Abstract
GSQ1530 is a compound derived from a newly identified class of antibiotics referred to as heteroaromatic polycyclic (HARP) antibiotics. The aim of this study was to assess the in vitro antimicrobial activity of GSQ1530. By using an NCCLS broth microdilution assay, the activities of GSQ1530 and other antibiotics were coevaluated against 215 clinical isolates. The MICs at which 90% of isolates are inhibited (MIC90s) of GSQ1530 for methicillin-susceptible Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) were 2 and 4 μg/ml, respectively. The MIC90s of GSQ1530 for the streptococci tested were 2 μg/ml or less, regardless of their susceptibilities to other antibiotics. The MIC90 of GSQ1530 for the enterococci tested (including vancomycin-resistant enterococci) was 4 μg/ml. No cross-resistance was found between GSQ1530 and other known antibiotics. In a separate assay, GSQ1530 demonstrated excellent activity against vancomycin-intermediate-susceptible staphylococci (MIC90, 1 μg/ml). The minimal bactericidal concentration test was conducted with 73 clinical isolates; GSQ1530 was cidal against streptococci and staphylococci but static against enterococci. An in vitro killing kinetic study revealed a time-dependent profile, with at least a 3-log reduction of bacterial growth within 6 h after exposure to four times the MICs of GSQ1530 for both S. aureus and Streptococcus pneumoniae. The checkerboard study showed that GSQ1530 had a synergistic interaction with rifampin against MRSA. The test medium was found to have little effect on in vitro antimicrobial potency. The MICs of GSQ1530 for gram-positive cocci were 4- to 32-fold higher in the presence of serum proteins. GSQ1530 has high levels of plasma protein binding (91 and 89% for rat and human plasma, respectively). These preliminary results demonstrate that GSQ1530, a representative compound of our novel HARP antibiotics, has broad-spectrum activity against gram-positive bacteria. This novel class of antibacterial compounds is profiled in vivo to assess the therapeutic potential in humans. Ongoing in vivo studies will assess whether this class of molecules has promising in vivo efficacy and safety profiles.
The rates of resistance of pathogenic microorganisms to commercialized antimicrobial agents are increasing with an alarming frequency. A significant percentage of clinical isolates, including species of streptococci, staphylococci, enterococci, enterobacteria, and Pseudomonas spp., are resistant to commonly used antibiotics, e.g., new β-lactams, glycopeptides, fluoroquinolones, and macrolides (3, 5, 10, 11, 12, 14). Therefore, there is an urgent medical need for antibiotics with novel antimicrobial mechanisms.
Heteroaromatic polycyclic (HARP) antibiotics, a class of small DNA-binding molecules, were discovered by screening a diverse library of DNA-binding molecules that preferentially target A/T-rich sites commonly found in bacterial promoters and replication origins (R. Bürli, M. Taylor, Y. Ge, E. Baird, S. Touami, and H. Moser, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1685, p. 241, 2001). This class of molecules exerts its antibacterial activity by binding to the minor groove of DNA, resulting in inhibition of DNA function and DNA-dependent RNA transcription (Y. Ge, J. Wu, and S. White, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1686, p. 241, 2001). Optimization of early hits has produced antimicrobial lead compounds with greatly improved potencies. GSQ1530 is a small DNA-binding molecule that was synthesized as part of the lead compound optimization program (Fig. 1). The objective of this study was to investigate the in vitro activity profiles of this class of molecules in detail by using GSQ1530 as a representative compound.
FIG.1.
Structure of GSQ1530. AcO−, acetate ion.
(This work was presented in part at the 41st Interscience Conference on Antimicrobial Agents and Chemotherapy, 16 to 19 December 2001, Chicago, Ill. [Y. Ge, S. Touami, I. Critchley, M. Taylor, E. Baird, R. Bürli, A. Pennell, and H. Moser, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-1687, p. 241, 2001].)
MATERIALS AND METHODS
Bacteria and agents.
The clinical isolates tested were recently collected (over the last 4 years) from hospitals throughout the United States. Recent clinical isolates of vancomycin-intermediate-susceptible staphylococci (VIS) were acquired from the Network on Antimicrobial Resistance in Staphylococcus aureus Repository. GSQ1530 was synthesized and purified as an acetate salt (Bürli et al., 41st ICAAC), dissolved in 100% dimethyl sulfoxide (10 mg/ml), and stored at −80°C. Antibacterial agents used as controls were purchased from qualified vendors or directly from the manufacturers. These antibiotics were prepared for testing according to the instructions of the manufacturers.
MIC and MBC tests.
