Abstract
Human and bovine milk inhibited the metabolic activity of Escherichia coli, as shown by luminescence monitoring of constructs expressing the luxCDABE genes. Inhibition was dependent on both xanthine oxidase (XO) activity and on the presence of nitrite, implying that XO-generated nitric oxide functions as an antibacterial agent.
Though the abundance of xanthine oxidase (XO) in milk has been recognized for more than a century (19), its physiological function has remained a matter of speculation. Isolated studies, however, have considered an antimicrobial role for XO; in these, purified XO was shown to inhibit bacterial growth in the presence of a reducing substrate, which presumed the generation of hydrogen peroxide (13, 18, 25).
Recently, the ability of XO to catalyze reactions that generate nitric oxide (NȮ) from inorganic nitrite was reported (11, 20). NȮ is formed only under anaerobic conditions; when oxygen is present, superoxide is also produced, which reacts with XO-generated NȮ to form peroxynitrite (12), a powerful bactericidal agent (6). These observations prompted us to investigate the antibacterial activities of milk, particularly under defined oxygen tensions and with reference to the involvement of nitrite. We used Escherichia coli cells transformed with a plasmid, pLITE27, which carries the luxCDABE operon from Photorhabdus luminescens controlled by the constitutive promoter. Microorganisms expressing the lux operon are able to emit light due to the activity of bacterial luciferase on reduced flavin mononucleotide and a long-chain aldehyde that produces flavin mononucleotide, acid, and blue-green light. Since reduced flavin mononucleotide production depends upon functional electron transport, only metabolically active cells can produce light (4). Therefore, lux operon-dependent bioluminescence is an extremely sensitive, nondestructive, real-time reporter of cell metabolism that has been successfully used to monitor antimicrobial effects (23). This technology confers many advantages over conventional plating techniques and has allowed us to demonstrate, for the first time, bacteriostatic activity for both bovine and human milk that is dependent on XO, low oxygen tensions, and nitrite.
E. coli isolate 16906 was transformed with the luxCDABE operon (23). Cells were grown overnight at 37°C on nutrient agar plates with 50 μg of ampicillin per ml, subcultured into minimal salts medium (8), and grown at 37°C with aeration for 3 h. Aliquots were mixed with an equal volume of 40% glycerol and frozen at −80°C. Before each assay, aliquots were pelleted by centrifugation and resuspended in fresh minimal salts medium at 37°C without glucose or a nitrogen source. Suspension of the cells in the nutrient-deprived medium caused the cells to cease active growth and enter stationary phase, where metabolic activity is at a steady low level. Measured light output was stable, as monitored by a specifically adapted luminometer. This instrument (LKB 1250) was modified so that the reaction cuvette could be continuously supplied with a defined mixture of air and nitrogen, thus allowing accurate control of oxygen tension, which was measured with a Clark-type oxygen electrode. Additions of substrate or inhibitors were made through additional injection ports, effecting minimal disturbance of either oxygen tension or signal output.
The effects of bovine or human milk on the metabolic activity of E. coli were studied under conditions of 0.63% oxygen. This oxygen concentration, known to be optimal for the generation of peroxynitrite by XO (12), had no significant effect on bacterial viability. Following incubation of the cells (3 to 5 min), the enzyme substrates pterin (10 μM) and sodium nitrite (20 mM) were added. Pterin was chosen over both xanthine and hypothine as the reducing substrate in order to avoid complications arising from the peroxynitrite-scavenging activity of urate (12). In the absence of milk, neither pterin nor nitrite had any significant effect on the metabolic activity as assessed by light emission. Fresh bovine or human milk (1 ml) was added to the cuvette and, as can be seen in Fig. 1, a sharp drop in light emission occurred, after which the cells appeared to recover over the next 4 min. However, approximately 5 min after the addition of the milk, a second, slower, and very significant drop in light output was recorded. This drop continued for approximately 5 min, after which a very slow but steady recovery was seen.
FIG. 1.
