Abstract
We investigated the significance of endogenous reactive oxygen species (ROS) produced by fungi treated with miconazole. ROS production in Candida albicans was measured by a real-time fluorogenic assay. The level of ROS production was increased by miconazole at the MIC (0.125 μg/ml) and was enhanced further in a dose-dependent manner, with a fourfold increase detected when miconazole was used at 12.5 μg/ml. This increase in the level of ROS production was completely inhibited by pyrrolidinedithiocarbamate (PDTC), an antioxidant, at 10 μM. In a colony formation assay, the decrease in cell viability associated with miconazole treatment was significantly prevented by addition of PDTC. Moreover, the level of ROS production by 10 clinical isolates of Candida species was inversely correlated with the miconazole MIC (r = −0.8818; P < 0.01). These results indicate that ROS production is important to the antifungal activity of miconazole.
Candidiasis is a life-threatening disease in patients with immune suppression. Azoles are used widely for the treatment of candidiasis, but some clinical isolates show resistance (11, 28). Further investigation of the mechanisms underlying the antifungal effects of azoles may aid in the development of new treatment strategies.
Azoles exert a cytostatic or cytotoxic effect via inhibition of synthesis reactions in the metabolic pathways of essential fungal cell membrane components including ergosterol (29, 30). Their primary target is the cytochrome P450-catalyzed 14α-demethylation of ergosterol precursors. Mutations or overexpression of 14α-demethylase, encoded by the ERG11 gene, as well as changes in the ergosterol synthesis pathway reportedly participates in the induction of resistance (14, 19, 20, 25).
Since it was found that miconazole, ketoconazole, and deacetylated ketoconazole were inserted in a lipid layer (6) and miconazole induced the release of K+ and intracellular ATP from Candida species (3, 4, 7), these agents may cause direct membrane damage.
Recent studies clarified that a decrease in drug concentration brought about by energy-dependent efflux pumps is usually involved in multidrug resistance including azole resistance (1, 2, 16, 26). This pumping system closely resembles a system in cancer cells based on the P-glycoprotein encoded by the MDR-1 gene. Thus, common biologic mechanisms shared with other eukaryotic cells are involved in the antifungal effects of azoles.
In eukaryotic cells, mitochondria are common organelles that represent an important source of reactive oxygen species (ROS). Many cellular stresses such as irradiation and cytotoxic drugs cause growth inhibition and the death of mammalian cells via endogenous ROS production (5, 15, 27). ROS produced by granulocytes or monocytes are known to exert activity against fungi (9, 31). Furthermore, recent studies demonstrated that Candida albicans possesses an ROS scavenger, superoxide dismutase; this suggests that fungi may require a cytoprotective mechanism against not only exogenous ROS but also endogenous, fungus-derived ROS (13, 17, 24). However, whether ROS production contributes to the antifungal effects of azoles is unclear. We therefore measured ROS production in fungi to investigate the relationship between ROS and the antifungal effect of miconazole.
MATERIALS AND METHODS
Strains and reagents.
C. albicans (ATCC 24433), C. glabrata (ATCC 2001), and C. tropicalis (ATCC 750) were used in this study. Each strain was initially cultured in Sabouraud liquid broth, modified (Becton Dickinson Microbiology Systems, Tokyo, Japan), at 35°C for 2 days and then cultured in Sabouraud dextrose agar (Becton Dickinson Microbiology Systems) for 2 days and subjected to the experiments. Four C. albicans and six C. glabrata isolates were freshly isolated from patients admitted to Sapporo Medical University Hospital. These clinical isolates were identified with a colorimetric plate (CHROMagar Candida; Kanto Chemical Co. Ltd., Tokyo, Japan) and stored at −70°C until the assay. Miconazole (Mochida, Inc., Tokyo, Japan) and fluconazole (Pfizer, Inc., Tokyo, Japan) were stocked at 2 and 10 mg/ml and appropriately diluted in buffered (0.165 M morpholinepropanesulfonic acid [MOPS; pH 7.0; Sigma, St. Louis, MO] buffer) RPMI 1640 medium (GIBCO BRL, Grand Island, N.Y.) at the time of use. Pyrrolidinedithiocarbamate (PDTC; Sigma) was stocked at 0.1 M in phosphate-buffered saline which did not contain calcium and magnesium [PBS(−)] and appropriately diluted in buffered RPMI 1640 medium.