The in vitro susceptibility tests (MIC and minimal bactericidal concentration [MBC] tests) were conducted by the NCCLS broth microplate assay (7, 8). For fastidious species, appropriate supplements and media were used according to standard protocols. For testing of clinical isolates other than VIS, premanufactured drug panel plates (Trek Diagnostic Systems Inc., Westlake, Ohio) that contained a total of six antimicrobial agents (see Table 1) were used. To determine the effects of protein and serum on in vitro antimicrobial properties, MIC tests were performed in the presence of 25% mouse serum (Equitech-Bio Inc., Ingram, Tex.) (either heat inactivated at 56°C for 30 min or non-heat inactivated) or 4% human plasma albumin (HPA; from a 30% HPA stock; Calbiochem-Novabiochem Corp., Pasadena, Calif.).
TABLE 1.
In vitro activities of GSQ1530 and other antibiotics against gram-positive pathogens
| Organism (no. of isolates) and compound | MIC (μg/ml)
|
||
|---|---|---|---|
| 50% | 90% | Range | |
| S. aureus, methicillin susceptible (21) | |||
| GSQ1530 | 1 | 2 | 0.5-4 |
| Linezolid | 2 | 4 | 1-4 |
| Vancomycin | 0.5 | 0.5 | 0.5-1 |
| Oxacillin | 0.25 | 0.5 | 0.12-2 |
| Quin/dalfoa | <0.12 | 0.25 | <0.12-0.25 |
| Moxifloxacin | 0.03 | 0.12 | 0.03-0.5 |
| S. aureus, methicillin resistant (19) | |||
| GSQ1530 | 1 | 4 | 1-4 |
| Linezolid | 4 | 4 | 1-16 |
| Vancomycin | 0.5 | 0.5 | 0.5-1 |
| Oxacillin | 8 | 16 | 4-16 |
| Quin/dalfo | <0.12 | 0.25 | <0.12-0.25 |
| Moxifloxacin | 0.12 | 8 | 0.03-8 |
| S. epidermidis, methicillin susceptible (10) | |||
| GSQ1530 | 2 | 2 | 2 |
| Linezolid | 1 | 2 | 1-16 |
| Vancomycin | 1 | 1 | 1 |
| Oxacillin | <0.03 | 0.12 | <0.03-0.12 |
| Quin/dalfo | <0.12 | <0.12 | <0.12 |
| Moxifloxacin | 0.06 | 0.06 | 0.06 |
| S. epidermidis, methicillin resistant (10) | |||
| GSQ1530 | 2 | 2 | 1-2 |
| Linezolid | 1 | 2 | 1-16 |
| Vancomycin | 1 | 1 | 11 |
| Erythromycin | >16 | >16 | 1->16 |
| Oxacillin | 2 | >32 | 1->32 |
| Quin/dalfo | <0.12 | <0.12 | <0.12 |
| Moxifloxacin | 0.06 | 2 | 0.03-2 |
| S. pneumoniae, penicillin susceptible (19) | |||
| GSQ1530 | 1 | 2 | 0.5-2 |
| Linezolid | 0.5 | 1 | <0.25-1 |
| Vancomycin | 0.25 | 0.25 | <0.03-0.25 |
| Oxacillin | <0.03 | 0.12 | <0.03-0.12 |
| Quin/dalfo | <0.12 | 0.25 | <0.12-0.25 |
| Moxifloxacin | 0.12 | 0.12 | 0.06-0.12 |
| S. pneumoniae, penicillin resistant (18) | |||
| GSQ1530 | 1 | 2 | 1-2 |
| Linezolid | 0.5 | 1 | <0.25-1 |
| Vancomycin | 0.12 | 0.25 | 0.12-0.25 |
| Oxacillin | <0.03 | 2 | <0.03-2 |
| Quin/dalfo | <0.12 | <0.03 | <0.03 |
| Moxifloxacin | 0.12 | 0.12 | 0.06-0.12 |
| S. pneumoniae, multidrug resistant (19) | |||
| GSQ1530 | 1 | 2 | 0.5-2 |
| Linezolid | 0.5 | 1 | <0.25-1 |
| Vancomycin | 0.12 | 0.25 | <0.03-0.25 |
| Oxacillin | <0.03 | 0.5 | <0.03-4 |
| Quin/dalfo | <0.25 | 0.5 | <0.12-0.5 |
| Moxifloxacin | 0.12 | 4 | 0.06-4 |
| Group A Streptococcus (20) | |||
| GSQ1530 | 1 | 1 | 0.5-1 |
| Linezolid | 1 | 1 | 0.5-2 |
| Vancomycin | 0.25 | 0.25 | 0.12-0.25 |
| Oxacillin | <0.03 | <0.03 | <0.03-0.06 |
| Quin/dalfo | <0.12 | <0.12 | <0.12 |
| Moxifloxacin | 0.12 | 0.12 | 0.06-0.25 |
