Abstract
Active surveillance for methicillin-resistant Staphylococcus aureus (MRSA) is among the strategies recommended by the Society for Healthcare Epidemiology of America for control of nosocomial MRSA infections. Infection control and laboratory personnel desire rapid, sensitive, and inexpensive methods to enhance surveillance activities. A multicenter study was performed to evaluate a new selective and differential chromogenic medium, BBL CHROMagar MRSA (C-MRSA) medium (BD Diagnostics, Sparks, MD), which enables recovery and concomitant identification of MRSA strains directly from nasal swab specimens taken from the anterior nares. Specimens were inoculated to C-MRSA and Trypticase soy agar with 5% sheep blood agar (TSA II, BD Diagnostics). Mauve colonies on C-MRSA at 24 h and 48 h and suspicious colonies on TSA II were confirmed as Staphylococcus aureus by Gram stain morphology and a coagulase test. In addition, the results of C-MRSA were compared to results of susceptibility testing (five different methods) of S. aureus strains isolated on TSA II. A total of 2,015 specimens were inoculated to C-MRSA and TSA II. Three hundred fifty-four S. aureus isolates were recovered; 208 (59%) were oxacillin (methicillin) susceptible and 146 (41%) were oxacillin resistant (MRSA). On C-MRSA, 139/146 or 95.2% of MRSA isolates were recovered, whereas recovery on TSA II was 86.9% (127/146) (P = 0.0027). The overall specificity of C-MRSA was 99.7%. When C-MRSA was compared to each susceptibility testing method, the sensitivity and specificity, respectively, were as follows: oxacillin MIC by broth microdilution, 94.4% and 96.7%; oxacillin screen agar, 94.3% and 96.7%; PBP2′ latex agglutination, 93.7% and 98.5%; cefoxitin disk diffusion, 95.0% and 98.1%; and mecA PCR, 95.1% and 98.1%. In this study, C-MRSA was superior to TSA II for recovery of MRSA from surveillance specimens obtained from the anterior nares and was comparable to conventional, rapid, and molecular susceptibility methods for the identification of MRSA isolates.
Methicillin-resistant Staphylococcus aureus (MRSA) remains an important nosocomial pathogen. According to the latest report from the National Nosocomial Infection Surveillance System, approximately 60% of all S. aureus nosocomial infections in intensive care units were methicillin resistant in 2003, representing an 11% increase in resistance compared to the preceding 5-year period (14). Infections caused by MRSA strains are associated with longer hospital stay, more days of antibiotic administration, and higher costs than infections caused by methicillin-susceptible Staphylococcus aureus (MSSA) strains (1, 7, 8, 10). More importantly, several studies (3, 7) and one large meta-analysis (5) have shown that patients who develop MRSA bacteremia have a higher mortality than patients with MSSA infections after adjusting for underlying severity of illness.
In the study by Davis et al. (6), patients colonized on admission to the hospital or who acquire MRSA infection during a hospital stay were more likely than patients colonized with MSSA to develop an infection. Active surveillance for MRSA in the nares of at-risk populations is an important component of the Society for Healthcare Epidemiology of America recommendations for control of nosocomial transmission of MRSA (12). Identification of patients and healthcare workers (in outbreak settings) colonized with MRSA, combined with contact precautions and improvements in hand hygiene, have been successful in reducing transmission and controlling spread (8, 11, 12, 15, 18). These and other guidelines, combined with the increase in community acquisition and colonization of MRSA, have led to an increase in the targeted surveyed population in many healthcare facilities. Hence, microbiology laboratories are under pressure to develop rapid but cost-effective surveillance methods.
This study was a multicenter clinical evaluation of the BBL CHROMagar MRSA (C-MRSA) medium (BD Diagnostics, Sparks, MD), a selective and differential agar, for detection of MRSA directly from surveillance cultures of the anterior nares. The goals of the study were (i) to compare the recovery of MRSA on C-MRSA to recovery on TSA II and (ii) to determine the performance of C-MRSA compared to that of conventional, rapid, and molecular susceptibility testing methods for the identification of MRSA isolates.