Determination of MICs.
The MICs of the antifungal reagents were determined with a colorimetric microdilution panel (ASTY; Kyokuto Pharmaceutical Industrial Co., Ltd., Tokyo, Japan), according to the instructions of the manufacturer and by a previously described method (23). Briefly, 20 μl of cells adjusted to a McFarland 0.5 standard in RPMI 1640 medium was added to RPMI 1640 medium containing 17.5 μg of resazurin per ml. Next, 500 μl of the solution was mixed with the medium that included the colorimetric reagent, and then 100 μl of the mixed solution was added to the wells of dried microdilution trays containing serial dilutions of the antifungal drugs. The plate was incubated at 35°C for 48 h, and the MICs were determined by reading of the color in each well (with a blue or purple color indicating the minimum concentration that inhibited growth compared to the growth in the control well).
Colony formation assay.
Drug sensitivity and the effect of the antioxidant PDTC on the fungicidal activities of the drugs were determined by a colony formation assay based on the macrodilution reference method (M27-A) of the National Committee for Clinical Laboratory Standards. Briefly, cells adjusted to a McFarland 0.5 standard with a reader (measurement at 530 nm; EAR 400; SLT-Labinstruments GmbH, Salzburg, Austria) were diluted 1:1,000 in RPMI 1640 medium buffered with MOPS. Next, 1 ml of diluted solution in a 15-ml polystyrene tube (Falcon) was treated with PDTC for 1 h or was left untreated and then incubated with several concentrations of drugs for 1 to 4 days. After the incubation, 20 μl of appropriately diluted solution was used for colony formation on Sabouraud dextrose agar (Becton Dickinson Microbiology Systems).
Measurement of ROS production.
Endogenous amounts of ROS were measured by a fluorometric assay with 2′,7′-dichlorofluorescin diacetate (DCFH-DA; Molecular Probes, Inc., Eugene, Oreg.). Briefly, the cells were adjusted to a McFarland 0.5 standard in 10 ml of PBS(−) and centrifuged at 2,500 × g for 15 min. The cell pellet was then suspended in PBS(−) and treated with appropriately diluted PDTC for 1 h or was left untreated. After the incubation with miconazole or fluconazole solution at 37°C for 1 h, 10 μM DCFH-DA in PBS(−) was added. The fluorescence intensities (FIs) of the resuspended cells were measured with a Spectrafluor instrument (excitation, 485 nm, emission, 538 nm; 37°C; SLT-Labinstruments) in a 96-well fluoroplate (FB; Wako Chemical Industry, Osaka, Japan) every 10 min. The kinetic measurement of ROS was continued for 4 h after administration of DCFH-DA.
Statistical analysis.
The statistical significance of differences was determined by Student's t test. A P value of <0.05 was considered to indicate significance. Correlation between two parameters was calculated by Spearman's correlation coefficient by rank test.
RESULTS
ROS production in C. albicans treated with miconazole.
