Abstract
The modifications to the vaginal habitat accompanying a change to vaginal flora in bacterial vaginosis (BV) are poorly understood. In this study enzymes involved in mucin degradation were measured, including a novel glycosulfatase assay. Women attending an emergency walk-in sexually transmitted disease clinic were studied. One high vaginal swab (HVS) was used to prepare a gram-stained smear to determine BV status, using Ison and Hay's criteria, and a separate swab was used for the purposes of the assays. The median glycosulfatase activity was 8.5 (range, −1.2 to 31.9) nmol h−1 1.5 ml−1 of HVS suspension in patients with BV compared to 0.5 (range, −0.7 to 9.4) nmol h−1 1.5 ml−1 of HVS suspension in patients without BV (P = <0.001). The median glycoprotein sialidase activity was 29.2 (range, −17 to 190) nmol h−1 1.5 ml−1 of HVS suspension in patients with BV compared to −1.1 (range, −41 to 48) nmol h−1 1.5 ml−1 of HVS suspension in patients without BV (P < 0.001). A rapid spot test for sialidase was positive in 22/24 patients with BV (sensitivity, 91.7%; 95% confidence interval [CI], 73 to 99%) and negative in 32/35 patients without BV (specificity, 91.4%; 95% CI, 76.9 to 98.2%) (P < 0.001). Glycosulfatase activity significantly correlated with both glycoprotein sialidase activity and the sialidase spot test (P = 0.006 and P < 0.001, respectively). The results are consistent with the hypothesis that the consortium of bacteria present in BV requires the ability to break down mucins in order to colonize the vagina and replace the normal lactobacilli.
Bacterial vaginosis (BV) is a condition in which commensal lactobacilli in the vagina are replaced by a profound overgrowth of a mixed microflora of anaerobes and Gardnerella vaginalis. It is strongly associated with preterm birth (6, 7, 14), endometritis, and pelvic inflammatory disease (12, 26), acquisition of Neisseria gonorrhoeae, Chlamydia trachomatis, and human immunodeficiency virus (3, 21, 30), and probably enhanced human immunodeficiency virus transmission (4, 15, 21). The causative link between BV colonization and associated pathology is not firmly established.
Mucolytic enzymes (mucinases), including those which degrade terminal carbohydrate residues or those which disrupt the mucin apoprotein primary structure, are produced by some bacteria associated with BV. Previous work has shown that the presence of glycosidases, including sialidase, is frequently associated with BV (1, 8, 14, 17, 33). Certain organisms related to those found in BV have been shown to produce sialidase (reviewed in reference 32). The detection of a variety of enzymes with mucin-degrading potential from BV organisms (1, 8, 17, 22, 32, 33) indicates that a complementary relationship may exist between the different types of bacteria and that synergistic-enzymatic activity is required to effect maximum control over the vaginal environment. Intensive production of hydrolytic enzymes in BV might lead to diminished mucosal barrier protection through disruption of secreted mucus viscoelasticity and deglycosylation of the apical cell surface glycocalyx. In addition, key enzymes produced in BV may degrade the mucosal barrier, mediate bacterial adhesion to host surfaces, and provide energy sources for the bacteria, thus enhancing bacterial colonization of the mucosa. However, the specificity and inducibility of bacterial mucinase action against human reproductive tract mucin have not yet been investigated in detail. Information on the source(s) and significance of the mucin-degrading enzymes may help to explain how the consortium of bacteria found in BV is able to colonize the vaginal niche.
In the present study, we have investigated whether glycosulfatase, another mucinolytic enzyme targeted against sulfated oligosaccharide side chains, is also associated with BV. Glycosulfatases (mucin-desulfating sulfatases) are known to be closely involved in mucin degradation in the gastrointestinal tract (19, 20, 27, 28), and several such enzymes have been characterized and/or cloned from Prevotella strain RS2 (34, 35). The removal of sulfate is thought to be one of the rate-limiting steps in sulfomucin degradation (18, 20), and the same principles apply to sialic acid removal due to sialidase action (8, 18, 32). We report here increased levels of glycosulfatase and sialidase activity in high vaginal swabs (HVSs) from patients with BV. The demonstration that both sulfatase and sialidase activities are frequently elevated in BV is consistent with the concept that mucin degradation may be necessary to support the colonization and maintenance of the vaginal microflora found in BV.
MATERIALS AND METHODS
Collection of clinical samples.
