Abstract
Serological diagnosis of Trypanosoma cruzi infection is hampered by issues related to test specificity due to the cross-reactivity of most antigens with proteins of related parasites such as Leishmania spp. The recombinant calflagins are considered relevant antigens for the diagnosis of infection by Trypanosoma cruzi. In the present work, we describe two genes coding for putative calflagins in Leishmania major with the N-terminal moieties presenting high similarity with T. cruzi genes. This fact raised questions about their role in some cross-recognition of this antigen by sera from Leishmania spp.-infected individuals. The complete T. cruzi calflagin and two fragments of the protein, consisting of 146 amino acids of the N-terminal and 65 amino acids of the C-terminal regions, were expressed and evaluated against a panel of sera, which included well-characterized samples from T. cruzi, and Leishmania-infected patients. We were able to show that sera from Leishmania (Viannia) braziliensis-infected individuals recognized the recombinant full-length calflagin. Both the N-terminal and the complete protein presented the same high sensitivity (98.5% of sera from T. cruzi-infected patients was detected) but different specificities (94% and 98%, respectively, when evaluated against sera from people not infected by T. cruzi, including 15 sera from people infected with L. braziliensis). The C-terminal fragment presented low sensitivity (70%) but 100% specificity. We propose the use of these antigens in two sequential assays to optimize the serological diagnosis of T. cruzi infection in humans in geographic areas where Leishmania spp. infection is coendemic.
American trypanosomiasis (or Chagas' disease) is an infectious disease that is endemic in the Americas, affecting 16 to 18 million people and putting 100 million people at risk (http://www.who.int/ctd/chagas/disease.htm). The causative agent, Trypanosoma cruzi, has a complex life cycle that occurs in triatomine insect vectors and mammals, including humans (5). Since vectorial transmission control is being practiced in several countries, one of the most important tools to control interhuman infection is its correct diagnosis in chronic asymptomatic blood donors (http://www.who.int/ctd/chagas/disease.htm). In view of these facts, a variety of T. cruzi antigens have been evaluated for enzyme-linked immunosorbent assay (ELISA) reactions in order to optimize the specificity and sensitivity of serological tests.
Most antigens consisting of homogenates, subcellular fractions, or complex mixtures of parasite proteins from both the epimastigote and trypomastigote stages show good performance in terms of sensitivity. However, frequently they have low specificity due to cross-reactivity of some components with proteins of other phylogenetically related protozoa, such as those from the genus Leishmania or T. rangelli, leading to misdiagnosis of the infection (8, 28). The search for antigens with a defined composition became a necessity, leading to the evaluation of a variety of native (1, 2, 9, 17, 22, 23, 30, 32, 36) or recombinant proteins (for a review, see reference 10). One main disadvantage, in relation to the first option, is that direct purification of proteins from total extracts of the parasite is generally difficult due to their low relative quantities. On the other hand, most of the recombinant antigens were not sensitive or specific enough to completely replace native antigens.
One of the most promising recombinant proteins for diagnosis is a T. cruzi flagellar calcium binding protein (11, 13, 15, 24, 27). It belongs to a family of proteins identified as calflagins (37) with orthologs in several trypanosomatids, such as T. brucei (20) and T. rangeli (27). Interestingly, no Leishmania orthologs have been described up to now. Several studies on the diagnostic profile of this recombinant protein identified its use as a valuable antigen for the serological detection of the infection in the indeterminate phase (14, 18, 25, 34, 35) and for therapy evolution follow-up (33). Several works also established that sera from Leishmania spp.-infected individuals have low levels of cross-reactivity with T. cruzi calflagins (14, 34, 35). Further, this cross-reactivity constitutes a relevant point due to the existence of geographic overlaps of the endemic areas for both infections (http://www.who.int/ctd/chagas/disease.htm).
In the present work, we identified in the Leishmania major genome database two genes coding for putative calflagins. The alignment of the deduced protein sequences showed an N-terminal region with high similarity to T. cruzi calflagin. This fact would explain some cross-reactivity of the Leishmania patient sera, as previously reported. In order to approach this question, we expressed the recombinant full-length calflagin (rC29) from T. cruzi as well as an N-terminal and a C-terminal portion and evaluated these peptides as antigens for diagnosis against a serum panel. We established that the use of two sequential serological assays, based on the complete calflagin and the C-terminal portion of the protein, could well result in an optimized routine for specific T. cruzi antibody detection in geographic areas where Leishmania spp. infections are coendemic.
MATERIALS AND METHODS
Chemicals.
All reagents were purchased from SIGMA (Saint Louis, Mo.) except where otherwise indicated.
Parasite cultures and total homogenates.
Epimastigotes of Trypanosoma cruzi (Tulahuen strain) were grown in liver infusion tryptose medium supplemented with 10% fetal calf serum (Cultilab, São Paulo, Brazil) (7). Total homogenates of epimastigotes (TH) were obtained by resuspension of the washed cells in five volumes of 1 mM Nα-p-tosyl-l-lysine chloromethyl ketone and 1 mM phenylmethylsulfonyl fluoride (PMSF) in distilled water, freeze and thaw (four cycles), and sonication (20 kHz, 30 W, 2 min).