| Group B Streptococcus (19) | |||
| GSQ1530 | 1 | 1 | 0.5-1 |
| Linezolid | 1 | 1 | 0.5-2 |
| Vancomycin | 0.25 | 0.25 | 0.12-0.5 |
| Oxacillin | 0.12 | 0.12 | 0.12-0.25 |
| Quin/dalfo | <0.12 | <0.12 | <0.12-0.25 |
| Moxifloxacin | 0.12 | 0.12 | 0.12-0.25 |
| Viridans group streptococci (20) | |||
| GSQ1530 | 2 | 2 | 0.5-4 |
| Linezolid | 0.5 | 1 | <0.25-1 |
| Vancomycin | 0.25 | 0.25 | 0.12-0.5 |
| Oxacillin | <0.03 | 0.5 | <0.03-4 |
| Quin/dalfo | <0.25 | 0.5 | <0.12-0.5 |
| Moxifloxacin | 0.12 | 4 | 0.06-4 |
| E. faecalis, vancomycin- susceptible (11) | |||
| GSQ1530 | 2 | 4 | 0.5-8 |
| Linezolid | 2 | 2 | 0.5-2 |
| Vancomycin | 1 | 1 | 0.5-2 |
| Oxacillin | 16 | 32 | 8-> 32 |
| Quin/dalfo | 4 | 4 | 2-16 |
| Moxifloxacin | 0.25 | 8 | 0.12-8 |
| E. faecalis, VIEb or VRE (9) | |||
| GSQ1530 | 2 | NDc | 1-8 |
| Linezolid | 2 | ND | 1-2 |
| Vancomycin | >32 | ND | 32-> 32 |
| Oxacillin | 16 | ND | 8-> 32 |
| Quin/dalfo | 16 | ND | 4-> 16 |
| Moxifloxacin | 2 | ND | 0.12->8 |
| E. faecium, VIE or VRE (20) | |||
| GSQ1530 | 1 | 4 | 0.5-4 |
| Linezolid | 2 | 2 | 1-2 |
| Vancomycin | >32 | >32 | 16-32 |
| Oxacillin | >32 | >32 | 4-32 |
| Quin/dalfo | 0.5 | 0.5 | 0.25-8 |
| Moxifloxacin | 0.12 | >8 | 0.12->8 |
Quin/dalfo, quinupristin-dalfopristin.
VIE, vancomycin-intermediate-susceptible enterococci.
ND, not determined.
In vitro killing-curve assay.
In vitro killing kinetic studies were performed by a previously described method (6). In brief, 106 CFU of fresh log-phase cells per ml were treated with appropriate concentrations of agents, and at the designated time point, bacterial cultures were harvested and plated in duplicate for viability counting. For groups treated with a high dose of drug (10 times the MIC), the bacteria were further diluted up to 10-fold before they were plated to reduce the concentration of drug carried over, and then 0.5 ml of the cultures was plated on 150-mm-diameter plates for colony counting.
PAE.
The log-phase bacteria were collected and adjusted to a concentration of 4 × 108 CFU/ml. After the treatment of the bacterial cultures in the presence of GSQ1530 at one or two times the MIC for 1 h, the cells were diluted 1:200 in fresh medium and then incubated and plated out at the appropriate time points for viability determination. The postantibiotic effect (PAE) was calculated by the standard equation, T − C, where T is the time required for the CFU count in the test culture to increase 10-fold above the count observed immediately after drug removal, and C is the time required for the count for the untreated control to increase 10-fold under the same conditions (6).
Synergistic study.
A standard checkerboard assay was performed by a well-established method (6). The fractional inhibitory concentration (FIC) index was calculated by a previously described method (6). An FIC index between two compounds less than or equal to 0.5 is considered synergism, an FIC index between 0.5 and 2 is considered indifference, and an FIC index equal to or more than 2 is considered antagonism.
Measurement of in vitro protein-binding affinity.
