Abstract
Transcriptional silencing of an amoebapore (ap-a) gene occurred in Entamoeba histolytica following the transfection of plasmids containing a DNA segment (473 bp) homologous to the 5′ upstream region of the gene (R. Bracha, Y. Nuchamowitz, and D. Mirelman, Eukaryot. Cell 2:295-305, 2003). This segment contains the promoter region of the ap-a gene, a T-rich stretch, followed by a truncated SINE1 (short interspersed element 1) that is transcribed from the antisense strand. Transfection of plasmids containing truncated SINE1 sequences which lack their 3′ regulatory elements upstream of the ap-a gene was essential for the downstream silencing of the ap-a gene while transfection with plasmids containing the entire SINE1 sequence or without the T-rich stretch promoted the overexpression of the ap-a gene. Both the T-rich stretch and sequences of the 5′ SINE1 were essential for the transcription of SINE1. RNA extracts from gene-silenced cultures showed small amounts of short (∼140-nucleotide), single-stranded molecules with homology to SINE1 but no short interfering RNA. Chromatin immunoprecipitation analysis with an antibody against methylated K4 of histone H3 showed a demethylation of K4 at the domain of the ap-a gene, indicating transcriptional inactivation. These results suggest the involvement of SINE1 in triggering the gene silencing and the role of histone modification in its epigenetic maintenance.
Transgene homology-dependent silencing of gene expression is a well-recognized phenomenon that has been studied in fungi, plants, and animals (6, 10, 12, 20, 27, 38). Gene-silencing mechanisms that are based on the recognition of nucleic acid sequence homology have been reported to occur via two diverse strategies: inactivation at the transcriptional level (TGS) or at the posttranscriptional level (PTGS) (26, 29). Gene silencing is usually accompanied by epigenetic changes such as DNA methylation, as well as chromatin structure changes via modifications of the amino-terminal tails of core histones by acetylation, methylation, phosphorylation, ADP-ribosylation, ubiquitination, or α-sumoylation (33, 35). Several studies have recently shown that the transcriptional activity of some non-long-terminal-repeat (non-LTR) transposable elements is sensitive to the presence of homologous transgenes, suggesting the involvement of homology-dependent gene-silencing mechanisms in their regulation (21, 22, 31). It has also been shown that transcriptional activation of retrotransposons can alter the expression of adjacent genes in wheat as well as in mice (23, 41). Primitive amitochondrial eukaryotic organisms such as Giardia and Entamoeba have been shown to harbor non-LTRs which are either long interspersed elements (LINEs) or short interspersed elements (SINEs) (7, 25, 36). These SINE1-like elements, also termed IE/Ehapt2 (11, 42), are noncoding retroposons that are widely dispersed in the Entamoeba histolytica genome and are abundantly transcribed. Their function is not yet known. Recent findings suggest that LINE-encoded enzymes may play a role in SINE mobilization (25).
The starting point of our work was the observation that transfection of E. histolytica trophozoites with a plasmid containing a genomic copy of the amoebapore gene (ap-a), including its upstream and downstream regulating elements, caused, instead of overexpression, a complete suppression of transcription of both the episomal and chromosomal genes (9). Nuclear run-on experiments revealed that gene silencing was at the transcription initiation level (TGS). Furthermore, removal of the plasmid from silenced trophozoites and cultivation of cloned, plasmid-less trophozoites (termed G3) in the presence of inhibitors of DNA methyltransferases such as 5-azacytidine (39) and zebularine or the histone deacetylase inhibitor trichostatin A (32) or butyrate failed to restore the expression of the ap-a gene (9). These observations prompted us to analyze the sequences present in the plasmid construct which triggered the silencing phenomenon. The analysis revealed that the 5′ flanking segment (473 bp) of the ap-a gene that was used in the plasmid construct included 140 bp of a neighboring SINE1 which is transcribed in the opposite orientation, and these were preceded by a unique T-rich stretch of 48 bp. Our present findings indicate that the transfection of a plasmid containing a truncated SINE1 and the T-rich region upstream of the 5′ regulating element of the ap-a gene enabled the suppression of transcription of the adjacent ap-a gene as well as the production of single-stranded RNA molecules of truncated SINE1. Gene silencing did not correlate with methylation of cytosine residues nor with the formation of short interfering RNA (siRNA) molecules; however, a modification in the histone methylation at the chromatin domain of the ap-a gene was detected.