The effects of bovine milk and human milk on the metabolic rates of E. coli are mediated by XO. E. coli isolate 16906, transformed with the luxCDABE operon, was resuspended in a luminometer cuvette under conditions of 0.63% oxygen as described in the text. Pterin (10 μM) and sodium nitrite (20 mM) were added, and subsequent light emissions were recorded before and after the addition of 1 ml of bovine (A) or human (B) milk. Measurements were repeated after pretreatment of milk with 25 μM oxypurinol. The traces shown are representative of at least six experiments.
In these experiments, the rapid fall and rise in light emission immediately following the addition of milk were deemed to be artefactual, given that they occur with boiled milk. While clearly independent of XO activity, these rapid changes probably reflect the quenching effects of milk on the luminescence signal.
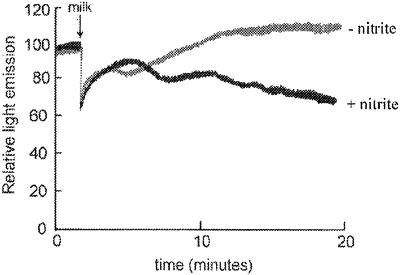
However, the second large fall in luminescence output was not observed when heat-treated milk was used. Furthermore, the effect was eliminated by the addition of oxypurinol, a specific inhibitor of XO (Fig. 1), and is therefore attributed to this enzyme. Oxypurinol alone appeared to have no effect on bacterial luminescence. The large fall in metabolic activity was also clearly dependent on the presence of nitrite (Fig. 2), which is consistent with the XO-mediated generation of NȮ. The latter mechanism was also demonstrated under hypoxic conditions (0.63% oxygen) known to be optimal for XO-catalyzed reduction of nitrite to NȮ (12). Such activity falls off rapidly as oxygen tensions increase, and, indeed, experiments conducted under conditions of 20% oxygen showed no significant oxypurinol-dependent impairment of bacterial metabolic activity. This finding argues against a major involvement of hydrogen peroxide (see below).
FIG. 2.
The antibacterial activity of human milk requires nitrite. E. coli isolate 16906, transformed with the luxCDABE operon, was resuspended in a luminometer cuvette under conditions of 0.63% oxygen as described in the text. Pterin (10 μM) and sodium nitrite (20 mM) were added, and subsequent light emissions were recorded before and after the addition of 1 ml of human milk. Measurements were repeated in the absence of nitrite.
This is the first real-time measurement of the effect of milk on bacterial metabolic activity and the first demonstration of the nitrite dependence of this effect. Previous studies have been concerned primarily with purified XO (13, 18, 25), although the results of two of these (18, 25) indicated that addition of the reducing substrate, hypoxanthine, led to decreased bacterial growth in samples of whole milk and that the antibacterial effects of purified XO were attributable to hydrogen peroxide. However, those investigations used high concentrations of XO (compared to those found in whole milk) and relied on colony counts to indicate bacterial viability, a method that would not have detected the transient bacteriostatic effects shown here.
An antibacterial role in the neonatal gut for XO-generated NȮ, and potentially peroxynitrite, is plausible for a number of reasons, both spatial and catalytic. XO is located on the outer surface of the milk fat globule membrane, and pathogenic bacteria, with the capacity to target epithelial membranes of the digestive tract, may well bind to similar antigens on the milk fat globule membrane, which is itself of epithelial cell origin (16, 21). This process will not only divert the bacteria from their primary target but will also bring them into intimate contact with XO. Contact of this type will be further promoted by the known affinity of XO for acidic polysaccharides (2), such as occur in many bacterial capsules (15). Therefore, the local concentrations of NȮ and peroxynitrite produced in the vicinity of the bacteria could potentially be very high and could account for the antibacterial effects seen.
The catalytic properties of XO are also well suited to its proposed antibacterial function. The optimal pH for anaerobic XO-catalyzed generation of NȮ is pH 6 or less (11), much lower than pH 8.8 (which is optimal for aerobic XO activity) and more suited to a role in the digestive tract. It has been suggested that the pH of the neonatal gut generally ranges between pH 4 and pH 6 (25), while others have suggested that the gut of a newborn immediately following birth is relatively alkaline, becoming progressively acidic during the first few days of life (24). Oxygen tensions are also low in the neonatal gut, and therefore the conditions used in the present study are both representative of physiological conditions suggested by others and ideal for NȮ generation by XO (25).