The protein assay was conducted by a previously described protocol (2). An aliquot of the stock solution of GSQ1530 or linezolid (control), each dissolved in methanol at 2 mg/ml, was added to 2 ml of rat plasma (Pel-Freez Biologicals, Rogers, Ark.) or human plasma (Plasma Care, Cincinnati, Ohio) to give a final concentration of 2.0 μg/ml. The spiked plasma samples were then incubated at 37°C for 20 min. An aliquot of 0.2 ml was taken out and saved at 4°C as the precentrifugation plasma control to measure the total drug concentration in the following test. The remaining 1.8 ml of the plasma sample was subjected to one round of high-speed centrifugation (300,000 × g for 3 h at 4°C) with an ultracentrifuge (model Optima TLX; Beckman, Fullerton, Calif.) with a Beckman 110K rotor. An aliquot of 0.2 ml of the supernatant was taken to determine the free drug concentration. Both the precentrifugation plasma sample and the supernatant were precipitated with acetonitrile that contained an internal standard and were then subjected to a round of centrifugation at 1,462 × g (with a model 6R centrifuge with a GH 3.8 rotor; Beckman) for 5 min. Finally, an aliquot of the supernatants from each of the samples was analyzed by liquid chromatography-mass spectrometry. The relative concentration was defined as the ratio of the peak area of the compound to that of the internal standard. The relative concentration was used to calculate the free drug percentage of percent binding to the protein. The limit of quantitation of the liquid chromatography-mass spectrometry method was 2 ng/ml. The relative standard deviation in the intraday variation was less than 10%, which was acceptable for this assay.
RESULTS
In vitro antibacterial potencies and spectra of activity.
The MICs of GSQ1530 and six other antibiotic agents for 215 gram-positive isolates were determined (Table 1). GSQ1530 displayed excellent activities against staphylococci, such as methicillin-susceptible S. aureus (MSSA), methicillin-resistant S. aureus (MRSA), methicillin-susceptible Staphylococcus epidermidis (MSSE), and methicillin-resistant S. epidermidis (MRSE), with MICs at which 90% of isolates are inhibited (MIC90s) ranging from 2 to 4 μg/ml. In a separate study (Table 2), GSQ1530 displayed excellent activity (MIC90, 1 μg/ml) against VIS (a total of 45 isolates), including the species S. aureus, Staphylococcus haemolyticus, and S. epidermidis. The strains of VIS showed significant resistance to the other comparative antibiotics tested (with the exception of linezolid), as indicated by the wide range of MICs for those isolates and the significant differences between the MIC50s and MIC90s. The MIC90s of GSQ1530 for Streptococcus pneumoniae, group A Streptococcus, group B Streptococcus, and viridans group streptococci were approximately 1 to 2 μg/ml, irrespective of their susceptibilities to the other antibiotics tested (Table 1). Enterococcus spp., including vancomycin-resistant enterococci (VRE), was also inhibited by GSQ1530 (MIC range, 1 to 4 μg/ml). In addition to the species delineated in Table 1, we also conducted limited tests with other gram-positive aerobic and anaerobic bacteria, including Bacillus spp., Listeria monocytogenes, Micrococcus spp., Corynebacterium spp., Clostridium spp., Peptostreptococcus spp., and Propionibaterium acnes. GSQ1530 displayed superior activities against those species, with the MICs ranging from 0.008 to 1 μg/ml (data not shown). Despite the superior activity of GSQ1530 against gram-positive bacteria, GSQ1530 was inactive against gram-negative bacteria, such as Escherichia coli, Haemophilus influenzae, Acinetobacter spp., and Pseudomonas aeruginosa (MICs, >64 μg/ml), but had weak activity against Moraxella catarrhalis (MICs, 16 to 64 μg/ml) (data not shown).
TABLE 2.
In vitro activities of GSQ1530 and other antibiotics against VIS
| Organism (no. of isolates)a | MIC (μg/ml)
|
||
|---|---|---|---|
| 50% | 90% | Range | |
| S. aureus (35) | |||
| GSQ1530 | 0.5 | 1 | 0.125-4 |
| Ampicillin | 64 | >64 | 2->64 |
| Erythromycin | >64 | >64 | 0.5->64 |
| Fusidic acid | 0.25 | 2 | 0.062->64 |
| Linezolid | 2 | 4 | 0.4-8 |
| Ofloxacin | 32 | >64 | 0.5->64 |
| Rifampin | 0.062 | >64 | 0.062->64 |
| Vancomycin | 4 | 8 | 2-16 |
| S. epidermidis (6) | |||
| GSQ1530 | 0.5 | NDb | 0.125-1 |
| Ampicillin | 32 | ND | 16-64 |
| Erythromycin | >64 | ND | >64 |
| Fusidic acid | 0.25 | ND | 0.125-0.25 |
| Linezolid | 2 | ND | 1-2 |
| Ofloxacin | 4 | ND | 0.5->64 |
| Rifampin | 0.062 | ND | 0.062->64 |
| Vancomycin | 4 | ND | 4-16 |
| S. haemolyticus (4) | |||
| GSQ1530 | 1 | ND | 1-2 |
| Ampicillin | >64 | ND | >64 |
| Erythromycin | >64 | ND | >64 |
| Fusidic acid | 0.062 | ND | 0.062-0.125 |
| Linezolid | 0.5 | ND | 0.5-2 |
| Ofloxacin | 16 | ND | 16->64 |
| Rifampin | 0.062 | ND | 0.062-0.125 |
| Vancomycin | 4 | ND | 2-8 |
The isolates were obtained from the Network on Antimicrobial Resistance in Staphylococcus aureus Repository, and all isolates were VIS.