MATERIALS AND METHODS
Study design.
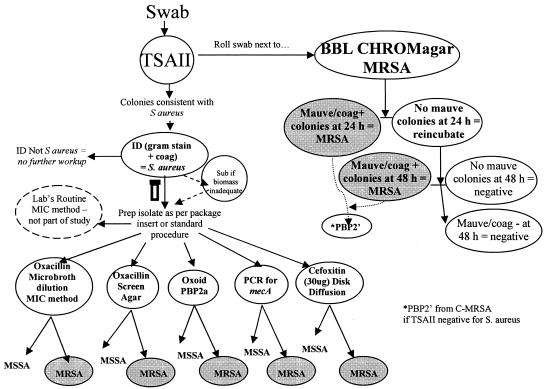
The study was designed to evaluate C-MRSA medium for the recovery and concomitant identification of MRSA isolates directly from surveillance specimens taken from the anterior nares. Four centers participated in a clinical trial following the manufacturer's defined protocol. Recovery of MRSA on C-MRSA was compared to recovery of MRSA on TSA II. Isolates identified as S. aureus on TSA II were tested for oxacillin susceptibility using five different methods. Oxacillin resistance was confirmed with the penicillin binding protein (PBP2′) latex agglutination test (Remel, Lenexa, KS) for S. aureus that grew on C-MRSA but not TSA II (Fig. 1). All participating sites obtained Institutional Review Board approval from their respective institutions prior to the initiation of the study. Completed data report forms were sent to the sponsor for data analysis.
FIG. 1.
CHROMagar MRSA medium clinical specimen study design. Shown is the algorithm demonstrating the study design for inoculation of nasal swab surveillance cultures to TSA II and C-MRSA media and subsequent workup of Staphylococcus aureus on TSA II or mauve colonies on C-MRSA.
Participating centers.
Four clinical centers representing geographically diverse regions within the United States participated in the study. Routine surveillance cultures were obtained from the anterior nares of inpatients and outpatients at the Johns Hopkins Hospital (JHH), Baltimore, MD, the University of Iowa Health Care (UI), Iowa City, IA, UCLA Medical Center (UCLA), Los Angeles, CA, and the Cleveland Clinic Foundation (CC), Cleveland, OH. Technologists performing testing at each site were trained by the sponsor, with emphasis placed on appropriate storage, inoculation, incubation, and examination of the medium as well as proper performance of antimicrobial susceptibility tests. All sites were required to pass quality control and a proficiency panel prior to participation in the clinical portion of the study.
Clinical specimens.
Surveillance swab specimens taken from the anterior nares were collected for the detection of MRSA carriage from patients at high risk of nosocomial infection, including intensive care unit patients and transplant recipients, as defined by infection control practices at each institution. Specimens were obtained and transported to each laboratory using the following transport devices: Copan transystem 138CQ dacron-tipped swabs and polyurethane foam pledget (Copan Diagnostics, Corona, Calif.) (CC); Fisherbrand transport swab with liquid Stuart's medium (Fisher Scientific, Pittsburgh, PA) (UI); BBL CultureSwab Plus (BD Diagnostics, Sparks, MD) (UCLA): BBL CultureSwab with liquid Stuart's medium (BD Diagnostics, Sparks, MD) (JHH).
Medium and specimen inoculation.
C-MRSA (BD Diagnostics, Sparks, MD) combines primary isolation from the clinical specimen with direct identification of MRSA in one step. C-MRSA contains the following ingredients: chromopeptone, 40 g/liter; sodium chloride, 25.0 g/liter; agar, 14 g/liter; a proprietary chromogen mix, 0.5 g/liter; and antifungal and antibacterial inhibitory agents, including 6 μg/ml cefoxitin. C-MRSA must be stored in the dark prior to inoculation and during incubation, as prolonged exposure to light may result in reduced coloration of bacteria. On C-MRSA, MRSA isolates appear as light mauve to mauve colonies at 16 to 48 h, while methicillin-resistant coagulase-negative staphylococci appear as white, beige, or light blue colonies. Other bacteria, including MSSA isolates, are inhibited or grow poorly.