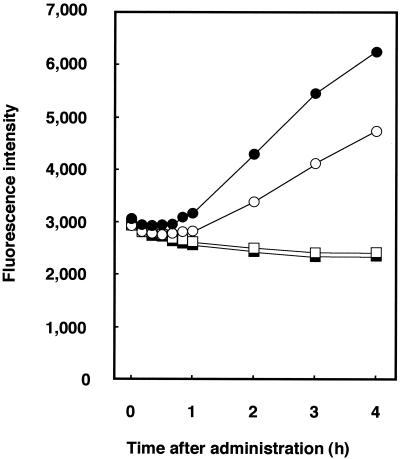
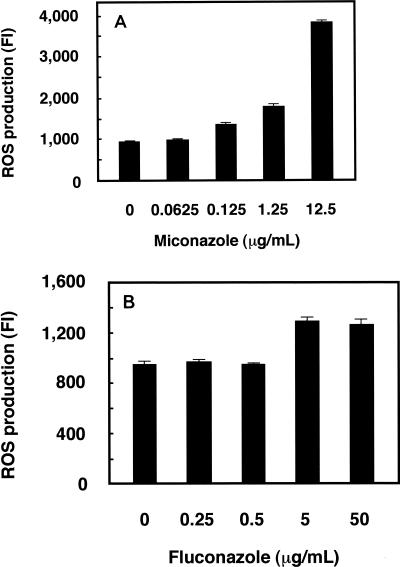
Preceding measurement of the level of ROS production, we initially determined the MICs of miconazole using an ASTY colorimetric microdilution panel. The MICs of miconazole were lower for C. albicans (0.125 μg/ml) than for C. glabrata (0.25 μg/ml) and C. tropicalis (2.0 μg/ml). We measured the endogenous level of ROS production induced by miconazole using the highly sensitive species C. albicans. Various cell densities, as determined by measurement of the number of McFarland standard units, were prepared, and kinetic measurement of FI was performed until 24 h after administration of fluorescent DCFH-DA. The increases in FI over time are shown in Fig. 1. The linearity of ROS production was good for times between 1 and 4 h after treatment, and the time point of 2 h was selected for subsequent experiments. The linearity of ROS production was also good for samples at McFarland 0.5 to 8 standards (data not shown). ROS production was calculated by subtracting the FI for cells not treated with DCFH-DA from the FI for cells treated with DCFH-DA. The level of ROS production with miconazole treatment increased in a dose-dependent manner, with a fourfold increase noted with miconazole at 12.5 μg/ml (Fig. 2A). Fluconazole treatment also augmented ROS production, although to a lesser extent (Fig. 2B).
FIG. 1.
Amounts of ROS in miconazole-treated C. albicans cells. Cells were treated with 1.25 μg of miconazole per ml and incubated with DCFH-DA for the indicated times. The level of ROS production was calculated by subtracting the FI for samples treated with DCFH-DA and that for cells not treated with DCFH-DA. Open circles, cells not treated with miconazole and incubated with DCFH-DA; filled circles, cells treated with miconazole and incubated with DCFH-DA; open squares, cells not treated with miconazole and not incubated with DCFH-DA; filled squares, cells treated with miconazole and not incubated with DCFH-DA. Each datum point represents the mean of three independent samples.
FIG. 2.
Effects of miconazole and fluconazole concentrations on ROS production in C. albicans. The level of ROS production was measured 2 h after treatment with the indicated concentrations of miconazole (A) and fluconazole (B). Data represent the means ± standard deviations for three independent samples.
Effect of PDTC treatment on miconazole-related ROS production and antifungal effect in C. albicans.
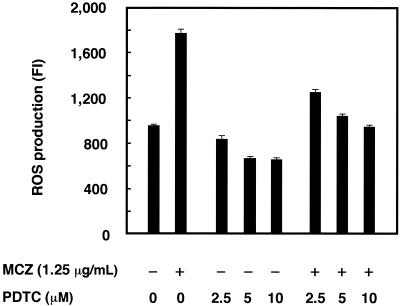
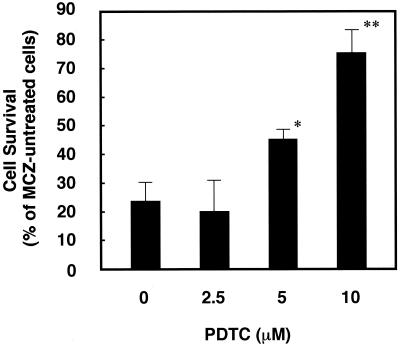
To investigate whether ROS production is directly involved in the antifungal effect of miconazole, we next examined the effect of an antioxidant on the net level of ROS production and antifungal activity in miconazole-treated cells. The net ROS production in cells induced by miconazole treatment was inhibited by addition of the antioxidant PDTC in a dose-dependent manner, with complete inhibition occurring with 10 μM PDTC (Fig. 3). Without miconazole treatment, PDTC inhibited the baseline level of ROS production 15 to 30%. We then examined whether PDTC treatment interferes with the antifungal effect of miconazole. In a colony formation assay, miconazole at the MIC caused a cytostatic effect (approximately 75% inhibition) at 4 days after treatment, as shown by an assay with an ASTY colorimetric microdilution panel. PDTC treatment prevented a miconazole-induced colony-inhibitory effect in a dose-dependent manner (Fig. 4).