ND, not determined.
Bactericidal activities of GSQ1530.
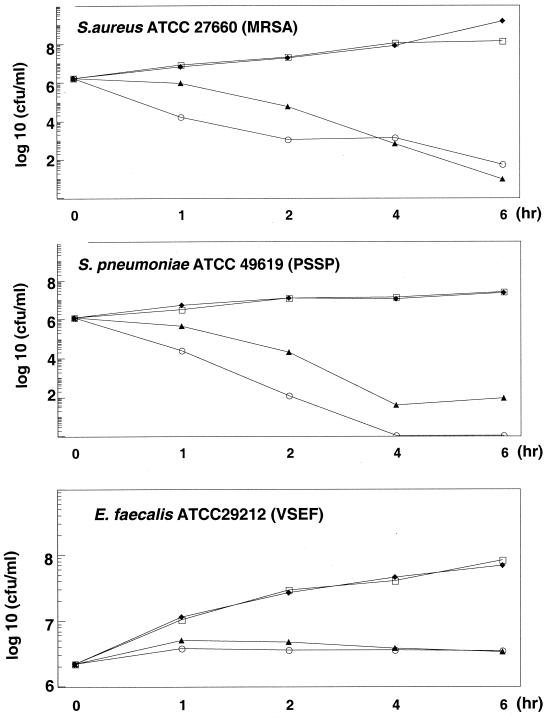
To assess the killing potency of GSQ1530, we conducted both MBC and killing-curve studies. MBCs were measured for a total of 67 clinical isolates, including isolates of MSSA (8 isolates), MRSA (10 isolates), MSSE (10 isolates), MRSE (10 isolates), S. pneumoniae (7 isolates), group A Streptococcus (10 isolates), and Enterococcus faecium (12 isolates). With the exception of Enterococcus spp., the differences between the MICs and the MBCs were less than 4 twofold dilutions for most of the streptococci and staphylococci tested, suggesting that GSQ1530 has cidal activity against these species. By contrast, the MBCs for Enterococcus faecalis were at least 3 twofold dilutions higher than the MICs. The killing kinetic assays were done with three species: S. aureus (MRSA), S. pneumoniae, and E. faecalis (Fig. 2). In the presence of 2.5 and 10 times the MICs of GSQ1530, more than 3-log reductions in the counts of S. aureus and S. pneumoniae were observed within the first 6 h. In the presence of GSQ1530 at 10 times the MIC, more than 106 CFU of pneumococcal cells per ml were rapidly eliminated to levels beyond the threshold of detection. No regrowth was noted over 24 h (data not shown). By contrast, the killing curve for E. faecalis showed neither growth nor a decline, indicating that GSQ1530 was static against this species.
FIG. 2.
In vitro killing kinetics of GSQ1530. The MICs for the species tested were as follows: 0.5 μg/ml for S. aureus ATCC 27660, 0.5 μg/ml for penicillin-susceptible S. pneumoniae (PSSP) ATCC 49619, and 1 μg/ml for vancomycin-susceptible E. faecalis (VSEF) ATCC 29212. □, control; ⧫, one-half the MIC; ▴, two times the MIC; ○, four times the MIC.
Medium and growth condition effects.
To investigate the influences of the growth medium and growth conditions on antimicrobial potency, we measured the changes in the MICs of GSQ1530 and the other (control) agents as a function of inoculum size, medium pH, medium ion concentration, and growth phase (Table 3). The growth phase, the ion concentration in the growth medium, and the inoculum size (1 × 105 to 5 × 107) did not significantly affect the in vitro activities of GSQ1530 against S. aureus and E. faecalis. With an extremely large inoculum (>108 CFU/ml), however, both GSQ1530 and the control antibiotics lost their activities (MICs, > 32 μg/ml). The pH of the medium appeared to directly influence the in vitro activity of GSQ1530 against S. aureus; the MICs decreased gradually from 2 to 0.031 μg/ml between pH 5.0 and pH 9.0 (Table 3). This trend was not observed for either of the control drugs (vancomycin and ofloxacin). In contrast, the effect of the medium pH on the activity of GSQ1530 against E. faecalis was not as dramatic as it was in the case of S. aureus. As demonstrated in Table 4, mouse serum and HPA were observed to have inhibitory effects on the in vitro antimicrobial activity of GSQ1530: the presence of 25% mouse serum or 4% HPA increased the MICs of GSQ1530 for MRSA, S. pneumoniae, and E. faecalis. A similar effect was also observed for fusidic acid, while mouse serum or 4% HPA had only a minimal effect on the in vitro antimicrobial activities of the other antibiotics tested. There was no difference in MICs between heat-inactivated and normal serum.