Sixty-one women attending an emergency walk-in clinic at the Milne Centre for Sexual Health who required a vaginal speculum examination were studied after informed consent was obtained. The age range of the women was 16 to 50 years. An HVS was obtained at speculum examination and placed into 1.5 ml Tris chloride buffer (25 mM, pH 7.4). Swabs were immediately vortexed in the buffer for 2 min, the swab squeezed out and removed, and the suspension placed on ice.
A separate swab was taken for microscopic diagnosis of BV. The swab was rolled onto a glass slide, air dried, heat fixed, and gram stained. Slides were classified according to Ison and Hay's criteria (10) as predominantly lactobacillus flora (grade I), mixed lactobacillus and BV-related organisms (grade II), or few or no lactobacilli and predominantly BV organisms present (grade III) (6, 25, 31). Slides were read independently by P. Horner and T. Crowley and results compared. Discordant findings were reviewed to reach agreement. Cathy Ison (Health Protection Agency, London) reviewed 10 slides on which there was ongoing debate. These were sent together with 10 slides on which there was consensus agreement. There was complete agreement between Ison's and our assessments of the latter 10 slides. Ison assessed all slides without knowledge of their identity, and her classification was taken as final. For the purposes of the analyses, BV was defined as grade III flora and grades I and II were defined as not having BV.
Quantitative glycosulfatase assay using 4-nitrophenyl 2-acetamido-2-deoxy-β-d-glucopyranoside 6-sodium sulfate (SO3-6-GlcNAc-1-PNP) substrate.
The enzyme assay involves the desulfation of SO3-6-GlcNAc-1-PNP by the glycosulfatase, followed by removal of 4-nitrophenol (PNP) from the 4-nitrophenyl 2-acetamido-2-deoxy-β-d-glucopyranoside (GlcNAc-1-PNP) by the hexosaminidase auxiliary enzyme, leaving 2-acetamido-2-deoxy-β-d-glucopyranoside (GlcNAc), and finally adjustment of the pH to 9.6 to measure PNP (2). 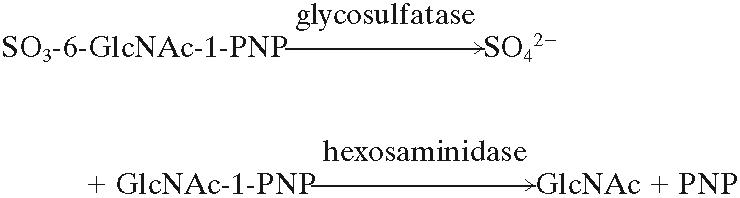 Assays were carried out in 96-well plates. SO3-6-GlcNAc-1-PNP (15 μL, 3.2 mM) and HVS suspension (15 μL) were added to duplicate wells (i). Tris chloride buffer, pH 7.4, was present at a 67 mM final concentration. Three controls were incubated in parallel, containing (ii) HVS suspension and buffer with no substrate (in duplicate), (iii) substrate and buffer without HVS suspension (in quadruplicate), and (iv) buffer alone (in quadruplicate). The contents of wells were mixed, placed in a moist chamber, and incubated at 37°C overnight with gentle circular rotation (50 rpm). After 18 to 22 h, 10 μl of Aspergillus oryzae extract (Sigma G7138) with a hexosaminidase activity sufficient to form 1.5 nmol product min−1, was added to wells i and iii and incubated for a further hour to convert GlcNAc-1-PNP produced by the bacterial glycosulfatase activity to GlcNAc and PNP. This choice of hexosaminidase source is crucial, since some commercial hexosaminidases will slowly remove PNP from SO3-6-GlcNAc-1-PNP. Then, 170 μl of sodium glycine buffer (0.5 M, pH 9.6) was added to all wells, and the absorbance difference was read between 405 nm (maximum absorbance of the PNP anion) and 620 nm (reference wavelength) using a Spectra Max Plus plate reader (Molecular Devices, Sunnyvale, CA). The absorbance due to the PNP production was corrected for light scattering and possible substrate hydrolysis by the auxiliary enzyme by subtracting the combined absorbances of well ii plus well iii from well i plus well iv and averaging the multiple readings.
