Abstract
Fluroquinolone antibiotics have been reported to have antiviral properties against RNA viruses, including hepatitis C virus (HCV). In the present study, five patients with advanced liver disease secondary to chronic HCV received 500 mg daily of oral ciprofloxacin for 30 days. Serum HCV-RNA levels and liver enzyme abnormalities remained largely unchanged. Thus, the role of fluoroquinolones as antiviral agents for chronic HCV in patients with advanced liver disease appears to be limited.
Hepatitis C (HCV) is a common cause of chronic liver disease, affecting approximately 200 million individuals worldwide (1). Cirrhosis and/or hepatocellular carcinoma is thought to occur in 20 to 30% of these individuals (8). To date, treatment of HCV is effective in only 40 to 60% of patients and can be associated with significant and potentially life-threatening side effects, particularly in those with advanced disease (5). For these individuals, liver transplantation represents the only therapeutic option. Unfortunately, posttransplant HCV recurrence is common if not inevitable (4). Because the natural history of HCV infection in the posttransplant period is largely determined by pretransplant viral loads, attention has focused on identifying means of lowering HCV-RNA levels prior to transplantation (3).
Fluoroquinolones are antimicrobial agents that have been reported to be effective in the treatment of conditions associated with liver failure, including portal systemic encephalopathy (PSE) and spontaneous bacterial peritonitis (2, 14) (S. Esposito, D. Barbra, D. Galanta, G. B. Gaeta, and O. Laghezza, Rev. Infect. Dis. 10:S197, 1996). They have also been reported to possess antiviral properties against both DNA and RNA viruses, including HCV (6, 10, 11). However, their value in HCV has largely been limited to patients with relatively mild, precirrhotic chronic hepatitis (7, 12, 13). If equally effective in patients with more advanced disease, these agents would represent an attractive therapeutic option for HCV-infected patients awaiting liver transplantation who require PSE and spontaneous bacterial peritonitis prophylaxis.
Five of 21 participants in a recent prospective, double-blind, placebo-controlled trial of ciprofloxacin treatment for subclinical PSE were known to have chronic HCV infections. Each patient had histologic and/or radiologic evidence of cirrhosis and had been listed for liver transplantation. The diagnosis of HCV was based on positive serologic (third-generation anti-HCV enzyme immunoassay) and virologic (HCV-RNA by PCR) testing. In all five cases, patients had been randomized to receive ciprofloxacin (500 mg orally twice a day for 30 days). None of the patients had received antiviral therapy in the past, and none was receiving immunosuppressive or other antimicrobial agents at the time of the study. All patients provided informed consent for participation in the subclinical PSE study and subsequently for HCV-RNA testing. The study was approved by the University of Manitoba Conjoint Ethics Committee for Human Experimentation.
Stored sera (−40°C) were available for testing from visits immediately prior to ciprofloxacin treatment (baseline) and again after 2 and 4 weeks of therapy. Posttreatment sera were also available at 4 and 8 weeks postciprofloxacin (follow-up samples). At each visit, sera were tested for liver enzymes (alanine and aspartate aminotransferases, alkaline phosphatase, and glutamyltransferase) and function tests (albumin, bilirubin, and prothrombin time/international normalized ratio) by standard laboratory procedures while stored sera were batched and tested for HCV-RNA quantitation by real-time PCR (see below) and genotyping by line probe assay (INNO-LiPA HCV II; Innogenetics, Toronto, Canada).
Measurement of HCV-RNA by real-time reverse transcriptase PCR (RT-PCR).
HCV-RNA levels were determined using a LightCycler-RNA Amplification Kit SYBR Green I (Roche Diagnostics GmbH). Forward and reverse primers (5′-GAGGAACTACTGTCTTCACGCAGAA-3′ and 5′-CTTTCGCGACCCAACACTACTC-3′) were designed to amplify a 229-bp segment of the 5′ noncoding region of HCV-RNA.
Twenty microliters of the PCR mixture contained LightCycler-RT-PCR Reaction Mix SYBR Green (Roche), 5 mM MgCl2, 0.25 μM forward primer, 0.5 μM reverse primer, LightCycler-RT-PCR Enzyme Mix, and template RNA. RT-PCR amplification was started with 30 min at 55°C for reverse transcription and followed by 30 s at 95°C for denaturation, which in turn was followed by 45 cycles of amplification at 57°C for 10 s and 72°C for 10 s. All reactions were performed in a LightCycler (Roche Diagnostics GmbH). The lower limit of detection for this assay is 1,000 viral copies/ml, and intrinsic variability < 10%.
Demographic and baseline laboratory findings of the five patients are provided in Table 1. The mean age was 53.2 ± 10.9 years. Baseline HCV-RNA levels ranged from 2.87 ×106 to 7.81 × 106 copies/ml (mean, 5.19 ± 2.18 × 106 copies/ml). All HCV were genotype 1a or -b.
TABLE 1.