MATERIALS AND METHODS
Strain and culture conditions.
Trophozoites of E. histolytica strain HM-1:IMSS were grown at 37°C in TYI-S-33 medium (13). Transfection of trophozoites was performed as previously described (19), and they were grown in the presence of the neomycin derivative G418.
Plasmid constructs.
The pEhActNeo shuttle vector, which served as the basic construct, contains the Neo gene, which confers resistance to G418, flanked by the 5′ and 3′ regulatory sequences of the amoeba actin-1 gene (3, 28) and the E. histolytica autonomous replication sequence, both cloned in pBluescript II SK(−). The plasmids containing the ap-a open reading frame (ORF) were constructed as follows (see also Fig. 1, 2, and 3). The sense primers from the 5′ upstream sequences of the ap-a gene (location upstream of the ATG is indicated in parentheses) were as follows: for psAP-10 (275 bp), 5′ACGAGCTCCTTATGTCATATATAGAATGTTC; for psAP-11 (848 bp), 5′CAGAGCTCCATCTATTCTTCCTAGCTCAG; and for plasmid psAP-15 (385 bp), 5′CAGAGCTCGAATTTGTACTAGGGTTTGTGTG. The antisense primer shared by the above plasmids is located on the 3′ regulatory sequence of the ap-a gene with a SacII site. Its sequence is 5′-TCCCCGCGGCTTCAGGATGGAACAG. Segments were generated by PCR from gDNA of strain HM-1:IMSS and cloned into the pEhActNeo shuttle vector. Plasmid pEhapa was generated by digestion of genomic clone Ehap (accession no. X-70851) kindly given by E. Tannich (BNI, Germany) with BamHI and HindIII, subcloned into an intermediate plasmid digested with SacI and BamHI, and subcloned into the above shuttle vector. A number of plasmids were prepared which contained different lengths of the 5′ upstream region of the ap-a gene, without including the ap-a ORF. The plasmids psAP-21, psAP-22, and psAP-23 were prepared similarly to that of plasmid psAP-2 as previously described (9) by PCR amplifications with primers designed at different locations of the 5′ upstream region (see also Fig. 2). Sense primers (location upstream of the ATG is indicated in parentheses) were as follows: for psAP-21 (453 bp), 5′TCCCCGCGGGCTTCCCTAATACATTCC; for psAP-22 (413 bp), 5′TCCCCGCGGCAGATAATAACTGGGAGAG; and for psAP-23 (385 bp), the sense psAP-15 primer (see above). As an antisense primer for all three constructs we used a primer starting directly upstream of the ATG of ap-a, 5′TCCCCGCGGGATTGTTTGTAAGATATG. Plasmid pSINE:CAT1 was constructed as follows: a PCR fragment containing 139 bp of the 5′ SINE1 and 283 bp upstream of SINE1 was generated using sense primer 5′TCCCCGCGGCACGGTGATGTGGCTG and antisense primer 5′CGGGGTACCCTTGCTGCACCCTTTG. The fragment was ligated into the KpnI site of the chloramphenicol acetyltransferase (CAT)-3′-actin fragment after XbaI and KpnI digestion of plasmid pEhHYG-tetR-O-CAT (18). The fused fragment was then cloned into SacII and XbaI sites of pBluescript II SK(+) and subcloned into SacI-SpeI sites of pEhActNeo. Plasmids pSINE:CAT2, pSINE:CAT3, and pSINE:CAT4 were generated using primers 5′TCCCCGCGGTTTTTTTTTTCATTTGAGTG, 5′TCCCCGCGGAAATAATAAAAGATCGAAGG, and 5′TCCCCGCGGAATTAGGAATGTATTAGGG, respectively, as sense primers together with the same antisense primer as used for pSINE:CAT1 mentioned above. Plasmid pTr:CAT was constructed by ligating the complementary synthetic oligonucleotides 5′GGTTTTTTTTTTCATTTGAGTGTTTTATATTTTTTTCTATTTTGTTTTTTAAATAATAAAGGTAC and 5′CTTTATTATTTAAAAAACAAAATAGAAAAAAATATAAAACACTCAAATGAAAAAAAAAACCGC to the KpnI site of the CAT:3′-actin fragment. Plasmid p5AP3:CAT was constructed by fusing the CAT:3′-actin fragment mentioned above to a PCR fragment generated using sense primer 5′TCCCCGCGGCTTCAGGATGGAACAG and antisense primer 5′CGGGGTACCCTTGCTGCACCCTTTG and using psAP-1 as template. Plasmid p5AP3:SINE is similar to p5AP3:CAT, except that the CAT:actin fusion was replaced by a PCR fragment generated with primers 5′GGACTAGTGCACTAAGAAGACTTCAACC and 5′CGGGGTACCAGGGATGGGATTAGTCTC, which contains the last 80 bp of SINE1 and an additional 372 bp downstream of its 3′ end.