Calflagin cDNA.
The calflagin cDNA used in this work was obtained as previously described (21).
Sequencing.
Plasmids were sequenced by the dideoxy chain termination method (29) using the BigDye Terminator kit v. 3.1 (Perkin Elmer, Foster City, CA) as described by the manufacturer's protocol. The reaction was read in an ABI Prism 377 automated DNA sequencer (Perkin Elmer).
Expression vector engineering.
Recombinant DNA techniques were performed using conventional protocols (3). Molecular biology reagents were purchased from Promega (Madison, WI). The full-length calflagin cDNA (C29 gene) was cloned in the EcoRI site of plasmid pMAL c2 (NEB, Beverly, MA), and this construction was named pMAL/C29FL. In order to express two fragments from the C29 gene, the pMAL/C29FL vector was digested with SalI, liberating a fragment of the gene coding for the 65 C-terminal amino acids. The digested plasmid was ligated again containing a fragment of the C29 gene coding for the 146 N-terminal amino acids of the protein. The excised fragment, coding for the 65 C-terminal amino acids, was subcloned in the SalI position of pMAL c2. Both constructions (named pMAL/C29N and pMAL/C29C, respectively) were used to transform Escherichia coli DH5α. The constructions were confirmed by PCR, restriction analysis, and sequencing. With pMAL/C29FL, pMAL/C29N, and pMAL/C29C plasmids, the complete C29 protein as well as a C-terminal portion and a N-terminal portion were produced as fusions with the maltose binding protein. pMAL c2 plasmid was used to produce maltose binding protein (MBP) as a control.
Protein expression.
E. coli single colonies containing the plasmids corresponding to each of the described constructions were grown in Luria-Bertani (LB) medium plus 0.1 mg/ml ampicillin overnight at 37°C, with agitation. The cultures were diluted 1:100 in fresh LB and incubated to an optical density at 600 nm (OD600) of 0.7. Expressions were induced with the addition of isopropyl-α-d-thiogalactopyranoside (Promega) at a final concentration of 1 mM. The cells were harvested by centrifugation after 3 h of induction.
Purification by affinity to amylose.
All other reagents in this section were from SIGMA. Cells were lysed by sonication (20 kHz, 30 W, 2 min) in the presence of 1 mM EDTA, 1 mM PMSF, 150 mM NaCl, 10 mM Tris-HCl, pH 8. The extracts were clarified by centrifugation at 16,000 × g for 10 min and applied to the column containing 1 ml of amylose-Sepharose resin (NEB, Beverly, MA). The column was washed with 10 column volumes of 1 mM EDTA, 1 mM PMSF, 150 mM NaCl, 10 mM Tris-HCl, pH 8. The fusion protein was eluted by adding 10 column volumes of elution buffer (10 mM maltose, 1 mM EDTA, 150 mM NaCl, 10 mM Tris-HCl, pH 8). The fractions were recovered, and the presence of the recombinant protein in each sample was determined by absorbance at 280 nm and by ELISA with specific antibodies.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
The samples were resuspended in loading buffer, subjected to SDS-12% PAGE (50 μg/lane), and stained with Coomassie brilliant blue according to Laemmli (19).
ELISA.
Total protein concentration was determined by using the Bradford method described elsewhere (4). Microtiter plates (Costar, Cambridge, MA) were sensitized with 1 μg of TH or the recombinant antigens, contained in a 100-μl solution of phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 1.4 mM NaH2PO4, 4.3 mM Na2HPO4, pH 7.4). The plates were incubated overnight at 4°C, washed three times with 0.01% Tween in PBS (PBS-T), and blocked for 1 h at 37°C with 1% bovine serum albumin in PBS. Microplates were incubated with different dilutions of sera (as stated in each case) in 1% low-fat milk in PBS. After being washed, they were incubated with anti-rabbit immunoglobulin G-peroxidase conjugate (Sigma, St. Louis, MO) diluted 1/1,000 in 1% milk in PBS. The reaction was developed using tetramethyl benzidine (TMB) in H2O2. All incubations were performed at 37°C for 60 min. Absorbances were read at 450 nm.
Human sera panel.
