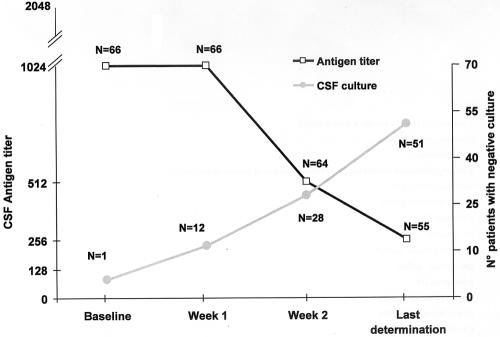
In a recent paper evaluating the significance of cryptococcal antigen test results for 29 Chinese human immunodeficiency virus (HIV)-negative patients affected by cryptococcal meningitis, Lu and colleagues (8) showed in all patients a decrease of antigen titer from the baseline following antifungal therapy and suggested that a decrease can be used to monitor antifungal therapy efficacy but cannot be used as an index of cure. We have reviewed our experience with 66 HIV-positive patients out of 118 with cryptococcal meningitis for whom at least three serial determinations (at baseline, day 7, and day 14) of cryptococcal antigen tests on cerebrospinal fluid (CSF) were available (1). A total of 440 determinations (range, 3 to 28 antigen determinations; median, 5 antigen determinations) were available, and for 55 patients the last determination was considered (median, 13 weeks; range, 2 to 84 weeks). In Fig. 1 is depicted the kinetics of CSF cryptococcal antigen together with the results of CSF culture. Overall, 53 patients (80%) showed a decrease of CSF antigen titer from the baseline (7 of whom had negative results), as follows: 27 cases of 1 to 3 dilutions, 16 cases of 4 to 6 dilutions, and 10 cases of 7 or more dilutions. However, 13 out of 15 of these patients for whom postmortem examination was available, despite evidence of several intravitam negative CSF cultures, still had cryptococcal meningitis or disseminated disease at autopsy (demonstrated by histopathology). Eight patients had an increase in the CSF antigen titer (four of 1 to 3 dilutions and four of 4 to 8 dilutions), and five showed stable (i.e., the same value) results throughout the follow-up. All the patients but two with an increase of CSF antigen titer had persistent positive CSF culture and died; four underwent autopsy showing disseminated cryptococcosis.
FIG. 1.
Change over time of CSF cryptococcal antigen titers and correlation with CSF cryptococcal cultures in 66 HIV-positive patients. Data of CSF antigen are median values. Data regarding CSF cultures refers to the total number of patients (n = 66), whereas those of CSF antigen titer are the number of patients for whom it was available.
Our experience regarding the role of cryptococcal antigen to monitor antifungal therapy in AIDS patients is in keeping with that previously reported by Powderly et al. (11), who showed the lack of any correlation of changes of CSF or serum cryptococcal antigen and the outcome of cryptococcal meningitis. However, a high CSF antigen level has been identified as a sign of poor prognosis in patients with AIDS (1, 7); interestingly, more recently Thay cohorts of HIV-positive patients with cryptococcal meningitis showed a significant positive correlation between CSF cryptococcal colony-forming units (CFU) and CSF cryptococcal antigen titers at baseline (P < 0.0001), but the rapid rate of decline in CSF CFU was not correlated with that in CSF cryptococcal antigen titers (2).
As shown in Table 1, regardless of the different hosts in whom cryptococcal meningitis is diagnosed, among all methods employed the cryptococcal CSF antigen had the best overall sensitivity (94.1%) followed by the serum antigen (93.6%). However, some differences were observed in the different categories of hosts, with lower sensitivity in AIDS and immunocompetent patients (92%) and higher sensitivity among the other immunocompromised hosts without HIV infection. Persistently elevated CSF cryptococcal antigen in HIV-infected patients carries a poor prognosis and indicates ongoing production of viable Cryptococcus neoformans in tissue. In conclusion, CSF cryptococcal antigen seems to be the best test for diagnosis of cryptococcal meningitis in terms of sensitivity, but it is an unreliable tool, at least among HIV-positive patients, to drive therapeutic monitoring, particularly in assessing the point of discontinuation of antifungal therapy in HIV-infected patients.
TABLE 1.
Efficiency of different techniques in the diagnosis of cryptococcal meningitis in different hostsa
| Reference | Country | No. of patients | Host condition | No. positive by CSF Ag (%) | No. positive by CSF culture (%) | No. positive by India ink (%) | No. positive by serum Ag (%) |
|---|---|---|---|---|---|---|---|
| 12 | Brazil | 65 | AIDS | 3/3 (100) | 65/65 (100) | 61/65 (93.8) | NR |
| 4 | United States | 89 | AIDS | 80/88 (90.9) | 89/89 (100) | 64/87 (73.5) | 70/71 (98.6) |
| 3 | Australia | 128 | AIDS | 112/128 (87.5) | 111/128 (86.7) | 93/128 (72.6) | NR |
| 1 | Italy | 119 | AIDS | 112/114 (98.2) | 115/118 (97.5) | 84/95 (88.4) | 111/112 (99.1) |
| Total | 401 | 307/333 (92.2) | 380/401 (94.7) | 302/375 (80.5) | 181/183 (98.9) | ||
| 12 | Brazil | 44 | Immunocompetent | 5/6 (83.3) | 26/30 (86.7) | 34/44 (77.2) | NR |
| 9 | Australia | 31 | Immunocompetent | 27/31 (87.1) | 26/31 (83.5) | 19/31 (61.3) | NR |
| 3 | Australia | 41 | Immunocompetent | 40/41 (97.6) | 39/41 (95.1) | 36/41 (87.8) | NR |
| Total | 116 | 72/78 (92.3) | 91/102 (89.2) | 89/116 (76.7) | 181/183 (98.9) | ||
| 12 | Brazil | 19 | HIV negative, immunocompromised | NR | 17/18 (94.4) | 14/18 (77.8) | NR |
| 6 | United States | 5 | Cancer patients | 5/5 (100) | 5/5 (100) | 5/5 (100) | NR |
| 10 | United States | 157 | HIV negative, immunocompromised | 144/149 (96.6) | 132/149 (89) | 68/133 (51) | 91/105 (87) |
| 5 | United Statesb | 122 | Organ transplant recipients | 37/37 (100) | 76/82 (93) | 38/47 (77) | 18/21 (86) |
| 13 | United States | 28 | Organ transplant recipients | 28/28 (100) | 21/28 (77) | 14/28 (50) | 20/22 (90.9) |
| Total | 331 | 214/219 (97.7) | 251/282 (89) | 139/231 (60.2) | 129/148 (87.2) | ||
| Overall total | 848 | 593/630 (94.1) | 722/785 (92) | 430/722 (59.6) | 310/331 (93.6) |
Ag, antigen; NR, not reported.