TABLE 3.
Effect of growth condition on antimicrobial activity of GSQ1530
| Growth condition | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
|
S. aureus ATCC 27660 (MRSA)
|
E. faecalis ATCC 29212
|
|||||
| GSQ1530 | Ofloxacin | Vancomycin | GSQ1530 | Ofloxacin | Vancomycin | |
| Growth phase | ||||||
| Stationary | 0.25 | 0.25 | 0.25 | 0.5 | 32 | 0.25 |
| Log | 0.25 | 0.125 | 0.25 | 0.5 | 16 | 0.25 |
| pH | ||||||
| CAMHBa (pH 5.0) | 8 | 1 | 1 | 2 | >32 | 0.5 |
| CAMHB (pH 6.0) | 1 | 0.25 | 0.5 | 1 | 8 | 0.25 |
| CAMHB (control, pH 7.2) | 0.25 | 0.125 | 0.5 | 1 | 8 | 0.25 |
| CAMHB (pH 8.0) | 0.062 | 0.25 | 0.5 | 1 | 16 | 0.5 |
| CAMHB (pH 9.0) | 0.031 | 0.5 | 2 | 0.5 | 32 | 2 |
| Ion concentration | ||||||
| CAMHB | 0.25 | 0.125 | 0.5 | 1 | 8 | 0.25 |
| CAMHB + 0.5 M NaCl | 0.25 | 0.5 | 1 | 0.25 | 8 | 0.25 |
| MHBb + MgCl2 + CaCl2c | 0.25 | 0.25 | 0.25 | 0.5 | 8 | 0.25 |
| Inoculum size (CFU/ml) | ||||||
| 1 × 105 to 2 × 105 | 0.125 | 0.125 | 0.25 | 0.5 | 4 | 0.25 |
| 1 × 105 to 2 × 106 | 0.25 | 0.125 | 0.5 | 1 | 8 | 0.25 |
| 1 × 105 to 2 × 107 | 0.25 | 0.25 | 1 | 2 | 32 | 0.25 |
CAMHB, cation-adjusted Mueller-Hinton broth.
MNB, Mueller-Hinton broth.
MgCl2, 22 mg/liter; CaCl2, 98 mg/liter.
TABLE 4.
Effects of serum and HPA on antimicrobial activity of GSQ1530
| Organism and growth condition | MIC (μg/ml)
|
||||||
|---|---|---|---|---|---|---|---|
| GSQ1530 | Vancomycin | Amoxacillin | Ofloxacin | Fusidic acid | Rifampin | Linezolid | |
| S. aureus ATCC 27660 (MRSA) | |||||||
| CAMHBa | 0.5 | 1 | >64 | 0.5 | 0.25 | ≤0.062 | 2 |
| CAMHB + 25% mouse serum | 4 | 1 | >64 | 0.5 | 8 | ≤0.062 | 2 |
| CAMHB + 25% inactivated serumb | 4 | 1 | >64 | 0.25 | 2 | ≤0.062 | 2 |
| CAMHB + 4% HPA | 4 | 1 | >64 | 0.25 | 16 | ≤0.062 | 4 |
| S. pneumoniae ATCC 49619 | |||||||
| CAMHB | 0.5 | 0.25 | 0.062 | 1 | 8 | 0.062 | 0.5 |
| CAMHB + 25% mouse serum | 2 | 0.25 | 0.25 | 1 | 64 | 0.25 | 1 |
| CAMHB + 25% inactivated serumb | 4 | 0.25 | 0.062 | 1 | 64 | 0.062 | 1 |
| CAMHB + 4% HPA | 2 | 0.25 | 0.062 | 1 | >64 | 0.062 | 0.5 |
| E. faecalis ATCC 29212 | |||||||
| CAMHB | 1 | 1 | 0.5 | 16 | 4 | 32 | 1 |
| CAMHB + 25% mouse serum | 16 | 1 | 2 | 8 | 64 | >64 | 2 |
| CAMHB + 25% inactivated serumb | 16 | 2 | 1 | 8 | >64 | >64 | 4 |
| CAMHB + 4% HPA | 8 | 1 | 4 | >64 | 32 | >64 | 1 |
CAMHB, cation-adjusted Mueller-Hinton broth.