Sixty-eight human sera samples characterized as positive (T) and 33 were characterized as negative (N) for the chagasic infection were obtained from blood bank asymptomatic donors. The T. cruzi infectious status was determined by using two different conventional tests: commercial ELISA (Chagatest ELISA) and indirect hemagglutination (Chagatest IHA), from Wiener (Rosario, Argentina), both of them based on epimastigote total homogenate antigens. Their serological status was established by the gold standard criteria: any sample was considered positive or negative based on concordant results from both reactions (16). All (N and T) individuals were negative for syphilitic infection and were also serologically negative for human immunodeficiency virus as well as hepatitis B and C. Fifteen sera from individuals infected with Leishmania (Viannia) braziliensis (L) were obtained from patients recruited at the Centro de Pesquisas Aggeu Magalhães, Fundação Oswaldo Cruz, Recife PE, Brazil, with clinical manifestations of cutaneous leishmaniasis. All of them are from a region where L. braziliensis is endemic (Pernambuco State, Brazil) (6), and were defined as epidemiologically negative for T. cruzi infection, since there are no reports of the presence of the insect vector or cases of infection. These patients have never been in contact with T. cruzi insect vectors nor traveled to areas where T. cruzi is endemic, and none of them have been submitted for blood transfusion or organ grafting.
Data analysis.
The data obtained as optical densities at 450 nm (OD450) were analyzed using computer graphics software (Microcal Origin 5.0). The cutoff values for ELISA were calculated as the mean OD450 of the true negative sera plus 3 standard deviations (SD). Positive and negative results in the ELISA tests were compared with the serologic status obtained by the respective reference technique, as described above. The statistical significances for comparisons among mean OD values were evaluated by the Student t test.
Sequence analysis and nucleotide sequence accession number.
The nucleotide sequence for rC29 has been deposited in the GenBank database under the accession number AF192890. Leishmania major genes reported in this work were deposited in the Sanger Center L. major genome database under systematic names LmF16.0910 and LmF16.0920. Using BioEdit Sequence Alignment Editor v. 7.0.1 with default parameters, we performed the translation and alignment of sequences.
RESULTS
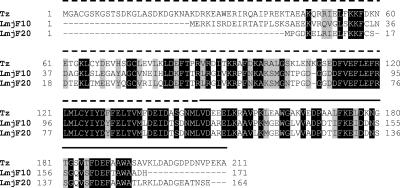
Some data in the literature indicate that the cross-reactivity of sera from Leishmania spp.-infected individuals when using recombinant calflagin to diagnose the infection of Chagas′ disease might be a relevant problem in regions defined as coendemic for both pathogens (14, 34, 35). A BLAST search using the T. cruzi calflagin amino acid sequence against the translated L. major genome project database at the Sanger Center (www.genedb.org) revealed two putative calflagins that may represent orthologous genes for the calcium binding protein (the systematic names at the Sanger Center L. major genome database are LmF16.0910 and LmF16.0920). Both proteins diverge from the T. cruzi calflagin in the extension of the N-terminal region, such that those from T. cruzi are larger than those from Leishmania. In spite of this difference, we were able to detect a block of amino acids between positions 110 and 149 with a high similarity, where the region corresponding to positions beyond 150 were more divergent (Fig. 1).
FIG. 1.
Alignment of the T. cruzi calflagin (Tz) and Leishmania major putative calflagins (LmjF10 and LmjF20). The dotted line marks the sequence of rC29N, and the continuous line marks the sequence of rC29C.
In view of this fact, we took advantage of the presence of the two SalI restriction sites on each side of the calflagin gene and an internal site at position 146 to generate a plasmid able to express the 146 N-terminal amino acids of the protein (dotted line in Fig. 1) and the 65 C-terminal amino acids (continuous line in Fig. 1).
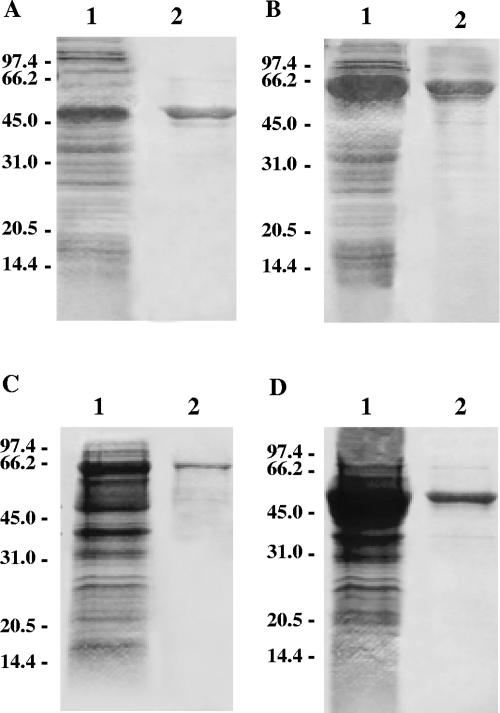
The expressions of each recombinant protein (rC29FL, rC29N, and rC29C) were established. The total extracts of the induced bacterial cultures were subjected to SDS-PAGE and stained with Coomassie. In all cases, predominant bands were observed with their respective molecular weights corresponding to those expected for the fusion proteins. The corresponding noninduced cultures were also subjected to SDS-PAGE as controls (data not shown). The recombinant proteins were purified through amylose-Sepharose resin affinity chromatography for bacterial extracts. Fractions corresponding to protein peaks were submitted to SDS-PAGE to evaluate the acquired protein quality. The major bands in the E. coli extracts were purified to apparent homogeneity (Fig. 2).