FIG. 3.
Effect of the antioxidant PDTC on ROS production in miconazole-treated C. albicans. Cells were incubated with or without the indicated concentrations of PDTC and 1.25 μg of miconazole per ml. Data represent the means ± standard deviations for three independent samples.
FIG. 4.
Effect of the antioxidant PDTC on miconazole (MCZ)-induced colony inhibition in C. albicans. Cells were incubated with or without the indicated concentrations of PDTC and 0.125 μg of miconazole per ml for 4 days, and the colonies were counted. The rate of cell survival is represented as a percentage of the survival rate for cells not treated with miconazole. Data represent the means ± standard deviations for three independent experiments. ∗, P < 0.05 by Student's t test; ∗∗, P < 0.02 by Student's t test.
ROS production in miconazole-treated clinical isolates.
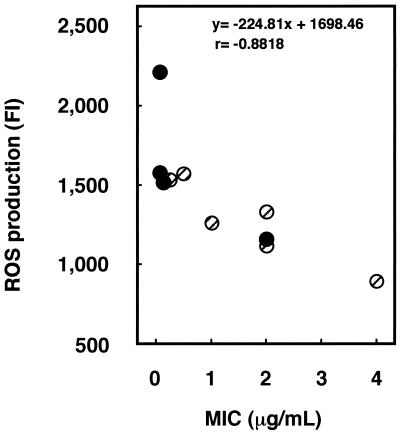
Since treatment with antioxidant inhibited the cytostatic action of miconazole, ROS may be an important mediator in the exhibition of the antifungal effect of miconazole. We therefore hypothesized that the miconazole sensitivities of clinical isolates can be estimated by determination of the level of ROS production as well as the MIC. We examined the relationship between the level of ROS production and miconazole sensitivity with 10 clinical isolates including C. albicans and C. glabrata. ROS production was detected in all miconazole-treated cells, with a strong inverse correlation (r = −0.8818; P < 0.01) between the MIC and the level of ROS production being detected (Fig. 5).
FIG. 5.
Relationship between miconazole sensitivity and ROS production in clinical isolates. The MICs for the isolates were assessed by a plate method with an ASTY colorimetric microdilution panel, and cells adjusted to a McFarland 0.5 standard were treated with 1.25 μg of miconazole per ml and subjected to measurement of the amount of ROS. The correlation between MIC and the level of ROS production was calculated by Spearman's correlation coefficient. Filled circles, C. albicans; hatched circles, C. glabrata.
DISCUSSION
Whether azole-treated fungi undergo growth inhibition and cell death via ROS production is not clear. ROS appears to be important, since fungi possess mitochondria. In this study we have provided for the first time evidence that azole induces intracellular ROS production in C. albicans. In our assay for detection of ROS, an increase in FI was apparent 120 min after DCFH-DA treatment, unlike administration of DCFH-DA in mammalian cell lines, in which the increase in FI peaks earlier, at 30 min. While ROS production in fungi has been considered difficult to measure, this is not true if the time lag of the reaction in fungi is considered. Recently, Lee et al. (18) used DCFH-DA to detect ionizing radiation-induced ROS production in Saccharomyces cerevisiae. Those investigators prepared a crude cell extract from S. cerevisiae which, when adjusted for protein concentration, was used to measure ROS production. Details concerning the recording time are unclear. The procedure used in the present study observes ROS production under more physiologic conditions, and real-time measurement is possible over a long period; viable cells rather than lysates are subjected directly to fluorometry. This assay system is simple, rapid, and well suited to the study of the significance of ROS production in fungi. The individual radical species involved in fungi, such as superoxide, hydroxyl radicals, and peroxynitrite, remain to be determined.