A single swab from the anterior nares was used to inoculate TSA II and then C-MRSA. One site (CC) plated the polyurethane foam pledget from the bottom of the swab transport tube, as the swabs were destructively used in the lab's routine testing. The pledget was extracted from the swab transport tube using sterile tweezers and inoculated directly to TSA II and then C-MRSA. All media were streaked for isolation and incubated at 35 to 37°C in a non-CO2 incubator. C-MRSA plates were incubated in the dark for 16 to 24 h (the majority of readings were after 20 h of incubation). TSA II and C-MRSA plates that were negative for MRSA and/or staphylococci at 16 to 24 h were incubated for an additional 20 to 24 h and reexamined.
Detection and identification of S. aureus and MRSA.
Recovery and identification of MRSA from TSA II was considered the reference method. Following incubation, the TSA II plates were examined for colonies suggestive of S. aureus. S. aureus was identified using conventional laboratory tests, including the Gram stain and coagulase testing. Susceptibility to oxacillin (methicillin) was determined for S. aureus isolates recovered on TSA II using five methods (see below and Fig. 1).
Recovery and identification of MRSA on C-MRSA was considered the test method. Following incubation, C-MRSA plates were examined for the presence of mauve-colored colonies. Mauve-colored colonies appearing at 16 to 24 h and 48 h (if negative at 24 h) were considered MRSA by colony morphology, Gram stain morphology, and coagulase testing. Oxacillin (methicillin) resistance was confirmed by the PBP2′ latex agglutination assay for S. aureus isolates that grew on C-MRSA but not on TSA II.
For recovery, a true positive was defined as an isolate whose oxacillin resistance was confirmed by all five susceptibility test methods or a mauve isolate recovered from C-MRSA whose resistance was determined only by PBP2′ latex. For any isolates that did not fulfill the above criteria, the mecA PCR was accepted as the “gold standard.”
Susceptibility testing.
S. aureus isolates recovered from TSA II had the following susceptibility testing completed: (i) broth microdilution MICs for oxacillin at concentrations ranging from 0.12 μg/ml to 4.0 μg/ml (PASCO, Wheat Ridge, CO); (ii) oxacillin salt agar screen (BD, Sparks, MD); (iii) PBP2′ latex agglutination (Remel, Lenexa, KS); (iv) cefoxitin disk diffusion; and (v) mecA gene detection by PCR. Oxacillin broth microdilution MICs, oxacillin salt agar screen, and cefoxitin disk (30 μg) diffusion tests were performed as described by the Clinical and Laboratory Standards Institute (4, 13). The PBP2′ latex agglutination test was performed per the manufacturer's instructions. PCR detection of the mecA gene was performed at three sites using a LightCycler instrument (Roche Molecular Systems, Pleasanton, CA) for amplification of the mecA gene. Two sites (JHH and UCLA) used Roche reagents per the manufacturer's instructions. A 315-bp fragment of the mecA gene and internal control were amplified by proprietary mecA gene primers. The other site (CC) used primers and probes from IT Biochem (Salt Lake City, UT). This site also performed the PCR testing on isolates from Iowa (Fig. 1).
The performance of the test method, C-MRSA, was compared independently to each testing method. All five susceptibility tests were not completed on all S. aureus isolates; thus, the numbers of isolates tested by each method differed slightly as follows: for oxacillin MIC, n = 336; for oxacillin screen agar, n = 336; for cefoxitin disk diffusion, n = 328; for PBP2′ latex agglutination, n = 331; and for mecA PCR, n = 334.
Data analysis.
Duplicate MRSA-positive patient samples were excluded from the final data analysis. For calculating recovery, a true positive MRSA isolate was defined as a MRSA isolate recovered from TSA II or a mauve isolate recovered from C-MRSA (only) that was positive for PBP2′ latex.