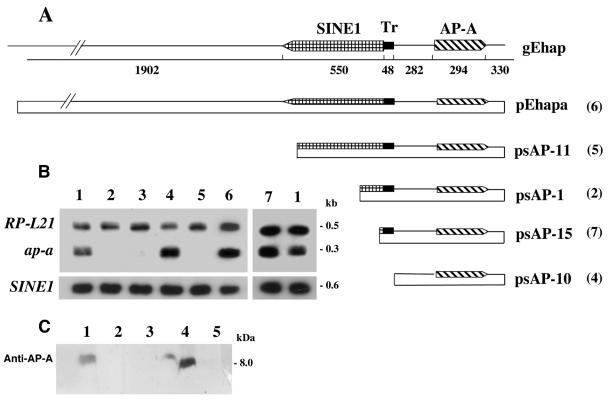
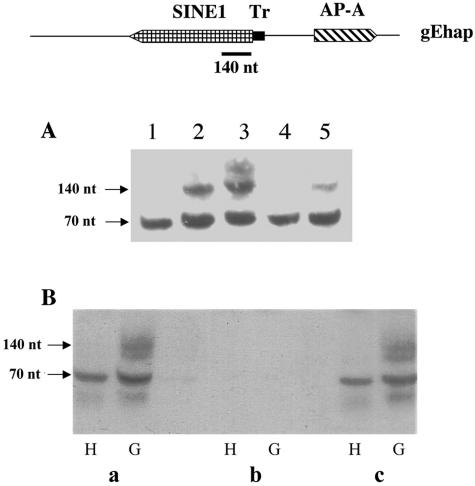
FIG. 1.
(A) gEhap (top): schematic diagram of the amoebapore and SINE1 genomic region. Arrowed boxes represent transcription orientation. The T-rich (Tr) region is represented by a solid bar. Plasmids: diagrams of the different fragments cloned into the pEhActNeo shuttle vector (length corresponds to the scheme above). pEhapa is derived from gEhap (accession no. X-70851). Numbers in parentheses represent the corresponding transfected trophozoites. (B) Northern blot analysis of amoebic RNA extracts. Lane 1, (both ends) parent strain HM1:IMSS; lane 2, transfected psAP-1; lane 3, plasmid-less G3; lane 4, psAP-10; lane 5, psAP-11; lane 6, pEhapa; lane 7, psAP-15. Blots were probed with radiolabeled DNA of the ap-a coding region, ribosomal protein gene EhRP-L21, and SINE1 probe. (C) Western blot of SDS-polyacrylamide gel reacted with anti-AP-A antibodies (samples 6 and 7 not shown).
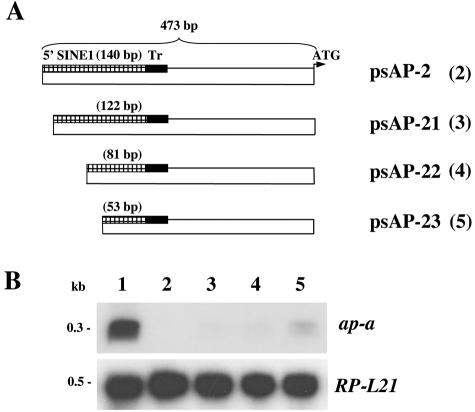
FIG. 2.