Heat inactivated at 56°C for 30 min.
Protein binding. We assessed the level of protein binding in rat and human plasma by a centrifugation assay. GSQ1530 appeared to have a strong affinity for binding to both rat and human plasma proteins (92 and 89% binding, respectively) compared with that (22%) for linezolid (to rat plasma), which was used as a control. Those values are consistent with a higher level of protein binding, as suggested above from the MICs determined in the presence of proteins.
In vitro PAE.
The in vitro PAEs of GSQ1530 against S. aureus and S. pneumoniae were examined. Only relatively short PAEs were observed: 0.1 and 0.4 h for MRSA and S. pneumoniae, respectively.
Synergism study.
A standard checkerboard study was conducted by a broth microplate method to determine the in vitro drug interaction between GSQ1530 and antibiotics of other classes, including vancomycin, ofloxacin, amoxicillin, linezolid, rifampin, and kanamycin, against MRSA and S. pneumoniae. GSQ1530 showed a synergistic interaction with rifampin against MRSA, with an FIC index of less than 0.4. No drug interactions were observed between GSQ1530 and representatives of the other classes of antibiotics tested, with FIC indices ranging from 1 to 2.
DISCUSSION
HARP antibiotics are among the most active DNA-binding antimicrobial molecules that have been reported to date. GSQ1530 is more active than other polypyrrole carboxamides that target the minor groove, such as distamycin and netropsin, against gram-positive bacteria (Y. Ge, and J. Wu, unpublished data). GSQ1530 demonstrated excellent potencies against gram-positive bacteria, including MRSA, vancomycin-intermediate S. aureus, MRSE, VRE, and penicillin-resistant S. pneumoniae (PRSP). The in vitro potencies and spectra of activity of GSQ1530 are comparable to those of drugs with activities against gram-positive organisms, such as vancomycin and linezolid: MIC90s are 2 to 4 μg/ml for all gram-positive pathogens (4, 9). In addition to its general effect, GSQ1530 also works effectively in vitro against VIS. As shown in this study, strains of VIS had reduced susceptibilities to vancomycin and also demonstrated multiple-drug-resistant profiles, with resistance to ofloxacin, ampicillin, fusidic acid, erythromycin, and rifampin. In contrast, only GSQ1530 and linezolid are consistently active against these isolates. To date no cross-resistance between GSQ1530 and other classes of antibiotics has been found. Unlike linezolid, the bactericidal spectrum of GSQ1530 is comparable to that of vancomycin: it is cidal against staphylococci (MRSA) and streptococci but static against enterococci. The time-dependent killing kinetics of GSQ1530 against MRSA and PRSP are similar to those of vancomycin, with the notable exception of the killing kinetics against S. pneumoniae, which shows a greater sensitivity to killing by GSQ1530. This rapid and complete bactericidal property against PRSP may give HARP antibiotics an advantage over other classes of antibiotics. In addition, preliminary data show that bacterial killing activity is replication dependent: GSQ1530 exerts its killing activity only against replicating cells (data not shown), suggesting that the antimicrobial mechanisms of this compound are not directed against the cell membrane and essential cellular respiratory systems. Although the proposed mode of action of this class of molecules (inhibition of DNA function and RNA transcription) cannot be used to directly explain its rapid cidal activity, it is assumed that molecules of this class are also able to trigger other modes of killing action, including the induction of autolysis or programmed death, at a relatively high dose.
GSQ1530 is not active against wild-type gram-negative species. However, it shows an inhibitory effect on an E. coli outer membrane-deficient mutant (MIC, 0.5 μg/ml) (data not shown), suggesting that GSQ1530 acts by inhibiting similar intracellular targets in both gram-positive and gram-negative species. The outer membrane barrier of gram-negative bacteria appears to limit the uptake of this molecule and inactivate it against those species.
In general, the medium used and the growth conditions seemed to have minimal effects on the in vitro antimicrobial activity of GSQ1530. Interestingly, basic conditions seem to enhance the antibacterial activity of GSQ1530. Conceivably, the molecule is deprotonated at high pH and is thus more readily taken up into cells. The in vitro activity of GSQ1530 was reduced in the presence of 25% mouse serum or a physiological concentration of HPA. It appears that some heat-insensitive components in serum interfered with the antimicrobial activity of this compound. This finding was confirmed by the observation that GSQ1530 had a higher percentage of plasma protein binding (over 89%) than the control antibiotic, linezolid (22%). Among several control antibiotics tested, only fusidic acid shared a similar behavior that has been well documented (1). No loss of activity of linezolid is noted in the presence of HPA. It is conceivable that the plasma protein binding of GSQ1530 reduces the concentration of free drug in the medium, resulting in higher MICs. Whether the higher level of protein binding of GSQ1530 has any adverse impact in an in vivo setting remains to be demonstrated.