Data management was through an SQL Server 2000 database, with analyses performed using SAS version 9.1.3 (SAS Institute Inc., Cary, N.C.). The denominator for comparing the identification of MRSA using C-MRSA to each susceptibility test method was the number of S. aureus strains isolated on TSA II that were subsequently identified as MRSA with the respective susceptibility test. C-MRSA comparisons to each susceptibility test method were analyzed separately. The total MRSA recovery between C-MRSA and TSA II was compared using McNemar's chi-square test of differences with a continuity correction at α = 0.05.
RESULTS
Overall recovery.
From 2,015 surveillance specimens, 354 S. aureus isolates were recovered; 208 (59%) were oxacillin (methicillin) susceptible and 146 (41%) were oxacillin resistant. C-MRSA detected 139/146 MRSA isolates for a 95.2% recovery rate (range by site, 90.9% to 95.3%), and TSA II detected 127/146 for an 86.9% recovery rate (range by site, 84.5% to 91.7%) (Table 1). The difference in rates of recovery from the two media was statistically significant (P value = 0.0027). At 24 h, 120 MRSA isolates were recovered (108 isolates from both media and 12 isolates from C-MRSA only). After an additional 24 h of incubation (48 h total), an additional 19 isolates were recovered (16 isolates from both media and 3 isolates from C-MRSA only). The time of recovery was not documented for the seven isolates recovered on TSA II only. For C-MRSA, 86% of MRSA isolates were recovered at 24 h and the remaining 14% were recovered at 48 h.
TABLE 1.
Recovery of MSSA and MRSA isolates on C-MRSA and TSA IIa
| Medium | Total no. (%) of MSSA isolates recovered (n = 208) | Total no. (%) of MRSA isolates recovered (n = 146) |
|---|---|---|
| C-MRSA | 0 (0) | 139 (95.2)b |
| TSA II | 208 (100) | 127 (86.9) |
The total number of specimens tested was 2,015.
Results are statistically significant (p = 0.0027 by McNemar's analysis).
The specificity of C-MRSA was 99.7% (1,863/1,869) at 24 h. There were six cultures with mauve colonies on C-MRSA that were identified as coagulase-negative Staphylococcus sp. (n = 4) and Corynebacterium sp. (n = 2). At 48 h, 68 cultures demonstrated mauve colonies on C-MRSA that were not MRSA isolates. These included 45 coagulase-negative Staphylococcus sp. and 23 Corynebacterium sp. At 48 h, a coagulase test must be performed on smooth, medium-sized mauve colonies, the colony morphology consistent with MRSA. Although not recommended by the manufacturer's package insert, laboratories may wish to perform a Gram stain on mauve colonies that do not have a morphology typical of MRSA. By excluding mauve colonies that were coagulase negative or had a Gram stain not consistent with MRSA at 48 h, the overall specificity of the C-MRSA remained at 99.7% (range by site, 98.9% to 100%).
CHROMagar performance versus specific methods of MRSA detection and identification.
There were five methods completed for susceptibility testing: oxacillin MIC, oxacillin screen agar, PBP2′ latex agglutination, cefoxitin disk diffusion, and mecA PCR (Tables 2 and 3). For the susceptibility data, the MRSA isolates that grew on CHROMagar alone were excluded from the data analysis. There were 339 S. aureus isolates tested by the various susceptibility methods. All five methods were completed for 326 isolates. There was 97.6% (318/326) agreement among all methods. Table 2 describes the percent agreement for MRSA and MSSA isolates. Seven isolates that grew as mauve colonies at 24 h or were mauve, slide coagulase-positive colonies at 48 h were considered “false positive” in this data set (Table 3). Two of the seven isolates were determined to be oxacillin (methicillin) resistant by PBP2′ latex, cefoxitin disk diffusion, and mecA PCR, suggesting an incorrect oxacillin MIC determination and a false negative oxacillin screen agar result. These two isolates were true MRSA isolates. Five of the seven S. aureus isolates that grew on the TSA II were oxacillin susceptible by all five tests. However, when PBP2′ was performed on mauve colonies from C-MRSA for each of these five isolates, all were PBP2′ positive. This likely indicated dual colonization of the patient with MRSA and MSSA isolates. The MRSA isolate, which was missed on the standard medium, was detected by using the selective medium. There was one specimen which grew a MRSA isolate based on a positive PBP2′ test. All other susceptibility testing on this isolate indicated oxacillin susceptibility. Therefore, the positive PBP2′ determination was considered a false positive.