Assays were carried out in 96-well plates. SO3-6-GlcNAc-1-PNP (15 μL, 3.2 mM) and HVS suspension (15 μL) were added to duplicate wells (i). Tris chloride buffer, pH 7.4, was present at a 67 mM final concentration. Three controls were incubated in parallel, containing (ii) HVS suspension and buffer with no substrate (in duplicate), (iii) substrate and buffer without HVS suspension (in quadruplicate), and (iv) buffer alone (in quadruplicate). The contents of wells were mixed, placed in a moist chamber, and incubated at 37°C overnight with gentle circular rotation (50 rpm). After 18 to 22 h, 10 μl of Aspergillus oryzae extract (Sigma G7138) with a hexosaminidase activity sufficient to form 1.5 nmol product min−1, was added to wells i and iii and incubated for a further hour to convert GlcNAc-1-PNP produced by the bacterial glycosulfatase activity to GlcNAc and PNP. This choice of hexosaminidase source is crucial, since some commercial hexosaminidases will slowly remove PNP from SO3-6-GlcNAc-1-PNP. Then, 170 μl of sodium glycine buffer (0.5 M, pH 9.6) was added to all wells, and the absorbance difference was read between 405 nm (maximum absorbance of the PNP anion) and 620 nm (reference wavelength) using a Spectra Max Plus plate reader (Molecular Devices, Sunnyvale, CA). The absorbance due to the PNP production was corrected for light scattering and possible substrate hydrolysis by the auxiliary enzyme by subtracting the combined absorbances of well ii plus well iii from well i plus well iv and averaging the multiple readings.
Sialidase spot test assay.
This was a modification of the previously described sialidase filter paper spot test (31) using 5-bromo-4-chloro-3-indolyl-α-d-N-acetylneuraminate cyclohexylamine salt (BCIN) as a substrate. A 3-mm disk of Whatman no. 1 filter paper was placed in the wells of a 96-well plate. The substrate (15 μl of 1.57 mM BCIN in 25 mM Tris chloride buffer, pH 7.4) and 10 μl of HVS suspension were added, and the plate was incubated at 37°C overnight (about 21 h) in a water-saturated atmosphere. The blue color on the filter due to 5-bromo-4-chloro-3-indole formation was estimated semiquantitatively, from 0 to +++. The color could be judged after 1 h, but it was convenient to record all of the spot test assays at one time, 3 h after the first subject's test was initiated. Color was checked again after overnight incubation but did not alter. If no development of color was noted, even after overnight incubation, the samples were classed as negative results. Assays which could not be definitely described as negative, i.e., were slightly tinted, were scored as +/−.
Quantitative glycoprotein sialidase assay using human alpha-1 acid glycoprotein as a substrate.
The hydrolysis of sialic acid from 1.0 mM human alpha-1 acid glycoprotein (AGP) (obtained from the Scottish National Blood Transfusion Service, Edinburgh, Scotland) by samples of HVS suspension was carried out as described previously (8). AGP is a serum glycoprotein containing sialic acid, which is a good model substrate for measuring the activity of some sialidases. After incubation, the free sialic acid was measured using the thiobarbituric acid assay. This assay measures (one or more) bacterial enzyme activities associated with the process of sialic acid removal from sialo-glycoproteins, involved in mucin degradation.
Some of the AGP sialidase measurements gave negative results. Further investigation of this phenomenon showed that some of the crude secretions that make up HVS samples contain an unknown substance or substances which react in the thiobarbituric acid assay to yield a colored product that absorbs at the control wavelength used during estimation of sialic acid.
Statistical methods.
Data collected on the enzyme activities of HVSs from subjects with BV and subjects without BV were analyzed using SPSS version 12. Two samples were excluded from the study, since their data were incomplete.
Both glycosulfatase activity and glycoprotein sialidase activities in HVSs appeared to have a log normal distribution; however, due to the presence of zero and negative values, the data were not transformed. Medians and ranges have been used to describe the data; however, means and standard deviations have also been included to facilitate any future sample size calculations. Correlations between continuous variables have been carried out using Spearman's rank correlation coefficient, or Kendall's tau in the case of ordered categorical variables, and scatter plots and box plots have been used to demonstrate the clinical significance.
To determine the statistical significance of differences between samples from subjects with BV and subjects without BV, a Mann-Whitney test was used, with data from intermediate (grade II) patients being included with data from subjects without BV.
Confidence intervals were calculated for sensitivities and specificities with STATA version 7, using the exact binomial method.
RESULTS
Of the 61 women in the study, 31 were graded as not having BV (grade I), 4 had intermediate flora (grade II), and 24 were graded as having BV (grade III). Two subjects were subsequently excluded from the trial, one because of an enzyme assay error and one because the HVS sample did not contain sufficient cells for diagnosis.