Demographic and baseline laboratory findings for the five patientsa
| Case no. | Age (yr)/sex | Usage of:
|
Amt of:
|
No. of HCV copies (106 copies/ml) | |||
|---|---|---|---|---|---|---|---|
| BT | IDU | ALT (0-40 IU/liter) | Total amt of bilirubin (2-18 μmol/liter) | Platelets (150 × 109-400 × 109/liter) | |||
| 1 | 40/male | − | + | 81 | 12 | 95 × 109 | 3.40 |
| 2 | 70/female | + | − | 24 | 64 | 92 × 109 | 2.87 |
| 3 | 53/male | − | + | 149 | 27 | 58 × 109 | 7.81 |
| 4 | 49/male | + | − | 134 | 26 | 10 × 109 | 4.79 |
| 5 | 54/female | − | + | 100 | 23 | 48 × 109 | 7.06 |
Numbers in parentheses indicate normal ranges. BT, blood transfusion; IDU, injection drug use. ALT, alanine aminotransferase.
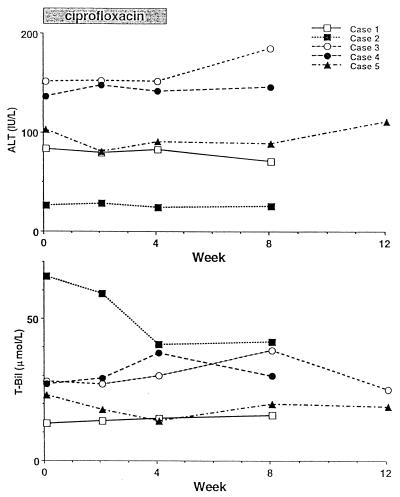
Baseline, treatment, and follow-up alanine aminotransferase and bilirubin levels as representative tests of liver enzyme and functional abnormalities, respectively, are provided in Fig. 1. No consistent changes in these or other liver biochemistry tests were evident throughout the study.
FIG. 1.
Serum alanine aminotransferase and total bilirubin levels during treatment and follow-up.
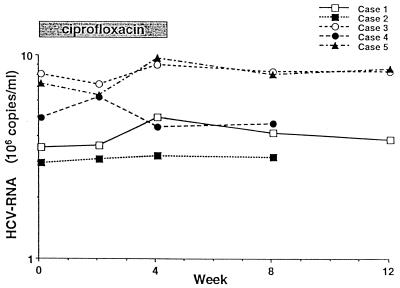
HCV-RNA levels are provided in Fig. 2. Once again, no consistent changes were evident. In two patients (cases 3 and 5), HCV-RNA levels decreased approximately 10% from baseline after 2 weeks of ciprofloxacin but then increased to levels at or slightly beyond baseline at the end of 4 weeks of therapy. The most striking change was a 34% increase in HCV-RNA levels at the end of 4 weeks of therapy (case 1). In this and all other patients, HCV-RNA levels trended towards baseline during the follow-up period. No adverse effects of therapy were reported.
FIG.2.
HCV-RNA levels during treatment and follow-up.
The results of this study indicate that, despite promising findings in patients with precirrhotic, chronic HCV infections, in patients with more advanced disease, ciprofloxacin neither lowers HCV-RNA levels nor improves liver biochemistry findings.
To date, there has been only one previous study documenting the effects of fluoroquinolones as single-agent therapy in patients with chronic HCV (12). In that study, five patients with HCV-induced chronic hepatitis and four patients with compensated cirrhosis received 100 to 900 mg of ofloxacin per day for 1 to 8 weeks. Regardless of viral genotype, HCV-RNA levels decreased by at least 1 log in four patients, three of whom had chronic hepatitis. Treatment was well tolerated at lower doses (<600 mg/day) but caused diarrhea, anorexia, and insomnia at higher doses (900 mg/day). The duration of antiviral effects was not reported.
In two additional studies, fluoroquinolones were used in combination with interferon (7, 13). In a study by Tsutsumi et al., 20 noncirrhotic interferon nonresponders were randomized to either 12 weeks of combination therapy with ofloxacin (600 mg/day) superimposed on a 6-month course of high-dose interferon or high-dose interferon alone (13). In the combination group, alanine aminotransferase values improved and HCV-RNA levels decreased to a greater extent than in those who received interferon alone. The only sustained HCV responses occurred in two patients who received combination therapy. In a similar study reported by Komatsu et al., 20 noncirrhotic interferon nonresponders were randomized to either 22 weeks of ofloxacin (600 mg/day) plus interferon (3 × 106 to 6 × 106 U/wk) or interferon alone (7). Once again, the only two sustained viral responders were in the combination group. Moreover, alanine aminotransferase levels were significantly lower during treatment in the combination group than in those who received interferon alone. However, not all studies have been supportive. In a more recent report by Negro et al., combination therapy with interferon and ofloxacin was ineffective both virologically and biochemically in 26 patients who had previously failed to respond to interferon alone (9).