We next showed that ROS production is directly involved in the cytostatic action of miconazole when miconazole is used at concentrations obtained clinically in serum by intravenous infusion. The extent to which ROS production contributes to the effect of miconazole has been unclear. In this study complete inhibition of miconazole-induced ROS production resulted in the restoration of 50 to 70% of cell viability, suggesting that ROS production is an important event, in addition to drug-induced inhibition of ergosterol synthesis.
Ansehn and Nilsson (3) showed that ketoconazole and tioconazole induce the release of the intracellular ATP from C. albicans, and Dufour et al. (12) demonstrated that miconazole inhibits the membrane ATPase activity of the yeast S. pombe. It has been reported that, in eukaryotic cells, ROS can initiate inhibition of membrane ATPase activity and depletion of cellular ATP (8). Therefore, the release of intracellular ATP and the inhibition of membrane ATPase activity by miconazole may be related to ROS production.
We found that fluconazole also induces ROS production in C. albicans (Fig. 2B). However, the augmentation effect of ROS production was less than that of miconazole. Ohkawa et al. (22) examined the antifungal activities of miconazole and fluconazole against 231 clinical isolates of Candida species and revealed that the 30% inhibitory concentrations of fluconazole were generally greater than those of miconazole. In addition, Odds et al. (21) demonstrated that fluconazole does not suppress the ATP concentration in C. albicans, while miconazole does. These results also indicate that ROS may be an important mediator for exhibition of the antifungal effects of azoles.
Lee et al. (18) demonstrated that the level of ROS production can be increased by irradiation and that cells with wild-type superoxide dismutase were more resistant than cells with mutant-type superoxide dismutase. Their data suggested that ROS are involved in the irradiation-mediated loss of viability. Daub et al. (10) previously reported that photosensitizers produce superoxide anion against Cercospora species and attack the nuclear membrane and cytoplasmic components by peroxidation. Thus, the production of increased levels of ROS is thought to be a common pathway underlying cellular damage induced by different types of stresses including exposure to azoles.
Finally, we examined the relationship between ROS production and miconazole sensitivity in 10 Candida isolates and found a strong inverse correlation between the level of ROS production and the MIC. This result indicates that ROS production acts as one factor that determines the sensitivities of isolates to miconazole. Furthermore, measurement of the level of ROS production could be useful in the development of an assay system to estimate resistance to azoles. Whether Candida species exhibit drug resistance via mechanisms such as scavenging of ROS by superoxide dismutases remains to be investigated.