Sulfatase activities in HVSs.
The 24 subjects with BV had a median glycosulfatase activity of 8.5 (range, −1.2 to 31.9) nmol PNP h−1 1.5 ml−1 of HVS suspension, while the 35 subjects without BV had a median activity of 0.5 (range, −0.7 to 9.4) nmol PNP h−1 1.5 ml−1 of HVS suspension (Table 1; Fig. 1). The sulfatase activities of the HVSs from the two groups were significantly different as analyzed by the Mann-Whitney U test (P < 0.001).
TABLE 1.
Sialidase and sulfatase activities found in HVSs from subjects with BV and subjects without BV
| Enzymatic activity measured | Value for group (nmol h−1 1.5 ml−1 of HVS suspension)
|
||||
|---|---|---|---|---|---|
| Subjects with BV
|
Subjects without BV
|
P valuea | |||
| Median range | Mean ± SD | Median range | Mean ± SD | ||
| Glycoprotein sialidase activity | 29.2 (−17-190) | 46.2 ± 54.8 | −1.1 (−41-48) | −1.0 ± 17.2 | <0.001 |
| Sulfatase activity | 8.5 (−1.2-31.9) | 11.5 ± 10.0 | 0.5 (−0.7-9.4) | 1.0 ± 1.8 | <0.001 |
Significance of data, analyzed by the Mann-Whitney U test.
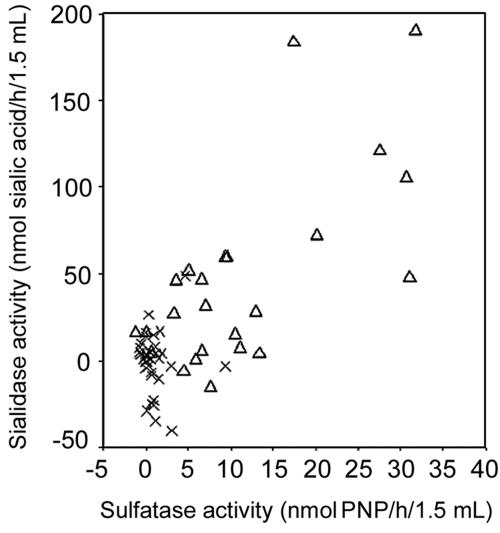
FIG. 1.
Comparison of sulfatase and glycoprotein sialidase activities from HVSs in subjects with BV (▵) and subjects without BV (x). Spearman's rank correlation coefficient between sialidase and sulfatase activity in subjects with BV was 0.54 (P = 0.006) and in subjects without BV was −0.22 (P = 0.20).
Glycoprotein sialidase activities in HVSs.
The 24 subjects with BV had a median activity of 29.2 (range, −17 to 190) nmol sialic acid h−1 1.5 ml−1 of HVS suspension, while the 35 subjects without BV had a median activity of −1.1 (range, −41 to 48) nmol sialic acid h−1 1.5 ml−1 of HVS suspension (Table 1; Fig. 1). The AGP sialidase activities of the HVSs from the two groups were significantly different as analyzed by the Mann-Whitney U test (P < 0.001).
Glycoprotein sialidase activity compared to glycosulfatase activity.
Comparison of the sialidase and glycosulphatase activities (Fig. 1) showed that only the individuals with BV showed a positive correlation. Cases without BV yielded a Spearman's rank correlation coefficient of −0.22 (P = 0.20), while a value of 0.54 (P = 0.006) was found for the group with BV.
Sialidase spot test for BV in HVSs.
In the present study, this test was slightly modified for use in a 96-well microtiter plate. The same fresh HVS samples were qualitatively assayed for BCIN sialidase activity in parallel with the quantitative glycoprotein sialidase test. A positive spot test was observed for 22 out of 24 subjects with BV (sensitivity, 91.7%; 95% confidence interval, 73 to 99%) and a negative spot test for 32 of 35 subjects without BV (specificity, 91.4%; 95% confidence interval, 76.9% to 98.2%) (P < 0.001, chi square).
Correlation between sialidase spot test and sulfatase activity.
Results of these two tests were significantly correlated, with a Kendall's tau coefficient of 0.573 (P < 0.001) (Fig. 2).