Because the mechanism(s) whereby fluoroquinolones inhibit viral replication remains to be defined, it is difficult to explain why ciprofloxacin was ineffective in our study. Possibilities to be considered include the following: (i) like interferon, the fluoroquinolones are disease stage specific (more effective in early than in late disease), (ii) the specific type and dose of the fluoroquinolone employed are important, (iii) the treatment period in our study (30 days) may have been inadequate, (iv) the response to fluoroquinolones may be genotype specific, and/or (v) our patient population was too small to detect a therapeutic benefit (type II error), particularly when response rates of only 40 to 60% are expected with more potent antiviral agents. Clearly, further studies are required to determine which of these possibilities will explain our findings.
In conclusion, although fluoroquinolone antimicrobial prophylaxis may be of benefit to patients with advanced liver disease who are at risk of developing hepatic encephalopathy and/or spontaneous bacterial peritonitis, their value as an antiviral agent in this setting appears to be limited.
Acknowledgments
We thank S. Zdanuk for her prompt and accurate typing of the manuscript.
This work was supported by the Health Sciences Centre Research Foundation, Winnipeg, Manitoba, Canada.
REFERENCES
- 1.Alter, M. J. 1997. Epidemiology of hepatitis C. Hepatology 26(Suppl. 1):625-655. [Google Scholar]
- 2.Campillo, B., C. Dupeyron, J. P. Richardet, N. Mangency, and G. Leluan. 1998. Epidemiology of severe hospital-acquired infections in patients with liver cirrhosis: effect of long-term administration of norfloxacin. Clin. Infect. Dis. 26:1066-1070. [DOI] [PubMed] [Google Scholar]
- 3.Feray, C., L. Caccamo, G. J. M. Alexander, et al. 1999. European collaborative study on factors influencing outcome after liver transplantation for hepatitis C. Gastroenterology 117:619-625. [DOI] [PubMed] [Google Scholar]
- 4.Ferrell, L., T. Wright, J. Roberts, N. Ascher, and J. Lake. 1992. Hepatitis C viral infection in liver transplant recipients. Hepatology 16:865-876. [DOI] [PubMed] [Google Scholar]
- 5.Heathcote, J. E., M. L. Shiffman, G. E. Cooksley, G. M. Dushieko, S. S. Lee, L. Balart, R. Reindollar, T. K. Reddy, T. L. Wright, A. Lin, J. Hoffman, and J. De Pamphilis. 2000. Peginterferon alfa-2a in patients with chronic hepatitis C and cirrhosis. N. Engl J. Med. 343:1673-1680. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda, S., M. Yazawa, and C. Nishimura. 1987. Antiviral activity and inhibition of topoisomerase by ofloxacin, a new quinolone derivative. Antivir. Res. 8:103-113. [DOI] [PubMed] [Google Scholar]
- 7.Komatsu, M., T. Ishii, T. Ono, T. Hoshino, T. Kuramitsu, T. Goto, T. Fujii, I. Toyoshima, M. Chiba, and O. Masamune. 1997. Pilot study of ofloxacin and interferon-alpha combination therapy for chronic hepatitis C without sustained response to initial interferon administration. Can J. Gastroenterol. 11:507-511. [DOI] [PubMed] [Google Scholar]
- 8.Liang, T. J., B. Rehermann, L. B. Seeff, and J. H. Hoofnagle. 2000. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann. Intern. Med. 132:296-305. [DOI] [PubMed] [Google Scholar]
- 9.Negro, F., P.-J. Male, L. Perrin, E. Gistra, and A. Hadengue. 1998. Treatment of chronic hepatitis C with α-interferon plus ofloxacin in patients not responding to α-interferon alone. J. Hepatol. 29:369-374. [DOI] [PubMed] [Google Scholar]
- 10.Nozaki-Renard, J., T. Iino, Y. Sato, Y. Marumoto, G. Ohta, and M. Furusawa. 1990. Fluoroquinolones protect the human lymphocyte CEM cell line from HIV-1-mediated cytotoxicity. Cell Struct. Funct. 15:295-299. [DOI] [PubMed] [Google Scholar]
- 11.Sumiyoshi, Y., T. Nishikawa, T. Watanabe, and K. Kano. 1983. Inhibition of retrovirus RNA-dependent DNA polymerase by novobiocin and nalidixic acid. J. Gen. Virol. 640:2329-2333. [DOI] [PubMed] [Google Scholar]
- 12.Takada, A., S. Takase, M. Tsutsumi, and M. Sawada. 1993. Effects of ofloxacin for type C hepatitis. Int. Hepatol. Commun. 1:272-277. [Google Scholar]
- 13.Tsutsumi, M., A. Takada, S. Takase, and M. Sawada. 1996. Effects of combination therapy with interferon and ofloxacin on chronic type C hepatitis: a pilot study. J. Gastroenterol. Hepatol. 11:1006-1011. [DOI] [PubMed] [Google Scholar]
- 14.Zhang, M., G. Song, and G. Y. Minuk. 1996. Effects of hepatic stimulator substance, herbal medicine, selenium/vitamin E, and ciprofloxacin on cirrhosis in the rat. Gastroenterology 110:1150-1155. [DOI] [PubMed] [Google Scholar]