(A) Schematic map of various plasmid constructs containing different lengths of the 5′ flanking region of the ap-a gene. The length of the truncated 5′ SINE is given in parentheses. (B) Northern blots hybridized with radiolabeled probes of ap-a and ribosomal protein L21 ORFs. Lane 1, parent strain HM-1:IMSS; lane 2, psAP-2; lane 3, psAP-21; lane 4, psAP-22; lane 5, psAP-23.
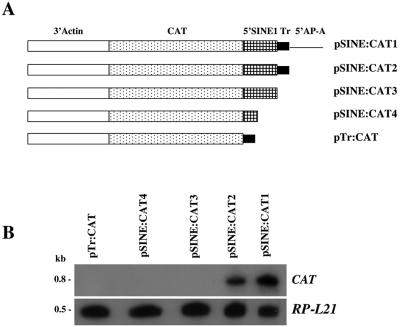
FIG. 3.
(A) Schematic map of the various plasmid constructs containing the CAT reporter gene and 3′ regulatory element of actin fused to different upstream regions of SINE1. (B) Northern blot analysis. RNA was extracted from HM1:IMSS transfectants with the different plasmids, probed with the radiolabeled CAT gene, and subsequently probed with a radiolabeled ribosomal protein gene, EhRP-L21.
Synthetic oligonucleotide probes.
Four synthetic oligonucleotide probes were prepared based on the first 140-nucleotide (nt) sequence (top strand) of the 5′ SINE1 that is present in the 473-bp upstream region of the ap-a gene. The sequences of the four synthetic probes which span the entire 140 bp are as follows (location upstream of the ATG of the ap-a gene is indicated in parentheses): A (−473), 5′CTTGCTGCACCCTTTG; B (−457), 5′CAGCTTCCCTAATACATTCCTAATTCAAAGGCACCGTCATAAC; C (−414), 5′CAGATAATAACTGGGAGAGTCGAAGTATGAATTTGTACTAG; D (−372), 5′GGTTTGTGTGTGGTGTTTCAGACGTGCCACCTTCGATCT (−333).
Northern blots.
Total RNA was prepared using the TRI reagent RNA isolation kit (Sigma). RNA was size fractionated on a 4% polyacrylamide denaturing gel containing 8 M urea and subsequently blotted to a nylon membrane. Using stringent conditions, hybridization was carried out with different probes randomly labeled using the Redi-Prime II kit (Amersham Life Science) and washed with 0.1% sodium dodecyl sulfate (SDS), 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate).
Enrichment for small RNA molecules.
A fraction enriched in small RNA molecules was prepared as described previously (9). In brief, extraction from freshly harvested trophozoites was performed with phenol-chloroform and the nucleic acids were precipitated with 3 volumes of absolute ethanol and 1/10 volume of 3 M sodium acetate at −20°C. The washed sediments were resuspended in 2× distilled water, and the solution was incubated on ice (30 min) with polyethylene glycol (molecular weight [MW], 8,000) at a final concentration of 5% and 500 mM NaCl, after which the high-MW nucleic acids were precipitated while the small RNAs remained in the solution. The supernatants were then precipitated with ethanol as described above. Low-MW RNAs (50 μg) were separated by electrophoresis in 0.5× Tris-borate-EDTA buffer through 17% polyacrylamide-7 M urea gels. End-labeled oligonucleotides of different lengths were prepared and used as size markers.
SDS-polyacrylamide gel electrophoresis and Western blots.
Soluble extracts from trophozoites prepared as previously described (9) were subjected to separation on a 20% polyacrylamide gel (9) under nonreducing conditions. Gels were blotted on a nitrocellulose membrane and subjected to immunoreactions with polyclonal antibodies prepared against high-pressure liquid chromatography-purified AP-A kindly supplied by M. Leippe, University of Kiel, Kiel, Germany. The blots were washed and incubated with horseradish peroxidase conjugated to donkey anti-rabbit immunoglobulin whole antibody (Amersham Pharmacia Biotech) and developed with an enhanced chemiluminescence kit (ECL) (Amersham, Little Chalfont, Buckinghamshire, United Kingdom). The procedure was carefully optimized using various protein and antibody concentrations.
Primer extension analysis.