Moreover, while the Km value for nitrite in XO-dependent NȮ production is in the millimolar region (11), this level can be achieved by enteric bacteria. In anaerobic culture, such bacteria can excrete millimolar levels of nitrite (9, 22) derived from dissimilatory nitrate reductase (7). It is an intriguing thought that, by generating a nitrite-rich microenvironment, enteric bacteria might initiate their own destruction. Finally, it is worth noting that XO activity of human milk, while generally very much lower than that of cows' milk (1), is exceptionally high in the first few weeks postpartum (5). This is precisely the period when antibacterial activity is required in the neonatal gut and when it both coincides with particularly high levels of nitrite (14) and correlates with the highest levels of XO-dependent NȮ generation found in human milk (25).
In summary, we have demonstrated antibacterial activity of bovine and human milk that is dependent on XO-catalyzed reduction of nitrite, leading presumably to NȮ and, eventually, peroxynitrite. These findings not only contribute significantly to the long-standing question of the physiological significance of XO in milk but are also relevant to the debate concerning the relative merits of breast- and bottle-feeding of infants. While it is well established that formula-fed infants in developing countries suffer from higher levels of gastrointestinal infections than do breast-fed infants (3, 10, 17), the reasons for this are not clear. A possible factor is XO activity, which is known to be lacking in formula feed (25).
Acknowledgments
We thank The Wellcome Trust and The Nuffield Foundation for financial support.
We acknowledge help from H. Macdonald. Cows' milk was kindly supplied by Brook Farm, South Gloucester, United Kingdom.
REFERENCES
- 1.Abadeh, S., J. Killacky, M. Benboubetra, and R. Harrison. 1992. Purification and partial characterization of xanthine oxidase from human milk. Biochem. J. 1117:25-32. [DOI] [PubMed] [Google Scholar]
- 2.Adachi, T., T. Fukushima, Y. Usami, and K. Hirano. 1993. Binding of human xanthine oxidase to sulphated glycosylaminoglycans on the endothelial cell surface. Biochem. J. 289:523-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaudry, M., R. Dufour, and S. Marcoux. 1995. Relation between infant feeding and infections during the first six months of life. J. Pediatr. 126:191-197. [DOI] [PubMed] [Google Scholar]
- 4.Billard, P., and M. S. DuBow. 1998. Bioluminescence-based assays for detection and characterization of bacteria and chemicals in clinical laboratories. Clin. Biochem. 31:1-14. [DOI] [PubMed] [Google Scholar]
- 5.Brown, A.-M., M. Benboubetra, M. Ellison, D. Powell, J. D. Reckless, and R. Harrison. 1995. Molecular activation-deactivation of xanthine oxidase in human milk. Biochim. Biophys. Acta 1245:248-254. [DOI] [PubMed] [Google Scholar]
- 6.Brunelli, L., J. P. Crow, and J. S. Beckman. 1995. The comparative toxicity of nitric oxide and peroxynitrite to Escherichia coli. Arch. Biochem. Biophys. 316:327-334. [DOI] [PubMed] [Google Scholar]
- 7.Cole, J. 1996. Nitrate reduction to ammonia by enteric bacteria: redundancy, or a strategy for survival during oxygen starvation. FEMS Microbiol. Lett. 136:1-11. [DOI] [PubMed] [Google Scholar]