A preliminary mechanistic study has demonstrated that the HARP antibiotic class of molecules kills bacteria by blocking DNA functions and other DNA-dependent enzymatic activities (Ge et al., 41st ICAAC, abstr. F-1686). Theoretically, GSQ1530 should have a good synergistic interaction with rifampin since the two drugs work on different targets of the bacterial transcriptional apparatus; GSQ1530 works by binding to bacterial promoters or other adjacent sequences to block bacterial transcription initiation and/or the elongation process, while rifampin exerts its antibacterial effect by directly binding to the β subunit of RNA polymerase to inactivate its enzymatic activity (13). Interestingly, such a synergistic effect was only observed against MRSA and was not observed against VRE or PRSP. The species dependence for this observed effect is not fully understood.
In conclusion, GSQ1530 is a representative compound of a new class of antimicrobials named HARP antibiotics. Members of this novel family of compounds exhibit broad-spectrum activity against common drug-resistant gram-positive pathogens. Focused lead compound optimization of GSQ1530 analogs is in progress to identify potential clinical candidates with improved profiles for the treatment of infections caused by gram-positive bacteria.
Acknowledgments
This study was funded in part by a DARPA grant (grant N65236-99-1-5427).
REFERENCES
- 1.Collignon, P., and J. Turnidge. 1999. Fusidic acid in vitro activity. Int. J. Antimicrob. Agents 12(Suppl. 2):S45-S58. [DOI] [PubMed] [Google Scholar]
- 2.Craig, W. A., and B. Suh. 1991. Protein binding and the antimicrobial effects: methods for the determination of protein binding, p. 367-402. In V. Lorian (ed.), Antibiotics in laboratory medicine. The Williams & Wilkins Co., Baltimore, Md.
- 3.Dennesen, P. J., M. J. Bonten, and R. A. Weinstein. 1998. Multiresistant bacteria as a hospital epidemic problem. Ann. Med. 30:176-185. [DOI] [PubMed] [Google Scholar]
- 4.Diekema, D. I., and R. N. Jones. 2000. Oxazolidinones: a review. Drugs 59:7-16. [DOI] [PubMed] [Google Scholar]
- 5.Hancock, R. E., and D. P. Speert. 2000. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and impact on treatment. Drug Resist. Update 3:247-255. [DOI] [PubMed] [Google Scholar]
- 6.Lorian, V. 1996. Antibiotics in laboratory medicine, 4th ed. The Williams & Wilkins Co., Baltimore, Md.
- 7.National Committee for Clinical Laboratory Standards. 1987. Methods for determining bactericidal activity of antimicrobial agents. Proposed guideline (M26-P). Approved standard (M11-A3). National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 8.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard (M7-A4). National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 9.Norrby, R. 2001. Linezolid—a review of the first oxazolidinone. Expert Opin. Pharmacother. 2:293-302. [DOI] [PubMed] [Google Scholar]
- 10.Novick, R. P., P. Schlievert, and A. Ruzin. 2001. Pathogenicity and resistance islands of staphylococci. Microbes Infect. 3:585-594. [DOI] [PubMed] [Google Scholar]
- 11.Prystowsky, J., F. Siddiqui, J. Chosay, D. L. Shinabarger, J. Millichap, L. R. Peterson, and G. A. Noskin. 2001. Resistance to linezolid: characterization of mutations in rRNA and comparison of their occurrences in vancomycin-resistant enterococci. Antimicrob. Agents Chemother. 45:2154-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts, M. C. 1998. Antibiotic resistance in oral/respiratory bacteria. Crit. Rev. Oral Biol. Med. 9:522-540. [DOI] [PubMed] [Google Scholar]
- 13.Sippel, A., and G. Hartmann. 1968. Mode of action of rafamycin on the RNA polymerase reaction. Biochim. Biophys. Acta 157:218-219. [DOI] [PubMed] [Google Scholar]
- 14.Wang, E. E., J. D. Kellner, and S. Arnold. 1998. Antibiotic-resistant Streptococcus pneumoniae. Implications for medical practice. Can. Fam. Physician 44:1881-1888. [PMC free article] [PubMed] [Google Scholar]