Primer extension was performed on RNA prepared from pSINE:CAT1-transfected cells using end-labeled (100,000 cpm/pmol) oligonucleotide 5′ATCAACGGTGGTATATCC corresponding to the antisense strand of the CAT gene. After being annealed at 60°C for 15 min, the sample was kept on ice for 1 min, 1 unit of reverse transcriptase (Expand RT; Roche Molecular Biochemicals) and 1 unit of RNase inhibitor (Promega) were added, and extension was performed at 42°C for 90 min. The reaction mixture was analyzed on a 6% polyacrylamide denaturing gel next to the DNA sequencing reaction performed on the pSINE:CAT1 plasmid with the same primer.
Analysis of DNA methylation.
The sodium bisulfite reaction on E. histolytica gDNA was performed according to the methods described by Warnecke (40). Primers were designed from the distal end of the 473-bp 5′ upstream region to the ap-a gene ORF region. The average size of amplification was ∼250 bp for both strands. The sequences chosen for the primers were from regions that do not undergo changes due to the bisulfite treatment, so as to reduce the variability of the results from different primers. To each of the reaction mixtures an internal DNA control was added, consisting of a segment of the CAT reporter gene that was prepared by PCR with either fully methylated 5′-cytosine residues or unmodified cytosines. The PCR-amplified, bisulfite-treated, and nontreated DNA was cloned, and 10 clones from each sample were sequenced using a 3700 DNA Analyzer from Perkin-Elmer.
ChIP.
The chromatin immunoprecipitation (ChIP) assay was performed as described previously (2) with a few modifications. Two cultures of E. histolytica trophozoites (3 × 107 to 4 × 107) of strain HM-1:IMSS and the ap-a-silenced G3 were grown for 48 h and cross-linked with 1% formaldehyde for 10 min at room temperature. The cells were collected and washed once with ice-cold phosphate-buffered saline, once with 5 ml of buffer I (10 mM HEPES, pH 6.5, 10 mM EDTA, 0.5 mM EGTA, 0.25% Triton X-100), and once with buffer II (10 mM HEPES, pH 6.5, 1 mM EDTA, 0.5 mM EGTA, 200 mM NaCl). The cells were then resuspended in 0.5 ml of lysis buffer (50 mM Tris, pH 8.1, 10 mM EDTA, 1% SDS) with proteinase inhibitors (1 μg of pepstatin A per ml, 1.5 μg of leupeptin per ml, 1 mM phenylmethylsulfonyl fluoride) and sonicated 8 times for 10 s each time in a sonicator (MICROSON, XL2005; Heat Systems) using setting 11. DNA was sheared by sonication to yield fragment sizes from 500 to 1,500 bp. Clarification of the extract was then carried out by centrifugation for 15 min in a microcentrifuge at 4°C. An aliquot of the extract was diluted 10-fold with dilution buffer solution (20 mM Tris, pH 8.1, 150 mM NaCl, 2 mM EDTA, 1% Triton X-100) to yield the solubilized chromatin and was precleared by addition of salmon sperm DNA (50 μg/ml), tRNA1 (100 μg/ml), bovine serum albumin (1 mg/ml), and 50 μl of a 50% suspension of protein A-Sepharose beads (Amersham) for 30 min on a rotator wheel. After a 15-min centrifugation at 4°C, the supernatant was transferred to a new tube and used for immunoprecipitation.
Immunoprecipitation.
Two microliters of an antibody against methylated K4 of histone H3 (Upstate Biotechnology) was added to 1 ml of the soluble chromatin (corresponding to 5 × 106 cells), and the mixture was incubated overnight at 4°C. Then 30 μl of a 50% suspension of protein A-Sepharose beads was added to the soluble chromatin solution and incubated at 4°C for 1 to 2 h in a rotating wheel. The beads were then washed sequentially, once with buffer III (20 mM Tris, pH 8.1, 2 mM EDTA, 1% Triton X-100, 0.1% SDS, and 150 mM NaCl), once with buffer IV (20 mM Tris, pH 8.1, 2 mM EDTA, 1% Triton X-100, 0.1% SDS, and 500 mM NaCl), once with LiCl buffer (10 mM Tris, pH 8.1, 0.25 M LiCl, 1% Nonidet P-40, 1% deoxycholate, 1 mM EDTA), and two times with TE (10 mM Tris, pH 8.1, 1 mM EDTA). The immune complexes were then eluted by incubating the beads two times with 250 μl of elution buffer (1% SDS, 0.1 M NaHCO3, 20 μg/ml of glycogen) for 15 min each at room temperature. To reverse cross-linking, the combined eluates were incubated with 0.2 M NaCl for 4 h at 65°C. The eluates were extracted once with phenol-chloroform and once with chloroform, precipitated with ethanol, and resuspended in 20 μl of TE. A 2-μl sample was used for PCR. The appropriate primer pairs were used for PCR. Primers for the ap-a, RP-L21 lgl1, and P-R genes were as described in Table 1. PCR products were separated by electrophoresis on 1.5% agarose gels and were stained with ethidium bromide.