REFERENCES
- 1.Alarco, A. M., I. Balan, D. Talibi, N. Mainville, and M. Raymond. 1997. AP1-mediated multidrug resistance in Saccharomyces cerevisiae requires FLR1 encoding a transporter of the major facilitator superfamily. J. Biol. Chem. 272:19304-19313. [DOI] [PubMed] [Google Scholar]
- 2.Albertson, G. D., M. Niimi, R. D. Cannon, and H. F. Jenkinson. 1996. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob. Agents Chemother. 40:2835-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ansehn, S., and L. Nilsson. 1984. Direct membrane-damaging effect of ketoconazole and tioconazole on Candida albicans demonstrated by bioluminescent assay of ATP. Antimicrob. Agents Chemother. 26:22-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beggs, W. H. 1983. Comparison of miconazole- and ketoconazole-induced release of K+ from Candida species. J. Antimicrob. Chemother. 11:381-383. [DOI] [PubMed] [Google Scholar]
- 5.Benhar, M., I. Dalyot, D. Engelberg, and A. Levitzki. 2001. Enhanced ROS production in oncogenically transformed cells potentiates c-jun N-terminal kinase and p38 mitogen-activated protein kinase activation and sensitization to genotoxic stress. Mol. Cell. Biol. 21:6913-6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brasseur, R., C. Vandenbosch, H. Van den Bossche, and J. M. Ruysschaert. 1983. Mode of insertion of miconazole, ketoconazole and deacylated ketoconazole in lipid layers. A conformational analysis. Biochem. Pharmacol. 32:2175-2180. [DOI] [PubMed] [Google Scholar]
- 7.Cope, J. E. 1980. Mode of action of miconazole on Candida albicans: effect on growth, viability and K+ release. J. Gen. Microbiol. 119:245-251. [DOI] [PubMed] [Google Scholar]
- 8.Cuzzocrea, S., D. P. Riley, A. P. Caputi, and D. Salvemini. 2001. Antioxidant therapy: a new pharmacological approach in shock, inflammation, and ischemia/reperfusion injury. Pharmacol. Rev. 53:135-159. [PubMed] [Google Scholar]
- 9.Danley, D. L., and A. E. Hilger. 1981. Stimulation of oxidative metabolism in murine polymorphonuclear leukocytes by unopsonized fungal cells: evidence for a mannose-specific mechanism. J. Immunol. 127:551-556. [PubMed] [Google Scholar]
- 10.Daub, M. E., G. B. Leisman, R. A. Clark, and E. F. Bowden. 1992. Reductive detoxification as a mechanism of fungal resistance to singlet oxygen-generating photosensitizers. Proc. Natl. Acad. Sci. USA 89:9588-9592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Denning, D. W., G. G. Baily, and S. V. Hood. 1997. Azole resistance in Candida. Eur. J. Clin. Microbiol. Infect. Dis. 16:261-280. [DOI] [PubMed] [Google Scholar]
- 12.Dufour, J. P., M. Boutry, and A. Goffeau. 1980. Plasma membrane ATPase of yeast. Comparative inhibition studies of the purified and membrane-bound enzymes. J. Biol. Chem. 255:5735-5741. [PubMed] [Google Scholar]
- 13.Hwang, C. S., G. Rhie, S. T. Kim, Y. R. Kim, W. K. Huh, Y. U. Baek, and S. O. Kang. 1999. Copper- and zinc-containing superoxide dismutase and its gene from Candida albicans. Biochim. Biophys. Acta 1427:245-255. [DOI] [PubMed] [Google Scholar]
- 14.Kelly, S. L., D. C. Lamb, D. E. Kelly, N. J. Manning, J. Loeffler, H. Hebart, U. Schumacher, and H. Einsele. 1997. Resistance to fluconazole and cross-resistance to amphotericin B in Candida albicans from AIDS patients caused by defective sterol delta5,6-desaturation. FEBS Lett. 400:80-82. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi, D., M. Sasaki, and N. Watanabe. 2001. Caspase-3 activation downstream from reactive oxygen species in heat-induced apoptosis of pancreatic carcinoma cells carrying a mutant p53 gene. Pancreas 22:255-260. [DOI] [PubMed] [Google Scholar]
- 16.Kolaczkowski, M., and A. Goffeau. 1997. Active efflux by multidrug transporters as one of the strategies to evade chemotherapy and novel practical implications of yeast pleiotropic drug resistance. Pharmacol. Ther. 76:219-242. [DOI] [PubMed] [Google Scholar]