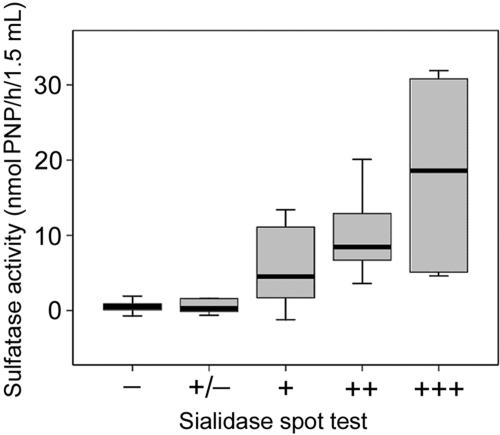
FIG. 2.
Comparison of sulfatase activities and spot test sialidase detection from HVSs with different BV gradings (Ison and Hay's criteria) (10), showing medians and ranges. Correlations were tested using Kendall's tau for ordered categorical variables, giving a coefficient of 0.573 (P < 0.001).
Correlation between sialidase spot test and sialidase activity.
Results of these two tests were significantly correlated, with a Kendall's tau coefficient 0.567 (P < 0.001) (Fig. 3).
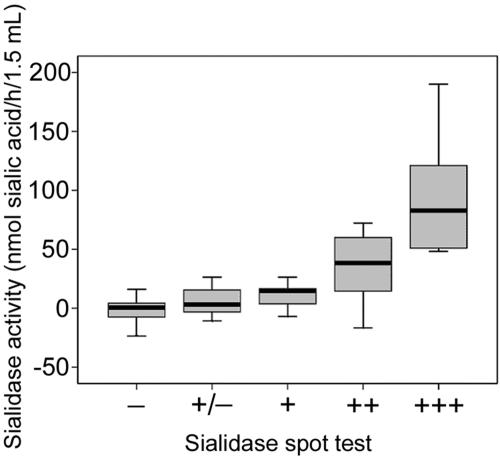
FIG. 3.
Comparison of AGP sialidase activity and spot test sialidase detection from HVSs with different BV gradings (Ison and Hay's criteria) (10), showing medians and ranges. Correlations were tested using Kendall's tau for ordered categorical variables, giving a coefficient of 0.567 (P < 0.001).
DISCUSSION
The novel finding in the present work is that a further enzyme involved in the overall process of mucin degradation is highly associated with BV. Glycosulfatase activity was significantly associated with BV. This enzyme has not previously been described in the microflora from the lower genital tract of women and can now be detected using a novel and specific substrate. In addition, the glycosulfatase activity from cases with BV was correlated with sialidase activity. The data presented suggest that both sialidase and glycosulfatase activities are important in the pathogenesis of BV. Both of these enzymes belong to families with multiple members showing a variety of specificities. This emphasizes the need to select and test substrates which will reflect the likely targets in the mucosal protective barrier.
This is a small study of patients at high risk of having a sexually transmitted infection (STI) who were attending an emergency clinic. BV is associated with STIs, including both C. trachomatis and N. gonorrhoeae, and it is therefore possible that other STIs could be confounding. The numbers with either of these infections was too small (six) to control for in the analyses. However, it is well documented that sialidase (1, 31, 33), hexosaminidase, galactosidase (8), and proline iminopeptidase (16, 17, 22) activities in the vaginal flora increase markedly in the majority of subjects diagnosed as having BV. Furthermore, the strong correlation of glycosulfatase activity with sialidase activity in the cases with BV and not in the individuals without BV suggests that the increased mucinase activities observed were indeed a consequence of BV and not due to other confounding STIs. The sulfatase assay clearly has potential to serve as a diagnostic marker for bacterial vaginosis. This needs to be assessed in a larger study involving the general population.
Further analysis of the glycosulfatase activity is now needed. The mechanism of PNP release from the assay substrate, SO3-6-GlcNAC-1-PNP, remains to be demonstrated. This release might be catalyzed by glycosulfatase enzymes from one or more bacteria, followed by hexosaminidase action. In addition, it cannot be discounted that there may be glycosidases that remove PNP from the sulfated substrate.
Some of the glycoprotein sialidase measurements gave negative results. Further investigation of this phenomenon showed that these samples contain an unknown substance(s) that generates a colored product that has higher absorption at the control wavelength in the thiobarbituric acid assay, resulting in negative values for some samples. From Fig. 3 it can be seen that these individuals all had a negative spot test, suggesting that this unknown contaminant is not hiding sialidase activity.