FIG. 2.
Expression and purification of the different constructions by SDS-PAGE. (A) MBP; (B) C29FL; (C) C29N; (D) C29C. In each case, lane 1 corresponds to whole lysates and lane 2 to purified proteins.
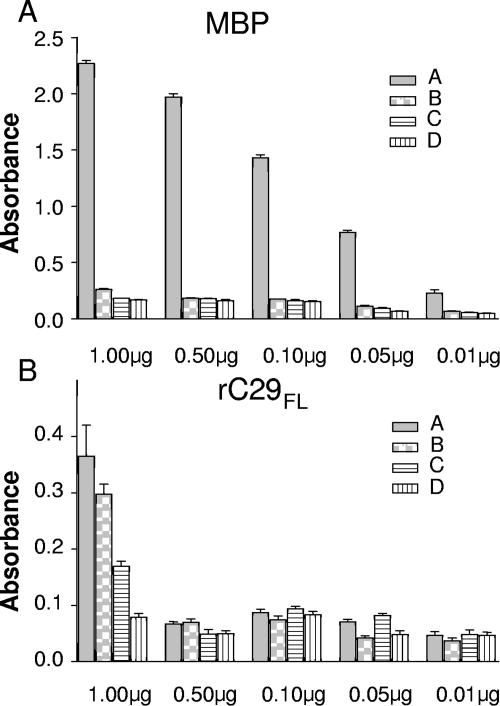
To exclude nonspecific reactivity against maltose binding proteins (MBP), we established the conditions of use for rC29 or its fragments fused to MBP for ELISA with human sera by comparing the performance of the rC29FL fusion protein with that of MBP alone. ELISA microplates were sensitized with different quantities of either rC29FL fusion protein or MBP. Both proteins were tested in parallel in order to optimize the reaction conditions for ELISA. Reactivity was evaluated by means of four well-characterized human sera, collected from infected and noninfected individuals. One of the positive sera was characterized as highly reactive, while the other was characterized as weakly reactive. The negative sera were characterized as strong and weak negative sera on the basis of their reactivity against T. cruzi total homogenate antigen. When the plates were sensitized with 1 μg of MBP, unspecific absorbencies in the assays were over 0.3 OD450 while negative sera were over 0.15 OD450. When plates were sensitized with 0.5 μg/well or less, nonspecific reactivity of positive and negative sera diminished to around 0.05 OD450. However, when the microplates were sensitized with less than 0.01 μg, the signal/noise ratio for the fusion protein diminished by approximately 50% for the highly reactive positive serum and 30% for the weakly reactive serum (Fig. 3).
FIG. 3.
Optimization of the fusion construction to be used for ELISA serological assay. The reactivity of four well-characterized sera against different concentrations of rC29FL (A) and maltose binding protein (B) was represented. The sera used were previously characterized as highly reactive (A), weakly reactive (B), strongly negative (with low unspecific reactivity) (C), and weakly negative (negative but presenting high background) (D) with respect to their reactivity against TH.
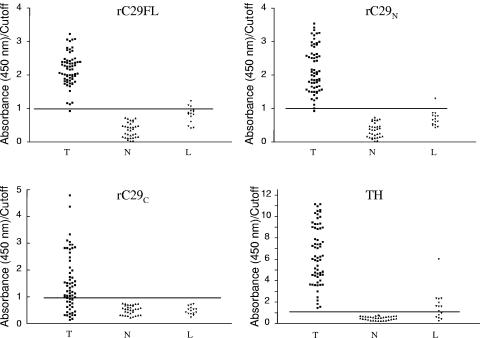
On the basis of the appropriate conditions for ELISA established above, we evaluated the performance of the three recombinant antigens here described (rC29FL, rC29N, and rC29C) and compared them with a complex mixture of proteins from T. cruzi epimastigotes (TH extract). The results obtained show that among the antigens that were evaluated in this work, rC29N displayed the best performance for T. cruzi infection diagnosis when a subpopulation of individuals infected with L. braziliensis is represented in the serological panel (Fig. 4). As can be seen in Fig. 4 and Table 1, TH displayed 100% sensitivity (the antigen was recognized by all the T sera), since no false-negative results were detected. Interestingly, 100% specificity was also observed when the healthy population of our panel (N sera) was considered. When the sera from patients infected with L. braziliensis (L sera) were included in the analysis, most of them behaved as reactive when using this system, giving rise to false-positive results and diminishing the specificity. As expected, the results in terms of specificity were best when rC29FL was used as antigen, but it must be remarked that 3 out of 15 L sera remained reactive. The serum corresponding to a T. cruzi-infected patient (T sera) was not detected as positive when using this ELISA test. When rC29C was evaluated, 100% specificity was obtained when including L sera; however, the sensitivity was poor (70%).
FIG. 4.