Review of published reports.
REFERENCES
- 1.Antinori, S., L. Galimberti, C. Magni, A. Casella, L. Vago, F. Mainini, M. Piazza, N. Nebuloni, M. Fasan, C. Bonaccorso, G. M. Vigevani, A. Cargnel, M. Moroni, and A. L. Ridolfo. 2001. Cryptococcus neoformans infection in a cohort of Italian AIDS patients: natural history, early prognostic parameters, and autopsy findings. Eur. J. Clin. Microbiol. Infect. Dis. 20:711-717. [DOI] [PubMed] [Google Scholar]
- 2.Brouwer, A. E., P. Teparrukkul, S. Pinpraphapora, R. A. Larsen, W. Chierakul, S. Peacock, N. Duy, N. J. White, and T. S. Harrison. 2005. Baseline correlation and comparative kinetics of cerebrospinal fluid colony-forming unit counts and antigen titers in cryptococcal meningitis. J. Infect. Dis. 192:681-684. [DOI] [PubMed] [Google Scholar]
- 3.Chen, S., T. Sorrell, G. Nimmo, B. Speed, B. Currie, D. Ellis, D. Marriott, T. Pfeiffer, D. Parr, K. Byth, and the Australasian Cryptococcal Study Group. 2000. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Clin. Infect. Dis. 31:499-508. [DOI] [PubMed] [Google Scholar]
- 4.Chuck, S. L., and M. A. Sande. 1989. Infections with Cryptococcus neoformans in the acquired immunodeficiency syndrome. N. Engl. J. Med. 321:794-799. [DOI] [PubMed] [Google Scholar]
- 5.Husain, S., M. M. Wagener, and N. Singh. 2001. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg. Infect. Dis. 7:375-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kontoyiannis, D. P., W. K. Peitsch, B. T. Reddy, E. E. Whimbey, X. Y. Han, G. P. Bodey, and K. V. I. Rolston. 2001. Cryptococcosis in patients with cancer. Clin. Infect. Dis. 32:e145-150. [DOI] [PubMed] [Google Scholar]
- 7.Larsen R. A., M. A. E. Leal, and L. S. Chan. 1990. Fluconazole compared with amphotericin B plus flucytosine for cryptococcal meningitis in AIDS: a randomized trial. Ann. Intern. Med. 113:183-187. [DOI] [PubMed] [Google Scholar]
- 8.Lu, H., Y. Zhou, Y. Yin, X. Pan, and X. Weng. 2005. Cryptococcal antigen test revisited: significance for cryptococcal meningitis therapy monitoring in a tertiary Chinese hospital. J. Clin. Microbiol. 43:2989-2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell, D. H., T. C. Sorrell, A. M. Allworth, C. H. Heath, A. R. McGregor, K. Papanaoum, M. J. Richards, and T. Gottlieb. 1995. Cryptococcal disease of the CNS in immunocompetent hosts: influence of cryptococcal variety on clinical manifestations and outcome. Clin. Infect. Dis. 20:611-616. [DOI] [PubMed] [Google Scholar]
- 10.Pappas, P. G., J. R. Perfect, G. A. Cloud, R. A. Larsen, G. A. Pankey, D. J. Lancaster, H. Henderson, C. A. Kaufmann, D. W. Hass, M. Saccente, R. J. Hamill, M. S. Holloway, R. M. Warren, and W. E. Dismukes. 2001. Cryptococcosis in human immunodeficiency virus-negative patients in the era of effective azole therapy. Clin. Infect. Dis. 33:690-699. [DOI] [PubMed] [Google Scholar]
- 11.Powderly, W. G., G. A. Cloud, W. E. Dismukes, and M. S. Saag. 1994. Measurement of cryptococcal antigen in serum and cerebrospinal fluid: value in the management of AIDS-associated cryptococcal meningitis. Clin. Infect. Dis. 18:789-792. [DOI] [PubMed] [Google Scholar]
- 12.Rozenbaum, R., and A. J. R. Goncalves. 1994. Clinical epidemiological study of 171 cases of cryptococcosis. Clin. Infect. Dis. 18:369-380. [DOI] [PubMed] [Google Scholar]
- 13.Wu, G., R. A. Vilchez, B. Eidelman, J. Fung, R. Kormos, and S. Kusne. 2002. Cryptococcal meningitis: an analysis among 5521 consecutive organ transplant recipients. Transplant Infect. Dis. 4:183-188. [DOI] [PubMed] [Google Scholar]