TABLE 2.
Detection of methicillin (oxacillin) resistance among S. aureus isolates by C-MRSA compared to five specific susceptibility testing methods
| Susceptibility test method being compared to CHROMagar MRSA medium | % Agreement of MRSA detection (no. of resistant isolates detected/ total no. of isolates) | % Agreement of MSSA detection (no. of susceptible isolates detected/ total no. of isolates) |
|---|---|---|
| Oxacillin MIC (n = 336) | 94.4 (117/124) | 96.7 (205/212) |
| Oxacillin screen agar (n = 336) | 94.3 (116/123) | 96.7 (206/213) |
| Cefoxitin disk (n = 328) | 93.7 (118/126) | 98.5 (202/205) |
| PBP2′ latex (n = 331) | 95.0 (115/121) | 98.1 (203/207) |
| mecA PCR (n = 334) | 95.1 (116/122) | 98.1 (208/212) |
TABLE 3.
Discrepant results between CHROMagar MRSA medium and five susceptibility methods for MRSA detection
| CHROMagar MRSA result (no. of isolates) | TSA II testinga
|
C-MRSA testing (PBP2′ latex) | ||||
|---|---|---|---|---|---|---|
| Oxacillin MIC | Oxacillin screen agar | Cefoxitin disk | PBP2′ latex | mecA PCR | ||
| Positive (2) | S | S | R | + | + | Not performed |
| Negative (1) | S | S | S | + | − | Not performed |
| Positive (5)b | S | S | S | − | − | + |
S, susceptible; R, resistant.
Mixed cultures with MSSA and MRSA isolates.
DISCUSSION
This study evaluated the performance of the newly formulated BBL CHROMagar MRSA medium, a selective and differential medium for the direct detection of MRSA isolates from surveillance swabs of the anterior nares without additional susceptibility testing. Surveillance specimens of the anterior nares contain variable amounts of primarily Corynebacterium sp. and coagulase-negative staphylococci that may inhibit or obscure recovery of S. aureus on nonselective media. C-MRSA recovered 95% of the MRSA isolates in this study, compared to a recovery rate of 86% for traditional medium. Eighty-six percent of the MRSA isolates recovered on C-MRSA were detected at 24 h.
The specificity of the medium allows for direct reporting at 24 h if a mauve colony morphologically resembling staphylococcus is visualized. At 48 h, a positive coagulase test must be completed before MRSA can be reported. Depending upon the amount of MRSA in the healthcare facility and the experience of the laboratory staff working with C-MRSA, a coagulase test at 24 h could be used to improve specificity to 100%.
This study also provided an opportunity to compare C-MRSA to several methods of susceptibility testing for detection of methicillin resistance among S. aureus isolates. Identification of mauve colonies on C-MRSA as MRSA isolates compared favorably to identification of S. aureus on TSA II and subsequent identification of these S. aureus isolates as MRSA isolates. Unresolved sensitivities ranged from 93.7% to 95%, and specificities ranged from 96.7% to 98.5%. Of note was the ability of C-MRSA to detect five dual infections with MSSA and MRSA missed by the TSA II agar.
This is the first multicenter study to evaluate the effectiveness of the BBL formulation of CHROMagar MRSA medium. This is the first Food and Drug Administration-cleared chromogenic medium specific for MRSA from surveillance swab specimens of the anterior nares. Selective media for S. aureus utilize fermentation of mannitol for the presumptive identification of S. aureus and require additional identification and susceptibility testing to confirm an isolate as MRSA. Mannitol salt agar (MSA) with oxacillin has been evaluated by several groups. Safdar et al. (16) evaluated three formulations of the medium as well as the use of multiple swab types for collection of nasal swab surveillance specimens. Using direct plating of specimens from 102 patients, they found that MSA with oxacillin (4 μg/ml) and 5% (vol/vol) lipovitellin had the best sensitivity for MRSA recovery at 90%.