TABLE 1.
Primers used for amplification of genes following ChIP
| Gene | Orientationa | Sequence |
|---|---|---|
| ap-a | S | 5′ GAAGATCTAAAATGAAAGCCATCGTC |
| ap-a | AS | 5′ ACGCGTCGACGCAAGCATGAATCTTAG |
| RP-L21 | S | 5′ CACTTCAACCATTCTTCAC |
| RP-L21 | AS | 5′ ATCTAAGAATTCCACTCACAAAACACCTTG |
| lgl-1 | S | 5′ CTAAGTGTAAAACATGTTGTAG |
| lgl-1 | AS | 5′ ACTCTTTTTGGAATTGCATAAC |
| P-R | S | 5′ ATGTCTGGTTGGTTTGGT |
| P-R | AS | 5′ GATTTCAACGACTGGTGG |
S, sense; AS, antisense.
RESULTS
Sequences of the neighboring SINE1 are required for amoebapore gene silencing.
We have previously reported that transfection of HM-1:IMSS trophozoites with a hybrid plasmid (psAP-1, Fig. 1A) which contained the ap-a ORF flanked by 473 bp at its 5′ end and by 330 bp at its 3′ regulatory region caused the transcriptional silencing of both the plasmid-encoded and the chromosomal ap-a gene rather than its overexpression (9). Furthermore, transfection with a plasmid (psAP-2) which contained only the 473-bp DNA fragment that was used as the 5′-flanking region (Fig. 2) also caused total gene silencing, and this occurred considerably faster (14 versus 28 days in culture, data not shown). These results indicated that the trigger for gene silencing was within the 5′ 473-bp fragment. Detailed analysis revealed that this fragment also encodes, in the opposite orientation, a truncated segment of a SINE1 and is preceded at its 5′ end by a short T-rich stretch (Fig. 1A). This T-rich region has a unique sequence that differs from all other T-rich regions found in the other homologous SINE1s (data not shown). As earlier reported (9), a plasmid (psAP-6) in which the 473-bp fragment was truncated by 122 bp at its 5′ end caused only a partial silencing whereas a plasmid (psAP-7) which was truncated at the putative promoter and 5′ untranslated region sequences of the ap-a gene (60 bp upstream of the ATG) did not cause any gene silencing (9). In order to better understand the minimal DNA sequence requirements for the induction of gene silencing and to distinguish between the ability of a plasmid construct to cause gene silencing and the ability to cause overexpression of the ap-a gene, we prepared additional plasmids in which we included different lengths of the 5′ flanking element with an identical ap-a ORF and a 3′ flanking segment (Fig. 1A) as well as plasmids which did not contain the ap-a ORF and had different lengths of the 5′ upstream region (Fig. 2A). Transfectants with plasmid psAP-10, in which the 48 bp of the T-rich region was omitted, or with plasmid pEhapa, which included a long 5′ flanking element (2,785 bp) that contained the entire 550 bp of the SINE1 as well as its 3′ regulatory region, both overexpressed AP-A (Fig. 1B and C). Furthermore, transfection with plasmid p5AP3:CAT, in which the CAT reporter gene, linked to the 3′ regulatory region of an actin gene, was fused to the 140 bp of the truncated SINE1 of psAP-1, also resulted in overexpression of AP-A (see Fig. 4A and C). Silencing of ap-a also did not occur following transfection of plasmid p5AP3:SINE, in which the 3′ end of the SINE1 was fused to the 140 bp of the truncated SINE1 of psAP-1 (see Fig. 4A and D). In