Prevention of vaginal colonization by bacteria associated with BV is believed to be due to the mucosal surface environment, including low pH, antimicrobial molecules secreted by the host and lactobacilli (5, 29), and the mucus and mucosal barrier. The present results implicate participation of the cervical mucins, glycoproteins, and glycolipids at the mucosal surface in BV pathology. Bacterial mucolytic activity in the vaginal habitat of women with BV indicates that these molecules are being actively altered or degraded. However, there is no evidence explaining how changes to these glycoconjugates contribute to the disease.
Several recent studies have demonstrated interactions of bacteria at mucosal surfaces and are pertinent to colonization by BV-associated bacteria. The role of mucins, glycoproteins, and glycolipids in the mucosal barrier as receptors in Helicobacter pylori adherence and persistence mechanisms in the stomach has been established. These molecules carry Lewis antigens as glycan ligands recognized by specific H. pylori adhesins (9, 13). Furthermore, the presence of mucins rich in terminal α-linked N-acetylglucosamine located in the gastric glands has been linked with a “natural antibiotic action” and explains why H. pylori is unable to colonize deep in the gastric glands (11). In the murine intestine selectively colonized with Bacteroides thetaiotaomicron, an adaptation to the dietary situation has been shown at the genomic level. Upregulation of the mucolytic enzymes is observed when polysaccharides are removed from the habitat and the endogenous host mucus becomes an alternative energy source (24). These examples highlight the significance of the glycoconjugates in the mucosal protective barrier and the need for more detailed structural information. In particular, a closer analysis of the glycan complement presented at the cervical and vaginal mucosal surfaces during the menstrual cycle (23) will be necessary to evaluate the physiological action of the mucinase activities released in BV. The results suggest that glycosulfatase can be added to the group of mucinase enzymes, including sialidase, hexosaminidase, galactosidase, and proline iminopeptidase, associated with BV. Each of these enzymes has a specificity that correlates with different stages in the process of mucin degradation (33).
The results of this study are consistent with the hypothesis that the consortium of bacteria present in BV requires the ability to break down mucins in order to colonize the vagina and replace the normal lactobacilli. In addition, changes in the physicochemical character of mucins as a result of this combined enzymatic degradation, including loss of negative charge, may lead to decreased effectiveness of the mucus barrier and its role in excluding pathogenic microorganisms, such as C. trachomatis and N. gonorrhoeae.
Acknowledgments
A.M.R. was the recipient of a Benjamin Meaker Visiting Fellowship from the University of Bristol Institute for Advanced Studies during the course of the research. The work was supported by grants from Tommy's the Baby Charity and from Tommy's Campaign (grant no. s30), 1 Kennington Rd, London SW, United Kingdom.
Informed consent was obtained from all patients used in this study.
We have no commercial or other association that might pose a conflict of interest.
REFERENCES
- 1.Briselden, A. M., B. J. Moncla, C. E. Stevens, and S. L. Hillier. 1992. Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated microflora. J. Clin. Microbiol. 30:663-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinch, K., G. B. Evans, R. H. Furneaux, P. M. Rendle, P. L. Rhodes, A. M. Roberton, D. I. Rosendale, P. C. Tyler, and D. P. Wright. 2002. Synthesis and utility of sulfated chromogenic carbohydrate model substrates for measuring activities of mucin-desulfating enzymes. Carbohydr. Res. 337:1095-1111. [DOI] [PubMed] [Google Scholar]
- 3.Cohen, C. R., A. Duerr, N. Pruithithada, S. Rugpao, S. Hillier, P. Garcia, and K. Nelson. 1995. Bacterial vaginosis and HIV seroprevalence among female commercial sex workers in Chiang Mai, Thailand. AIDS 9:1093-1097. [DOI] [PubMed] [Google Scholar]