Simor et al. (17) compared MSA with oxacillin (2 μg/ml) to oxacillin resistance screening agar base (ORSAB) (Oxoid, Basingstoke, Hampshire, England). They evaluated 455 specimens, including nasal swabs, perineum, tissue, sputum, and urine. After 24 h of incubation, 76% of MRSA isolates were evident on ORSAB and 64% were evident on MSA (17). At 48 h, recovery for ORSAB was 98% and that for MSA was 96% (17). Twenty-six percent and 44% of all mannitol-fermenting colonies on ORSAB and MSA, respectively, were found not to be MRSA. Both media require definitive susceptibility testing for confirmation of methicillin resistance. Becker et al. (2) found a lower specificity of mannitol-fermenting colonies on the ORSAB. One hundred thirty of the 266 isolates were MRSA isolates, thus resulting in a predictive value of 48.9%. Kluytmans et al. (9) compared ORSAB to CHROMagar Staph aureus (CSA; CHROMagar Microbiology, Paris, France) for the identification of S. aureus. A total of 624 clinical strains of S. aureus and coagulase-negative staphylococci were initially plated to each medium containing no antibiotics. For detection of S. aureus, the sensitivities at 24 h were comparable (CSA, 98.6%; ORSAB, 97.1%), but the specificity of CSA was significantly higher than that of ORSAB (CSA, 97.1%; ORSAB, 92.1%). After the addition of oxacillin to CSA and ORSAB, the isolates were retested. The sensitivities of the media to detect MRSA after 48 h of incubation were 77.5% and 91.4% for CSA and ORSAB, respectively. The above studies differ from our study, as the specificity of colony color on MSA and ORSAB are lower than the 99.7% specificity of C-MRSA. The ability of the above-mentioned media to recover MRSA was decreased with the addition of antibiotics. We recovered additional MRSA isolates using the selective C-MRSA versus the nonselective TSA II.
The cost of C-MRSA ($4.59/plate list price) is higher than that of nonselective conventional media for isolation of S. aureus from swab surveillance specimens. However, there are significant benefits gained by using C-MRSA that include identification of most MRSA isolates at 24 h without additional susceptibility testing, enhanced recovery of MRSA, and suppression of MSSA and other non-MRSA species that might be present in the nose. These all contribute to substantial labor and materials cost savings. Two hundred eight of 354 (59%) S. aureus isolates in our study were MSSA that were inhibited on C-MRSA. From these data from routine clinical specimens, it is apparent that use of C-MRSA would result in a significant reduction in the numbers of coagulase and susceptibility tests performed in a clinical laboratory.
As laboratories rise to help meet the challenge of control of MRSA in the healthcare facilities and now in the community, rapid diagnostic methods are needed. For many, C-MRSA can fulfill this need, as demonstrated in this study. C-MRSA was superior to TSA II for recovery of MRSA from swab surveillance cultures of the anterior nares, and it may shorten the time to detection of MRSA by 24 to 48 h, depending upon the methods used.
Acknowledgments
We acknowledge the following staff and microbiologists for their contribution to the clinical trial and manuscript preparation: Clara Lema and Hollie Snyder, JHH; Meredith Erwin, Barry Buschelman, and Linda Aker, University of Iowa Microbiology Laboratory; Farzaneh Sooudipour, Linda Sueyoshi, and Karen Ward, UCLA Microbiology Laboratory; and Sarah Arthur and Paula Johnson from BD Diagnostics.
This study was funded by BD Diagnostics, Sparks, MD.
REFERENCES
- 1.Abramson, M. A., and D. J. Sexton. 1999. Nosocomial methicillin-resistant and methicillin-susceptible Staphylococcus aureus primary bacteremia: at what costs? Infect. Control Hosp. Epidemiol. 20:408-411. [DOI] [PubMed] [Google Scholar]
- 2.Becker, A., D. Forster, and E. Kniehl. 2002. Oxacillin screening agar base for the detection of methicillin-resistant Staphylococcus aureus. J. Clin. Microbiol. 40:4400-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blot, S. I., K. H. Vandewoude, E. A. Hoste, and F. A. Colardyn. 2002. Outcome and attributable mortality in critically ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch. Intern. Med. 162:2229-2235. [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2005. M100-S15. Performance standard for antimicrobial susceptibility testing; 15th informational supplement. Clinical and Laboratory Standards Institute, Wayne, Pa.