- 8.Davis, R. W., D. Botstein, and J. R. Roth. 1980. Advanced bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 9.DeMoss, J. A., and P.-Y. Hsu. 1991. NarK enhances nitrate uptake and nitrite excretion in Escherichia coli. J. Bacteriol. 173:3303-3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgieff, M., Y. Piovanetti, J. Queenan, and the American Academy of Pediatrics Work Group on Breastfeeding. 1997. Breast feeding and the use of human milk. Pediatrics 100:1035-1038. [DOI] [PubMed] [Google Scholar]
- 11.Godber, B. L. J., J. J. Doel, G. P. Sapkota, D. R. Blake, C. R. Stevens, R. Eisenthal, and R. Harrison. 2000. Reduction of nitrite to nitric oxide catalysed by xanthine oxidoreductase. J. Biol. Chem. 275:7757-7763. [DOI] [PubMed] [Google Scholar]
- 12.Godber, B. L. J., J. J. Doel, J. Durgan, R. Eisenthal, and R. Harrison. 2000. A new route to peroxynitrite: a role for xanthine oxidoreductase. FEBS Lett. 475:93-96. [DOI] [PubMed] [Google Scholar]
- 13.Green, D. E., and R. Pauli. 1943. The antibacterial action of the xanthine oxidase system. Proc. Soc. Exp. Biol. Med. 54:148-150. [Google Scholar]
- 14.Iizuka, T., M. Sasaki, K. Oishi, S. Uemura, M. Koike, and M. Shinozaki. 1999. Non-enzymatic nitric oxide generation in the stomachs of breastfed neonates. Acta Paediatr. 88:1053-1055. [DOI] [PubMed] [Google Scholar]
- 15.Jann, K., and B. Jann. 1997. Capsules of Escherichia coli,p. 113-143. In M. Sussman (ed.) Escherichia coli: mechanisms of virulence. Cambridge University Press, Cambridge, United Kingdom.
- 16.Keenan, T. W., and S. Patton. 1995. The structure of milk: implications for sampling and storage. A. The milk fat globule membrane, p. 5-50. In R. G. Jensen (ed.), Handbook of milk composition. Academic Press, New York, N.Y.
- 17.Kovar, M. G., M. K. Serdula, J. S. Marks, and D. W. Fraser. 1984. Review of the epidemiologic evidence for an association between infant feeding and infant health. Pediatrics 74(Suppl.):615-638. [PubMed] [Google Scholar]
- 18.Lipmann, F., and C. R. Owen. 1943. The antibacterial effect of enzymatic xanthine oxidation. Science 98:246-248. [DOI] [PubMed] [Google Scholar]
- 19.Massey, V., and C. M. Harris. 1997. Milk xanthine dehydrogenase: the first one hundred years. Biochem. Soc. Trans. 25:750-755. [DOI] [PubMed] [Google Scholar]
- 20.Millar, T. M., C. R. Stevens, N. Benjamin, R. Eisenthal, R. Harrison, and D. R. Blake. 1998. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett. 427:225-228. [DOI] [PubMed] [Google Scholar]
- 21.Patton, S., and T. W. Keenan. 1975. The milk fat globule membrane. Biochim. Biophys. Acta 415:273-309. [DOI] [PubMed] [Google Scholar]
- 22.Rowe, J. J., T. Ubbink-Kok, D. Molenaar, W. N. Konings, and A. J. M. Driessen. 1994. NarK is a nitrate extrusion system involved in anaerobic nitrate respiration by Escherichia coli. Mol. Microbiol. 12:579-586. [DOI] [PubMed] [Google Scholar]
- 23.Salisbury, V., A. Pfoest, H. Wiesinger-Mayr, R. Lewis, K. E. Bowker, and A. P. MacGowan. 1999. Use of a clinical Escherichia coli isolate expressing lux genes to study the antimicrobial pharmacodynamics of moxiflaxacin. J. Antimicrob. Chemother. 43:829-832. [DOI] [PubMed] [Google Scholar]
- 24.Sondheimer, J. M., D. A. Clark, and E. P. Gervaise. 1985. Continuous gastric pH measurement in young and older healthy preterm infants receiving formula and clear liquid feedings. J. Pediatr. Gastroenterol. Nutr. 4:352-355. [DOI] [PubMed] [Google Scholar]
- 25.Stevens, C. R., T. M. Millar, J. G. Clinch, J. M. Kanczler, T. Bodamyali, and D. R. Blake. 2000. Antibacterial properties of xanthine oxidase in human milk. Lancet 356:829-830. [DOI] [PubMed] [Google Scholar]