Evaluation of the reactivity of sera in the panel against rC29FL, rC29N, rC29C, and TH. The scatter plots represent the reactivity in ELISA against the different antigens evaluated with respect to the cut-off value defined as the mean OD of N sera plus 3 SD (OD/cutoff ratio) in each case. T, sera of patients infected by T. cruzi; N, sera of patients not infected either by T. cruzi or by Leishmania spp.; L, sera of patients infected by Leishmania spp. but not infected by T. cruzi.
TABLE 1.
Sensitivity and specificity values for rC29FL, rC29N, rC29C, and TH antigens against the different subpopulations of sera in the panel
| Criterion | rC29FL (%) | rC29C (%) | rC29N (%) | TH (%) |
|---|---|---|---|---|
| Sensitivitya | 98.5 | 70 | 98.5 | 100 |
| Specificityb | 94 | 100 | 98 | 73 |
| Specificityc | 100 | 100 | 100 | 100 |
| Specificityd | 80 | 100 | 93.5 | 13 |
No. of T sera, 68.
Including 33 N sera and 15 L sera.
Only 33 N sera (without L sera).
Only 15 L sera (without N sera).
The performance of the recombinant antigens against sera that could have given rise to false-positive results can be inferred from a statistical comparison of panel sera behavior against the three recombinant proteins herein evaluated. The mean OD values and their distribution for the three groups and for the three antigens were analyzed (Table 2). When protein rC29FL or rC29N was used as antigen, the mean OD values obtained for the T sera and the T. cruzi-negative sera (N sera) were similar. However, L sera showed a diminished reactivity when tested against rC29N with respect to rC29FL (the mean OD value was 0.514 for rC29N and 0.650 for rC29FL). The distribution pattern of the OD values, when rC29C was used as antigen, was different from that presented with rC29FL and rC29N antigens. When the mean OD values obtained for N and L sera against the three antigens were compared, it was determined that L sera recognized rC29FL and rC29N in a nonspecific way with respect to the recognition of N population (P < 0.001). Interestingly, when N and L populations were evaluated against rC29C, the differences between these two groups were not significant (P = 0.667).
TABLE 2.
Comparisons among the OD values obtained for the three groups of sera assayed with the three recombinant antigens
| ODs and P value | rC29FL | rC29C | rC29N |
|---|---|---|---|
| Mean OD for T sera | 1.734 | 0.590 | 1.641 |
| Mean OD for N sera | 0.278 | 0.199 | 0.266 |
| Mean OD for L sera | 0.650 | 0.191 | 0.514 |
| Comparison between the OD values corresponding to L sera and N sera (95% of confidence) | 0.372 ± 0.051 | 0.0086 ± 0.0199 | 0.248 ± 0.050 |
| P value | <0.0001 | 0.6675 | <0.0001 |
DISCUSSION
Calflagin is the name given by Wu et al. to a group of flagellar calcium binding proteins present in most trypanosomatids (37). The function of this protein remains largely unknown, but it has been hypothesized that it participates in an acyl-calcium switch system (12), and this proposal is supported by experimental data showing a Ca2+-dependent conformational change (26). Since it was cloned for the first time, its utility in the diagnosis of T. cruzi infection has been well established (14, 18, 25, 33-35), including the possibility of using its gene sequence for PCR diagnosis (31). The rapid reduction of antibodies against calflagin in patients that respond to trypanocidal treatment has also been demonstrated, indicating that calflagin may be used in therapy follow-up (33). It is worth emphasizing that several isoforms of calflagin were tested, by a number of research groups (14, 18, 25, 34, 35), against panels of sera, including samples from Leishmania spp.-infected patients. In all these studies, the authors detected low levels of cross-reactivity for the sera from patients infected by Leishmania. However, Passos et al. reported that the reactivity of patient sera against this protein in a geographic area that was coendemic for T. cruzi and Leishmania spp. infections were significantly higher than the prevalence of chagasic infection (25). These authors determined the reactivity percentage of 335 sera from patients presenting clinical manifestations of cutaneous and mucocutaneous leishmaniasis. The percentage of positive samples in immunofluorescence, hemagglutination, ELISA using T. cruzi crude extract, and ELISA based on an isoform of calflagin were 46, 26, 12, and 6.9%, respectively. The data indicated that ELISA with calflagin was the most specific. Nevertheless, the prevalence of T. cruzi infection in the population studied had been determined to be 2 to 4.5%, below the 6.9% seroprevalence value obtained with ELISA using this antigen. The authors speculated that this fact might be due to a cross-reactivity of part of the sera from Leishmania spp.-infected individuals with calflagin.
On the basis of these results, we hypothesized the presence of orthologous genes for this protein in Leishmania. In fact, a BLAST search revealed the presence of two sequences that present similarities of 73 to 75% with T. cruzi calflagins and included highly conserved regions. Based on this fact and on the availability of appropriate restriction sites in the starting construction for expressing the full-length protein, we prepared two constructions: one to express the 146 N-terminal amino acids (rC29N) and the other to express the 65 C-terminal amino acids (rC29C).