- 5.Cosgrove, S. E., G. Sakoulas, E. N. Perencevich, M. J. Schwaber, A. W. Karchmer, and Y. Carmeli. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin. Infect. Dis. 36:53-59. [DOI] [PubMed] [Google Scholar]
- 6.Davis, K. A., J. J. Stewart, H. K. Crouch, C. E. Florez, and D. R. Hospenthal. 2004. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin. Infect. Dis. 39:776-782. [DOI] [PubMed] [Google Scholar]
- 7.Engemann, J. J., Y. Carmeli, S. E. Cosgrove, V. G. Fowler, M. Z. Bronstein, S. L. Trivette, J. P. Briggs, D. J. Sexton, and K. S. Kaye. 2003. Adverse clinical and economic outcomes attributable to methicillin resistance among patients with Staphylococcus aureus surgical site infection. Clin. Infect. Dis. 36:592-598. [DOI] [PubMed] [Google Scholar]
- 8.Farr, B. M. 2004. Prevention and control of methicillin-resistant Staphylococcus aureus infections. Curr. Opin. Infect. Dis. 17:317-322. [DOI] [PubMed] [Google Scholar]
- 9.Kluytmans, J., A. Van Griethuysen, P. Willemse, and P. Van Keulen. 2002. Performance of CHROMagar selective medium and oxacillin resistance screening agar base for identifying Staphylococcus aureus and detecting methicillin resistance. J. Clin. Microbiol. 40:2480-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopp, B. J., D. E. Nix, and E. P. Armstrong. 2004. Clinical and economic analysis of methicillin-susceptible and -resistant Staphylococcus aureus infections. Ann. Pharmacother. 38:1377-1382. [DOI] [PubMed] [Google Scholar]
- 11.Kotiliainen, P., M. Routamaa, R. Peltonen, J. Oski, E. Rintala, O Meurman, O. P. Lehtonen, E. Eerola, S. Salmenlinna, J. Vuopio-Varkila, and T. Rossi. 2003. Elimination of epidemic methicillin-resistant Staphylococcus aureus from a university hospital and district institutions, Finland. Emerg. Infect. Dis. 9:169-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muto, C. A., J. A. Jernigan, B. E. Ostrowsky, H. M. Richet, W. R. Jarvis, J. M. Boyce, and B. M. Farr. 2003. SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus. Infect. Control Hosp. Epidemiol. 24:362-386. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed. Approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.National Nosocomial Infections Surveillance System. 2004. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am. J. Infect. Control 32:470-485. [DOI] [PubMed] [Google Scholar]
- 15.Pittet, D., S. Hugonnet, S. Harbarth, P. Mourouga, V. Sauvan, S. Touveneau, and T. V. Perneger. 2000. Effectiveness of a hospital-wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet 356:1307-1312. [DOI] [PubMed] [Google Scholar]
- 16.Safdar, N., L. Narans, B. Gordon, and D. G. Maki. 2003. Comparison of culture screening methods for detection of nares carriage of methicillin-resistant Staphylococcus aureus: a prospective study comparing 32 methods. J. Clin. Microbiol. 41:3163-3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simor, A. E., J. Goodfellow, L. Louie, and M. Louie. 2001. Evaluation of a new medium, oxacillin resistance screening agar base, for the detection of methicillin-resistant Staphylococcus aureus from clinical samples. J. Clin. Microbiol. 39:3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verhoef, J., D. Beaujean, H. Blok, A. Baars, A. Meyler, C. van der Werken, and A. Weersink. 1999. A Dutch approach to methicillin resistant Staphylococcus aureus. Eur. J. Clin. Microbiol. Infect. Dis. 18:461-466. [DOI] [PubMed] [Google Scholar]