- 4.Hashemi, F. B., M. Ghassemi, K. A. Roebuck, and G. T. Spear. 1999. Activation of human immunodeficiency virus type 1 expression by Gardnerella vaginalis. J. Infect. Dis. 179:924-930. [DOI] [PubMed] [Google Scholar]
- 5.Hawes, S. E., S. L. Hillier, J. Benedetti, C. E. Stevens, L. A. Koutsky, P. Wolner-Hanssen, and K. K. Holmes. 1996. Hydrogen peroxide-producing lactobacilli and acquisition of vaginal infections. J. Infect. Dis. 174:1058-1063. [DOI] [PubMed] [Google Scholar]
- 6.Hay, P. E., R. F. Lamont, D. Taylor-Robinson, D. J. Morgan, C. Ison, and J. Pearson. 1994. Abnormal bacterial colonisation of the genital tract and subsequent preterm delivery and late miscarriage. Br. Med. J. 308:295-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillier, S. L., R. P. Nugent, D. A. Eschenbach, M. A. Krohn, R. S. Gibbs, D. H. Martin, M. F. Cotch, R. Edelman, J. G. Pastorek II, A. V. Rao, et al. 1995. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. N. Engl. J. Med. 333:1737-1742. [DOI] [PubMed] [Google Scholar]
- 8.Howe, L., R. Wiggins, P. W. Soothill, M. R. Millar, P. J. Horner, and A. P. Corfield. 1999. Mucinase and sialidase activity of the vaginal microflora: implications for the pathogenesis of preterm labour. Int. J. STD AIDS 10:442-447. [DOI] [PubMed] [Google Scholar]
- 9.Ilver, D., A. Arnqvist, J. Ogren, I. M. Frick, D. Kersulyte, E. T. Incecik, D. E. Berg, A. Covacci, L. Engstrand, and T. Boren. 1998. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science 279:373-377. [DOI] [PubMed] [Google Scholar]
- 10.Ison, C. A., and P. E. Hay. 2002. Validation of a simplified grading of Gram stained vaginal smears for use in genitourinary medicine clinics. Sex. Transm. Infect. 78:413-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawakubo, M., Y. Ito, Y. Okimura, M. Kobayashi, K. Sakura, S. Kasama, M. N. Fukuda, M. Fukuda, T. Katsuyama, and J. Nakayama. 2004. Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science 305:1003-1006. [DOI] [PubMed] [Google Scholar]
- 12.Korn, A. P., G. Bolan, N. Padian, M. Ohm-Smith, J. Schachter, and D. V. Landers. 1995. Plasma cell endometritis in women with symptomatic bacterial vaginosis. Obstet. Gynecol. 85:387-390. [DOI] [PubMed] [Google Scholar]
- 13.Mahdavi, J., B. Sonden, M. Hurtig, F. O. Olfat, L. Forsberg, N. Roche, J. Angstrom, T. Larsson, S. Teneberg, K. A. Karlsson, S. Altraja, T. Wadstrom, D. Kersulyte, D. E. Berg, A. Dubois, C. Petersson, K. E. Magnusson, T. Norberg, F. Lindh, B. B. Lundskog, A. Arnqvist, L. Hammarstrom, and T. Boren. 2002. Helicobacter pylori SabA adhesin in persistent infection and chronic inflammation. Science 297:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGregor, J. A., J. I. French, W. Jones, K. Milligan, P. J. McKinney, E. Patterson, and R. Parker. 1994. Bacterial vaginosis is associated with prematurity and vaginal fluid mucinase and sialidase: results of a controlled trial of topical clindamycin cream. Am. J. Obstet. Gynecol. 170:1048-1060. [DOI] [PubMed] [Google Scholar]
- 15.Olinger, G. G., F. B. Hashemi, B. E. Sha, and G. T. Spear. 1999. Association of indicators of bacterial vaginosis with a female genital tract factor that induces expression of HIV-1. AIDS 13:1905-1912. [DOI] [PubMed] [Google Scholar]
- 16.Olmsted, S. S., L. A. Meyn, and S. L. Hillier. 2001. Production of mucin degrading enzymes by vaginal bacteria. Int. J. STD AIDS 12:68-69.11589801 [Google Scholar]
- 17.Olmsted, S. S., L. A. Meyn, L. C. Rohan, and S. L. Hillier. 2003. Glycosidase and proteinase activity of anaerobic gram-negative bacteria isolated from women with bacterial vaginosis. Sex. Transm. Dis. 30:257-261. [DOI] [PubMed] [Google Scholar]