- 17.Lamarre, C., J. D. LeMay, N. Deslauriers, and Y. Bourbonnais. 2001. Candida albicans expresses an unusual cytoplasmic manganese-containing superoxide dismutase (SOD3 gene product) upon the entry and during the stationary phase. J. Biol. Chem. 276:43784-43791. [DOI] [PubMed] [Google Scholar]
- 18.Lee, J. H., I. Y. Choi, I. S. Kil, S. Y. Kim, S. Y., E. S. Yang, and J. W. Park. 2001. Protective role of superoxide dismutases against ionizing radiation in yeast. Biochim. Biophys. Acta 1526:191-198. [DOI] [PubMed] [Google Scholar]
- 19.Marichal, P., L. Koymans, S. Willemsens, D. Bellens, P. Verhasselt, W. Luyten, M. Borgers, F. C. Ramaekers, F. C. Odds, and H. V. Bossche. 1999. Contribution of mutations in the cytochrome P450 14alpha-demethylase (Erg11p, Cyp51p) to azole resistance in Candida albicans. Microbiology 145:2701-2713. [DOI] [PubMed] [Google Scholar]
- 20.Marichal, P., H. Vanden Bossche, F. C. Odds, G. Nobels, D. W. Warnock, V. Timmerman, C. Van Broeckhoven, S. Fay, and P. Mose-Larsen. 1997. Molecular biological characterization of an azole-resistant Candida glabrata isolate. Antimicrob. Agents Chemother. 41:2229-2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odds, F. C., S. L. Cheesman, and A. B. Abbott. 1986. Antifungal effects of fluconazole (UK 49858), a new triazole antifungal, in vitro. J. Antimicrob. Chemother. 18:473-478. [DOI] [PubMed] [Google Scholar]
- 22.Ohkawa, M., S. Tokunaga, M. Takashima, T. Nishikawa, H. Hisazumi, S. Fujita, and R. W. Wheat. 1990. In vitro susceptibility testing of Candida isolates from clinical specimens to four antifungal agents. Chemotherapy 36:396-402. [DOI] [PubMed] [Google Scholar]
- 23.Pfaller, M. A., S. Arikan, M. Lozano-Chiu, Y. Chen, S. Coffman, S. A. Messer, R. Rennie, C. Sand, T. Heffner, J. H. Rex, J. Wang, and N. Yamane. 1998. Clinical evaluation of the ASTY colorimetric microdilution panel for antifungal susceptibility testing. J. Clin. Microbiol. 36:2609-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhie, G. E., C. S. Hwang, M. J. Brady, S. T. Kim, Y. R. Kim, W. K. Huh, Y. U. Baek, B. H. Lee, J. S. Lee, and S. O. Kang. 1999. Manganese-containing superoxide dismutase and its gene from Candida albicans. Biochim. Biophys. Acta 1426:409-419. [DOI] [PubMed] [Google Scholar]
- 25.Sanglard, D., F. Ischer, L. Koymans, and J. Bille. 1998. Amino acid substitutions in the cytochrome P-450 lanosterol 14alpha-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob. Agents Chemother. 42:241-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanglard, D., K. Kuchler, F. Ischer, J. L. Pagani, M. Monod, and J. Bille. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki, M., D. Kobayashi, and N. Watanabe. 2001. Augmented adriamycin sensitivity in cells transduced with an antisense tumor necrosis factor gene is mediated by caspase-3 downstream from reactive oxygen species. Jpn. J. Cancer Res. 92:983-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van den Bossche, H., P. Marichal, and F. C. Odds. 1994. Molecular mechanisms of drug resistance in fungi. Trends Microbiol. 2:393-400. [DOI] [PubMed] [Google Scholar]
- 29.Van den Bossche, H., G. Willemsens, W. Cools, W. F. Lauwers, and L. Le Jeune. 1978. Biochemical effects of miconazole on fungi. II. Inhibition of ergosterol biosynthesis in Candida albicans. Chem. Biol. Interact. 21:59-78. [DOI] [PubMed] [Google Scholar]
- 30.Van den Bossche, H., G. Willemsens, W. Cools, P. Marichal, and W. Lauwers. 1983. Hypothesis on the molecular basis of the antifungal activity of N-substituted imidazoles and triazoles. Biochem. Soc. Trans. 11:665-667. [DOI] [PubMed] [Google Scholar]
- 31.Vazquez, N., T. J. Walsh, D. Friedman, S. J. Chanock, and C. A. Lyman. 1998. Interleukin-15 augments superoxide production and microbicidal activity of human monocytes against Candida albicans. Infect. Immun. 66:145-150. [DOI] [PMC free article] [PubMed] [Google Scholar]