strain in GenBank that is highly similar to the assumed E. histolytica-specific episomal repeat (accession number AJ306927). This calls for a reevaluation of the episomal repeats as targets for differential molecular diagnosis of amebiasis.
In conclusion, this work identified the TaqMan targeting the 18S rRNA gene as a superior real-time PCR assay for specific and quantitative diagnosis of amebiasis. The SYBR Green approach offered a good alternative to the TaqMan assay and may be especially attractive for those who already have the conventional PCR assay running and want to convert to the real-time format.
Acknowledgments
We are indebted to Barth Reller and Megan Reller for providing two of the liver aspirates used in the assay evaluations and to student Jeremy Long for performing some of the SYBR Green experiments. We also thank Stephanie Johnston for providing invaluable information about the clinical specimens, Iaci Moura for exquisite technical assistance, and Dennis Juranek and Norman J. Pieniazek for the thorough revision of the manuscript.
The use of trade names is for identification only and does not imply endorsement by the Public Health Service or by the U.S. Department of Health and Human Services.
REFERENCES
- 1.Acuna-Soto, R., J. Samuelson, P. De Girolami, L. Zarate, F. Millan-Velasco, G. Schoolnick, and D. Wirth. 1993. Application of the polymerase chain reaction to the epidemiology of pathogenic and nonpathogenic Entamoeba histolytica. Am. J. Trop. Med. Hyg. 48:58-70. [DOI] [PubMed] [Google Scholar]
- 2.Bell, A. S., and L. C. Ranford-Cartwright. 2002. Real-time quantitative PCR in parasitology. Trends Parasitol. 18:337-342. [PubMed] [Google Scholar]
- 3.Bhattacharyaa, S., I. Soma, and A. Bhattacharyab. 1998. The Ribosomal DNA plasmids of entamoeba. Parasitol. Today 14:181-185. [DOI] [PubMed] [Google Scholar]
- 4.Blessmann, J., H. Buss, P. A. Nu, B. T. Dinh, Q. T. Ngo, A. L. Van, M. D. Alla, T. F. Jackson, J. I. Ravdin, and E. Tannich. 2002. Real-time PCR for detection and differentiation of Entamoeba histolytica and Entamoeba dispar in fecal samples. J. Clin. Microbiol. 40:4413-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark, C. G., and L. S. Diamond. 1992. Differentiation of pathogenic Entamoeba histolytica from other intestinal protozoa by riboprinting. Arch. Med. Res. 23:15-16. [PubMed] [Google Scholar]
- 6.da Silva, A. J., F. J. Bornay-Llinares, I. N. Moura, S. B. Slemenda, J. L. Tuttle, and N. J. Pieniazek. 1999. Fast and reliable extraction of protozoan parasite DNA from fecal specimens. Mol. Diagn. 4:57-64. [DOI] [PubMed] [Google Scholar]
- 7.da Silva, A. J., and N. J. Pieniazek. 2003. Latest advances and trends in PCR-based diagnostic methods, p. 397-412. In D. Dionisio (ed.), Textbook-atlas of intestinal infections in AIDS. Springer-Verlag Italia, Milan, Italy.
- 8.Diamond, L. S., and C. G. Clark. 1993. A redescription of Entamoeba histolytica Schaudinn, 1903 (Emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. J. Eukaryot. Microbiol. 40:340-344. [DOI] [PubMed] [Google Scholar]
- 9.Diamond, L. S., D. R. Harlow, and C. C. Cunnick. 1978. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72:431-432. [DOI] [PubMed] [Google Scholar]
- 10.Evangelopoulos, A., G. Spanakos, E. Patsoula, N. Vakalis, and N. Legakis. 2000. A nested, multiplex, PCR assay for the simultaneous detection and differentiation of Entamoeba histolytica and Entamoeba dispar in faeces. Ann. Trop. Med. Parasitol. 94:233-240. [DOI] [PubMed] [Google Scholar]
- 11.Furrows, S. J., A. H. Moody, and P. L. Chiodini. 2004. Comparison of PCR and antigen detection methods for diagnosis of Entamoeba histolytica infection. J. Clin. Pathol. 57:1264-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garfinkel, L. I., M. Giladi, M. Huber, C. Gitler, D. Mirelman, M. Revel, and S. Rozenblatt. 1989. DNA probes specific for Entamoeba histolytica possessing pathogenic and nonpathogenic zymodemes. Infect. Immun. 57:926-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haque, R., I. K. Ali, S. Akther, and W. A. Petri, Jr. 1998. Comparison of PCR, isoenzyme analysis, and antigen detection for diagnosis of Entamoeba histolytica infection. J. Clin. Microbiol. 36:449-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haque, R., N. U. Mollah, I. K. Ali, K. Alam, A. Eubanks, D. Lyerly, and W. A. Petri, Jr. 2000. Diagnosis of amebic liver abscess and intestinal infection with the TechLab Entamoeba histolytica II antigen detection and antibody tests. J. Clin. Microbiol. 38:3235-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katzwinkel-Wladarsch, S., T. Loscher, and H. Rinder. 1994. Direct amplification and differentiation of pathogenic and nonpathogenic Entamoeba histolytica DNA from stool specimens. Am. J. Trop. Med. Hyg. 51:115-118. [DOI] [PubMed] [Google Scholar]