contrast, transfectants with plasmid psAP-11, which contained 880 bp of the 5′ flanking region, i.e., a truncated segment of SINE1 (516 bp without its 3′ regulatory region), and psAP-1, which also has a truncated (140 bp) SINE, were suppressed in the expression of the ap-a gene (Fig. 1B and C). Transfectants with psAP-15 (Fig. 1A and B), in which the 5′ upstream region of the AP-A gene included the T-rich region but only a shorter segment (55 bp) of the transcribed SINE, were not silenced and had an AP-A expression similar to that of the parent strain without overexpression of AP-A. All the transfected as well as the nontransfected control cultures transcribe the multiple genomic SINE1s (11) (Fig. 1B). As previously shown (9), transfections with plasmid psAP-2, which contains the 473-bp 5′ flanking region but lacks the ORF of ap-a, also caused silencing. Transfectants with plasmids psAP-21, psAP-22, and psAP-23, which contain shorter lengths of the 5′ flanking region (Fig. 2), clearly show that the length of the SINE1 sequences present is critical for the gene silencing, and whereas plasmids psAP-21 and plasmid psAP-22 induced full silencing like psAP-2, plasmid psAP-23, which is analogous in length to psAP-15 (55 bp of the 5′ SINE) and very similar to the previously described psAP-6 (9), which had only 20 bp of the SINE, caused only a partial silencing of the genomic ap-a. In summary our results indicate that downstream silencing of the ap-a gene will occur only following transfection with plasmids such as psAP-1 and psAP-11, which contain a truncated SINE1 of at least 80 bp and lacking the 3′ regulatory element. On the other hand, transfection with plasmids that contained the entire SINE1 including its 3′ regulatory elements (pEhapa), a truncated SINE1 fused to its 3′ regulatory elements (p5AP3:SINE), or a heterologous 3′ regulatory element fused to the CAT gene and the truncated SINE1 as in p5AP3:CAT promoted the overexpression of the amoebapore gene. Overexpression was also seen with plasmid psAP-10, which was devoid of the T-rich region (Fig. 1). In addition, plasmids in which the length of the truncated SINE was <80 bp (psAP-23, psAP-15, and psAP-6) were only partially silenced.
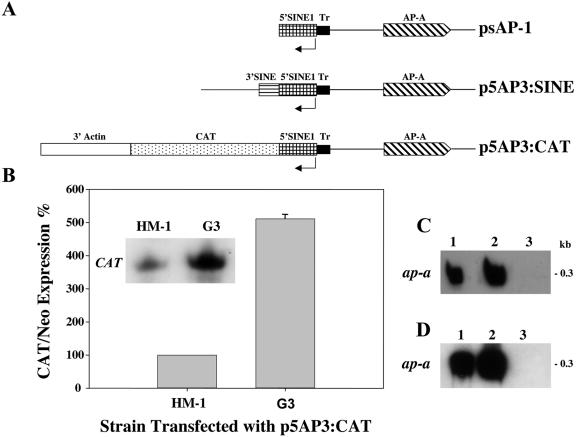
FIG. 4.
(A) Schematic map of the various chimeric plasmids used for transfection of parent strain HM-1:IMSS and ap-a-silenced G3 trophozoites. (B) Expression levels of CAT transcripts in trophozoites of strain HM-1:IMSS and silenced G3 which were transfected with p5AP3:CAT plasmid. Quantitative analysis of CAT RNA levels, which were normalized for plasmid copy number. The densitometric analysis was normalized by defining CAT/Neo ratios of expression in transfected strain HM-1:IMSS as 100%. (C) Northern blot analysis of amoebic RNA extracts. Lane 1, HM-1:IMSS; lane 2, p5AP3:CAT transfected into HM-1:IMSS; lane 3, p5AP3:CAT in G3. (D) Northern blot analysis of amoebic RNA extracts. Lane 1, HM-1:IMSS; lane 2, p5AP3:SINE transfected into HM-1:IMSS; lane 3, psAP-1 transfected into HM-1:IMSS. Blots in panels C and D were hybridized with radiolabeled probes of ap-a.
Silencing of Ehap-a gene did not occur in two other E. histolytica cultures.