The constructions were expressed as fusions to the E. coli MBP and purified by affinity chromatography. A comparative assay using the MBP alone or the rC29FL allowed us to establish the conditions for ELISA with human sera in which the MBP fused to the antigen of interest showed minimal interference.
Finally, the three recombinant proteins as well as a total extract of epimastigote proteins were assayed on a pilot scale against a human serum panel that included sera from L. braziliensis patients. The results herein presented are in agreement with previous reports establishing that calflagin as a whole molecule behaves as a sensitive antigen, and it may be specific in regions where infection by Leishmania spp. is not an endemic problem (specificity, 100%). However, some specificity problems were detected when a population infected with L. braziliensis is represented (specificity, 94%, with this value calculated from 1/5 of the L sera). When rC29C was evaluated, the specificity value was 100% but the sensitivity was unsatisfactory (70%). The rC29N antigen presented the same level of sensitivity as the whole protein, but the specificity values, in spite of having been optimized, were still considered unsatisfactory (98% when including L sera). When taken together, these results led us to propose the diagnosis optimization of T. cruzi infection in areas where Leishmania spp. is endemic by designing a sequential two-step diagnosis routine. To this end, whole calflagin could be used as a first screening test and rC29C as a confirmatory assay to discard false-positive cases due to Leishmania spp. The antigen rC29N, which shows higher specificity than the whole protein, could be used for the diagnosis of T. cruzi infections from areas where Leishmania is not endemic, where the probability of the occurrence of false positives does not justify the use of a confirmatory assay.
In conclusion, Leishmania presents orthologous genes in relation to T. cruzi calflagin. The putative proteins appear to be responsible for the positivity of T. cruzi antigen in sera from L. braziliensis patients. In preliminary evaluations, we were able to successfully identify a subregion of the flagellar calcium binding protein of T. cruzi that resulted in a 100% specific antigen for the diagnosis of the T. cruzi infection in humans, which shows improved specificity over the whole protein. Despite being an optimized antigen, in terms of diagnosis specificity we considered that it was not specific enough to propose its use as a single criterion for the diagnosis of T. cruzi infection in areas where Leishmania spp. are endemic. For these regions, we propose a two-step diagnostic routine for the specific determination of chagasic infection: the use of the whole calflagin as a first screening test for positive serology and rC29C as a confirmatory assay to discard false-positive cases due to Leishmania spp. infections. This proposal will be further evaluated by using a larger panel of sera in order to validate “in the field” the two-step diagnostic routine.
The strategy for optimizing the serological diagnosis of T. cruzi infection herein described, together with the analysis of the influence of the expression system on the diagnostic profile (21), may be applied to other proteins with high sensitivity and low specificity. This could lead to the engineering of synthetic genes to be expressed in defined systems whose products may result in optimized systems for the serological diagnosis of T. cruzi infection.
Acknowledgments
We are indebted to Walter Colli for his critical reading of the manuscript.
This work was partially founded by ANPCyT (PICT No. 2002/00057). Throughout this work, I.S.M. was a fellow from the Universidad Nacional del Litoral and A.M.S. was a fellow from the Argentinian National Research Council (CONICET).
The authors do not have a commercial or any other association that might pose a conflict of interest with the publication of this article.
Footnotes
C.R. and G.C. contributed equally to this work.
REFERENCES
- 1.Aguillon, J. C., R. Harris, M. C. Molina, A. Colombo, C. Cortes, T. Hermosilla, P. Carreno, A. Orn, and A. Ferreira. 1997. Recognition of an immunogenetically selected Trypanosoma cruzi antigen by seropositive chagasic human sera. Acta Trop. 63:159-166. [DOI] [PubMed] [Google Scholar]
- 2.Almeida, I. C., D. T. Covas, L. M. Soussumi, and L. R. Travassos. 1997. A highly sensitive and specific chemiluminescent enzyme-linked immunosorbent assay for diagnosis of active Trypanosoma cruzi infection. Transfusion 37:850-857. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel, F., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1995. Short protocols in molecular biology, 4th ed. John Wiley & Sons Inc., Cold Spring Harbor, N.Y.