- 18.Roberton, A. M., and A. P. Corfield. 1999. Mucin degradation and its significance in inflammatory conditions of the gastrointestinal tract, p. 222-261. In G. W. Tannock (ed.), Medical importance of the normal microflora. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 19.Roberton, A. M., C. McKenzie, N. Scharfe, and L. Stubbs. 1993. A glycosulphatase that removes sulphate from mucus glycoprotein. Biochem. J. 293:683-689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberton, A. M., and D. P. Wright. 1997. Bacterial glycosulfatases and sulfomucin degradation. Can. J. Gastroenterol. 11:361-366. [DOI] [PubMed] [Google Scholar]
- 21.Schmid, G., L. Markowitz, R. Joesoef, and E. Koumans. 2000. Bacterial vaginosis and HIV infection. Sex. Transm. Infect. 76:3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoonmaker, J. N., B. D. Lunt, D. W. Lawellin, J. I. French, S. L. Hillier, and J. A. McGregor. 1991. A new proline aminopeptidase assay for diagnosis of bacterial vaginosis. Am. J. Obstet. Gynecol. 165:737-742. [DOI] [PubMed] [Google Scholar]
- 23.Sheehan, J. K., M. Howard, D. Thornton, E. Chantler, and I. Carlstedt. 1995. Physical and biochemical changes in cervical mucins through the ovulatory cycle. Glycoconj. J. 12:494. [Google Scholar]
- 24.Sonnenburg, J. L., J. Xu, D. D. Leip, C.-H. Chen, B. P. Westover, J. Weatherford, J. D. Buhler, and J. I. Gordon. 2005. Glycan foraging in vivo by an intestine-adapted bacterial symbiont. Science 307:1955-1959. [DOI] [PubMed] [Google Scholar]
- 25.Spiegel, C. A., R. Amsel, and K. K. Holmes. 1983. Diagnosis of bacterial vaginosis by direct Gram staining of vaginal fluid. J. Clin. Microbiol. 18:170-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sweet, R. L. 1995. Role of bacterial vaginosis in pelvic inflammatory disease. Clin. Infect. Dis. 20:S271-S275. [DOI] [PubMed] [Google Scholar]
- 27.Tsai, H. H., A. D. Dwarakanath, C. A. Hart, J. D. Milton, and J. M. Rhodes. 1995. Increased faecal mucin sulphatase activity in ulcerative colitis: a potential target for treatment. Gut 36:570-576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai, H. H., D. Sunderland, G. R. Gibson, C. A. Hart, and J. M. Rhodes. 1992. A novel mucin sulphatase from human faeces: its identification, purification and characterization. Clin. Sci. 82:447-454. [DOI] [PubMed] [Google Scholar]
- 29.Vallor, A. C., M. A. Antonio, S. E. Hawes, and S. L. Hillier. 2001. Factors associated with acquisition of, or persistent colonization by, vaginal lactobacilli: role of hydrogen peroxide production. J. Infect. Dis. 184:1431-1436. [DOI] [PubMed] [Google Scholar]
- 30.Wiesenfeld, H. C., S. L. Hillier, M. A. Krohn, D. V. Landers, and R. L. Sweet. 2003. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clin. Infect. Dis. 36:663-668. [DOI] [PubMed] [Google Scholar]
- 31.Wiggins, R., T. Crowley, P. J. Horner, P. W. Soothill, M. R. Millar, and A. P. Corfield. 2000. Use of a 5-bromo-4-chloro-3-indolyl-α-d-N-acetylneuraminic acid in a novel spot test to identify sialidase activity in vaginal swabs from women with bacterial vaginosis. J. Clin. Microbiol. 38:3096-3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiggins, R., S. J. Hicks, P. W. Soothill, M. R. Millar, and A. P. Corfield. 2001. Mucinases and sialidases: their role in the pathogenesis of sexually transmitted infections in the female genital tract. Sex. Transm. Infect. 77:402-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wiggins, R., M. R. Millar, P. W. Soothill, S. J. Hicks, and A. P. Corfield. 2002. Application of a novel human cervical mucin-based assay demonstrates the absence of increased mucinase activity in bacterial vaginosis. Int. J. STD AIDS 13:755-760. [DOI] [PubMed] [Google Scholar]
- 34.Wright, D. P., C. G. Knight, S. G. Parkar, D. L. Christie, and A. M. Roberton. 2000. Cloning of a mucin-desulfating sulfatase gene from Prevotella strain RS2 and its expression using a Bacteroides recombinant system. J. Bacteriol. 182:3002-3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright, D. P., D. I. Rosendale, and A. M. Roberton. 2000. Prevotella enzymes involved in mucin oligosaccharide degradation and evidence for a small operon of genes expressed during growth on mucin. FEMS Microbiol. Lett. 190:73-79. [DOI] [PubMed] [Google Scholar]