- 16.Kebede, A., J. Verweij, W. Dorigo-Zetsma, E. Sanders, T. Messele, L. van Lieshout, B. Petros, and T. Polderman. 2003. Overdiagnosis of amoebiasis in the absence of Entamoeba histolytica among patients presenting with diarrhoea in Wonji and Akaki, Ethiopia. Trans. R. Soc. Trop. Med. Hyg. 97:305-307. [DOI] [PubMed] [Google Scholar]
- 17.Mirelman, D., Y. Nuchamowitz, and T. Stolarsky. 1997. Comparison of use of enzyme-linked immunosorbent assay-based kits and PCR amplification of rRNA genes for simultaneous detection of Entamoeba histolytica and E. dispar. J. Clin. Microbiol. 35:2405-2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novati, S., M. Sironi, S. Granata, A. Bruno, S. Gatti, M. Scaglia, and C. Bandi. 1996. Direct sequencing of the PCR amplified SSU rRNA gene of Entamoeba dispar and the design of primers for rapid differentiation from Entamoeba histolytica. Parasitology 112:363-369. [DOI] [PubMed] [Google Scholar]
- 19.Nunez, Y. O., M. A. Fernandez, D. Torres-Nunez, J. A. Silva, I. Montano, J. L. Maestre, and L. Fonte. 2001. Multiplex polymerase chain reaction amplification and differentiation of Entamoeba histolytica and Entamoeba dispar DNA from stool samples. Am. J. Trop. Med. Hyg. 64:293-297. [DOI] [PubMed] [Google Scholar]
- 20.Pinheiro, S. M., R. M. Carneiro, I. S. Aca, J. I. Irmao, M. A. Morais, Jr., M. R. Coimbra, and L. B. Carvalho, Jr. 2004. Determination of the prevalence of Entamoeba histolytica and E. dispar in the pernambuco state of northeastern Brazil by a polymerase chain reaction. Am. J. Trop. Med. Hyg. 70:221-224. [PubMed] [Google Scholar]
- 21.Romero, J. L., S. Descoteaux, S. Reed, E. Orozco, J. Santos, and J. Samuelson. 1992. Use of polymerase chain reaction and nonradioactive DNA probes to diagnose Entamoeba histolytica in clinical samples. Arch. Med. Res. 23:277-279. [PubMed] [Google Scholar]
- 22.Roy, S., M. Kabir, D. Mondal, I. K. Ali, W. A. Petri, Jr., and R. Haque. 2005. Real-time-PCR assay for diagnosis of Entamoeba histolytica infection. J. Clin. Microbiol. 43:2168-2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma, A. K., S. Chibbar, G. Bansal, U. Kaur, and H. Vohra. 2003. Evaluation of newer diagnostic methods for the detection and differentiation of Entamoeba histolytica in an endemic area. Trans. R. Soc. Trop. Med. Hyg. 97:396-397. [DOI] [PubMed] [Google Scholar]
- 24.Tanyuksel, M., and W. A. Petri, Jr. 2003. Laboratory diagnosis of amebiasis. Clin. Microbiol. Rev. 16:713-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verweij, J. J., R. A. Blange, K. Templeton, J. Schinkel, E. A. Brienen, M. A. van Rooyen, L. van Lieshout, and A. M. Polderman. 2004. Simultaneous detection of Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum in fecal samples by using multiplex real-time PCR. J. Clin. Microbiol. 42:1220-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verweij, J. J., J. Blotkamp, E. A. Brienen, A. Aguirre, and A. M. Polderman. 2000. Differentiation of Entamoeba histolytica and Entamoeba dispar cysts using polymerase chain reaction on DNA isolated from faeces with spin columns. Eur. J. Clin. Microbiol. Infect. Dis. 19:358-361. [DOI] [PubMed] [Google Scholar]
- 27.Verweij, J. J., F. Oostvogel, E. A. Brienen, A. Nang-Beifubah, J. Ziem, and A. M. Polderman. 2003. Prevalence of Entamoeba histolytica and Entamoeba dispar in northern Ghana. Trop. Med. Int. Health 8:1153-1156. [DOI] [PubMed] [Google Scholar]
- 28.Verweij, J. J., J. Vermeer, E. A. Brienen, C. Blotkamp, D. Laeijendecker, L. van Lieshout, and A. M. Polderman. 2003. Entamoeba histolytica infections in captive primates. Parasitol. Res. 90:100-103. [DOI] [PubMed] [Google Scholar]
- 29.Walsh, J. A. 1986. Problems in recognition and diagnosis of amebiasis: estimation of the global magnitude of morbidity and mortality. Rev. Infect. Dis. 8:228-238. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. 1997. Amoebiasis. Wkly. Epidemiol. Rec. 72:97-99.9100475 [Google Scholar]
- 31.Yap, E. P. H., Y.-M. O. Lo, K. A. Flemming, and J. O. D. McGee. 1994. False-positives and contamination in PCR, p. 249-258. In H. G. Griffin and A. M. Griffin (ed.), PCR technology. Current innovations. CRC Press, Boca Raton, Fla.
- 32.Zengzhu, G., R. Bracha, Y. Nuchamowitz, I. W. Cheng, and D. Mirelman. 1999. Analysis by enzyme-linked immunosorbent assay and PCR of human liver abscess aspirates from patients in China for Entamoeba histolytica. J. Clin. Microbiol. 37:3034-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]