- 4.Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 5.Brener, Z. 1973. Biology of Trypanosoma cruzi. Annu. Rev. Microbiol. 27:347-382. [DOI] [PubMed] [Google Scholar]
- 6.Brito, M. E., M. G. Mendonca, Y. M. Gomes, M. L. Jardim, and F. G. Abath. 2000. Identification of potentially diagnostic Leishmania braziliensis antigens in human cutaneous leishmaniasis by immunoblot analysis. Clin. Diagn. Lab. Immunol. 7:318-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camargo, E. P. 1964. Growth and differentiation in Trypanosoma cruzi. I. Origin of metacyclic trypanosomes in liquid media. Rev. Inst. Med. Trop. Sao Paulo 12:93-100. [PubMed] [Google Scholar]
- 8.Camargo, M. E., E. L. Segura, I. G. Kagan, J. M. Souza, R. J. da Carvalheiro, J. F. Yanovsky, and M. C. Guimaraes. 1986. Three years of collaboration on the standardization of Chagas' disease serodiagnosis in the Americas: an appraisal. Bull. Pan. Am. Health Org. 20:233-244. [PubMed] [Google Scholar]
- 9.Carbonetto, C. H., E. L. Malchiodi, M. Chiaramonte, E. Durante de Isola, C. A. Fossati, and R. A. Margni. 1990. Isolation of a Trypanosoma cruzi antigen by affinity chromatography with a monoclonal antibody. Preliminary evaluation of its possible applications in serological tests. Clin. Exp. Immunol. 82:93-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.da Silveira, J. F., E. S. Umezawa, and A. O. Luquetti. 2001. Chagas disease: recombinant Trypanosoma cruzi antigens for serological diagnosis. Trends Parasitol. 17:286-291. [DOI] [PubMed] [Google Scholar]
- 11.Engman, D. M., K. H. Krause, J. H. Blumin, K. S. Kim, L. V. Kirchhoff, and J. E. Donelson. 1989. A novel flagellar Ca2+-binding protein in trypanosomes. J. Biol. Chem. 264:18627-18631. [PubMed] [Google Scholar]
- 12.Godsel, L. M., and D. M. Engman. 1999. Flagellar protein localization mediated by a calcium-myristoyl/palmitoyl switch mechanism. EMBO J. 18:2057-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godsel, L. M., C. L. Olson, Z. G. Lacava, and D. M. Engman. 1995. Comparison of the 24 kDa flagellar calcium-binding protein cDNA of two strains of Trypanosoma cruzi. J Eukaryot. Microbiol. 42:320-322. [DOI] [PubMed] [Google Scholar]
- 14.Godsel, L. M., R. S. Tibbetts, C. L. Olson, B. M. Chaudoir, and D. M. Engman. 1995. Utility of recombinant flagellar calcium-binding protein for serodiagnosis of Trypanosoma cruzi infection. J. Clin. Microbiol. 33:2082-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez, A., T. J. Lerner, M. Huecas, B. Sosa-Pineda, N. Nogueira, and P. M. Lizardi. 1985. Apparent generation of a segmented mRNA from two separate tandem gene families in Trypanosoma cruzi. Nucleic Acids Res. 13:5789-5804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guhl, F., C. Jaramillo, J. C. Carranza, and G. A. Vallejo. 2002. Molecular characterization and diagnosis of Trypanosoma cruzi and T. rangeli. Arch. Med. Res. 33:362-370. [DOI] [PubMed] [Google Scholar]
- 17.Kirchhoff, L. V., A. A. Gam, R. A. Gusmao, R. S. Goldsmith, J. M. Rezende, and A. Rassi. 1987. Increased specificity of serodiagnosis of Chagas' disease by detection of antibody to the 72- and 90-kilodalton glycoproteins of Trypanosoma cruzi. J. Infect. Dis. 155:561-564. [DOI] [PubMed] [Google Scholar]
- 18.Krautz, G. M., J. D. Peterson, L. M. Godsel, A. U. Krettli, and D. M. Engman. 1998. Human antibody responses to Trypanosoma cruzi 70-kD heat-shock proteins. Am. J. Trop. Med. Hyg. 58:137-143. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 20.Maldonado, R. A., J. Linss, N. Thomaz, C. L. Olson, D. M. Engman, and S. Goldenberg. 1997. Homologues of the 24-kDa flagellar Ca(2+)-binding protein gene of Trypanosoma cruzi are present in other members of the Trypanosomatidae family. Exp. Parasitol. 86:200-205. [DOI] [PubMed] [Google Scholar]
- 21.Marcipar, I. S., M. L. Olivares, L. Robles, A. Dekanty, A. Marcipar, and A. M. Silber. 2004. The diagnostic performance of recombinant Trypanosoma cruzi ribosomal P2beta protein is influenced by its expression system. Protein Expr. Purif. 34:1-7. [DOI] [PubMed] [Google Scholar]
- 22.Marcipar, I. S., E. Welchen, C. Roodveldt, A. J. Marcipar, and A. M. Silber. 2003. Purification of the 67-kDa lectin-like glycoprotein of Trypanosoma cruzi, LLGP-67, and its evaluation as a relevant antigen for the diagnosis of human infection. FEMS Microbiol. Lett. 220:149-154. [DOI] [PubMed] [Google Scholar]
- 23.Martinez, J., O. Campetella, A. C. Frasch, and J. J. Cazzulo. 1991. The major cysteine proteinase (cruzipain) from Trypanosoma cruzi is antigenic in human infections. Infect. Immun. 59:4275-4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ouaissi, A., T. Aguirre, B. Plumas-Marty, M. Piras, R. Schoneck, H. Gras-Masse, A. Taibi, M. Loyens, A. Tartar, A. Capron, et al. 1992. Cloning and sequencing of a 24-kDa Trypanosoma cruzi specific antigen released in association with membrane vesicles and defined by a monoclonal antibody. Biol. Cell 75:11-17. [DOI] [PubMed] [Google Scholar]
- 25.Passos, V. M., A. C. Volpini, E. M. Braga, P. A. Lacerda, A. Ouaissi, M. V. Lima-Martins, and A. U. Krettli. 1997. Differential serodiagnosis of human infections caused by Trypanosoma cruzi and Leishmania spp. using ELISA with a recombinant antigen (rTc24). Mem. Inst. tOswaldo Cruz 92:791-793. [DOI] [PubMed] [Google Scholar]
- 26.Pinto, A. P., P. T. Campana, L. M. Beltramini, A. M. Silber, and A. P. Araujo. 2003. Structural characterization of a recombinant flagellar calcium-binding protein from Trypanosoma cruzi. Biochim. Biophys. Acta 1652:107-114. [DOI] [PubMed] [Google Scholar]
- 27.Porcel, B. M., E. J. Bontempi, J. Henriksson, M. Rydaker, L. Aslund, E. L. Segura, U. Pettersson, and A. M. Ruiz. 1996. Trypanosoma rangeli and Trypanosoma cruzi: molecular characterization of genes encoding putative calcium-binding proteins, highly conserved in trypanosomatids. Exp. Parasitol. 84:387-399. [DOI] [PubMed] [Google Scholar]
- 28.Saez-Alquezar, A., A. O. Luquetti, J. Borges-Pereira, E. F. Moreira, M. Gadahela, M. T. Garcia Zapata, and A. H. Strugo Arruda. 1997. Estudo multicéntrico: validação do desempenho de conjuntos diagnósticos de hemaglutinação indirecta disponíveis no Brasil para o diagnóstico serológico da infeção pelo Trypanosoma cruzi. Revista de Patologia Tropical 26:343-374. [Google Scholar]
- 29.Sanger, F., S. Nicklen, and A. R. Coulson. 1977. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 74:5463-5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scharfstein, J., M. Schechter, M. Senna, J. M. Peralta, L. Mendonca-Previato, and M. A. Miles. 1986. Trypanosoma cruzi: characterization and isolation of a 57/51,000 m.w. surface glycoprotein (GP57/51) expressed by epimastigotes and bloodstream trypomastigotes. J. Immunol. 137:1336-1341. [PubMed] [Google Scholar]
- 31.Silber, A., J. Bua, B. Porcel, E. Segura, and A. Ruiz. 1997. Trypanosoma cruzi: specific detection of parasites by PCR in infected humans and vectors using a set of primers (BP1/BP2) targeted to a nuclear DNA sequence. Exp. Parasitol. 85:225-232. [DOI] [PubMed] [Google Scholar]
- 32.Solana, M. E., A. M. Katzin, E. S. Umezawa, and C. S. Miatello. 1995. High specificity of Trypanosoma cruzi epimastigote ribonucleoprotein as antigen in serodiagnosis of Chagas' disease. J. Clin. Microbiol. 33:1456-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sosa Estani, S., E. L. Segura, A. M. Ruiz, E. Velazquez, B. M. Porcel, and C. Yampotis. 1998. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas' disease. Am. J. Trop. Med. Hyg. 59:526-529. [DOI] [PubMed] [Google Scholar]
- 34.Umezawa, E. S., S. F. Bastos, M. E. Camargo, L. M. Yamauchi, M. R. Santos, A. Gonzalez, B. Zingales, M. J. Levin, O. Sousa, R. Rangel-Aldao, and J. F. da Silveira. 1999. Evaluation of recombinant antigens for serodiagnosis of Chagas' disease in South and Central America. J. Clin. Microbiol. 37:1554-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umezawa, E. S., S. F. Bastos, J. R. Coura, M. J. Levin, A. Gonzalez, R. Rangel-Aldao, B. Zingales, A. O. Luquetti, and J. F. da Silveira. 2003. An improved serodiagnostic test for Chagas' disease employing a mixture of Trypanosoma cruzi recombinant antigens. Transfusion 43:91-97. [DOI] [PubMed] [Google Scholar]
- 36.Umezawa, E. S., M. S. Nascimento, N. Kesper, Jr., J. R. Coura, J. Borges-Pereira, A. C. Junqueira, and M. E. Camargo. 1996. Immunoblot assay using excreted-secreted antigens of Trypanosoma cruzi in serodiagnosis of congenital, acute, and chronic Chagas' disease. J. Clin. Microbiol. 34:2143-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu, Y., J. Deford, R. Benjamin, M. G. Lee, and L. Ruben. 1994. The gene family of EF-hand calcium-binding proteins from the flagellum of Trypanosoma brucei. Biochem. J. 304:833-841. [DOI] [PMC free article] [PubMed] [Google Scholar]