Transfections with plasmid psAP-2 (9) of trophozoites of avirulent E. histolytica strain Rahman or of another isolate of E. histolytica strain HM-1:IMSS, which has been subcultured for many years in the lab, did not cause silencing of the ap-a gene (data not shown). An independent and successful silencing of the ap-a gene by the same plasmid, psAP-2, was obtained, however, with another isolate of strain HM-1:IMSS (independently acquired from Mexico) in the lab of S. Stanley (44). DNA sequence comparison of the 473-bp 5′ upstream regions of the ap-a gene in silenced HM-1:IMSS and nonsilenced Rahman trophozoites revealed no sequence differences, although recently some divergence has been detected in the mapping of SINEs in the genomes of various E. histolytica strains including strain Rahman (34).
The SINE1 neighboring the ap-a gene is actively transcribed.
The genome of E. histolytica has a few hundred copies of the SINE1 which are highly transcribed (25, 36, 42). The transcription of the neighboring SINE1 is from the orientation opposite to that of the ap-a gene. In order to find out if the particular SINE1 that neighbors the ap-a gene is transcribed, we prepared chimeric plasmids containing the CAT reporter gene flanked at the 5′ end with either a fragment of 422 bp (51 bp upstream of the ap-a ORF) or a shorter one which included 140 bp of the SINE1 as well as the 48-bp T-rich region. Both plasmids were flanked at their 3′ ends with a 3′ downstream segment of an actin gene (pSINE:CAT1 and pSINE:CAT2, respectively, Fig. 3A). Transfectants of strain HM-1:IMSS showed that the CAT gene was expressed. The 5′ upstream region required for the transcription of the SINE was investigated using a number of chimeric CAT-containing plasmid constructs (pSINE:CAT3 and pSINE:CAT4, Fig. 3A) in which the 48 bp of the T-rich region of the SINE1 was omitted. Transfectants with such plasmids were incapable of expressing CAT, suggesting a promoter role for this T-rich region (Fig. 3B). Furthermore, a plasmid construct (pTr:CAT, Fig. 3) in which the SINE1-transcribed sequences were omitted and the T-rich region was directly ligated to the CAT ORF also failed to express the CAT gene, which suggests that the 5′ end of the SINE contains an internal promoter. Analysis of the transcription initiation site by primer extension of the SINE-CAT transcripts revealed that the initiation sites are located 6 and 9 nucleotides downstream of the T-rich region (not shown). In conclusion our results demonstrate that both the T-rich region and a putative internal promoter region located at the 5′ end of SINE need to be present to promote its transcription. This finding correlates with the finding that silencing of the ap-a gene also requires both the T-rich region and sequences of the 5′ end of the SINE. This was shown by the partial ability of transfectants psAP-23, psAP-15, and psAP-6 (9), which contain the T-rich region but lack most of the SINE sequences, to induce silencing (Fig. 1 and 2).
Transfections of the silenced plasmid-less G3 trophozoites with the plasmid p5AP3:CAT (Fig. 4A) showed a significantly higher expression of CAT (∼5-fold) than that seen in transfected strain HM-1:IMSS (Fig. 4B). The transcript levels of the ribosomal protein L21 gene and the Neo resistance gene were used for normalization. A similar significantly higher level of CAT expression was observed after transfection of plasmid pSINE:CAT1 (Fig. 3A) into G3 trophozoites (data not shown). We have previously reported (9) that transfection of G3 trophozoites with plasmid pTS-1, in which the CAT reporter gene is under the promoter of the ribosomal protein gene gLE3, gave expression of CAT in G3 similar to that in strain HM-1:IMSS, indicating that other amoebic promoters are not activated. These results are interesting because they demonstrate that the locking of the episomal expression of ap-a in G3 trophozoites is accompanied by an increase in the transcription activity of the adjacent SINE which is in the opposite orientation.
Extracts of silenced trophozoites contain single-stranded short SINE1 RNA fragments.
Most of the transcriptional gene-silencing mechanisms have been shown to involve the formation of small RNA molecules (6). The most commonly known are siRNA molecules of 21 to 25 nt which are the digestion products of double-stranded RNA by a Dicer enzyme and have homology to transcripts of the ORF of the silenced gene (20, 27, 38). Bioinformatic searches of the E. histolytica genome databases have not revealed a Dicer-like enzyme, although a gene with similarity to a motif of RNase III has been identified (1). In addition, other enzymes known to be involved in the gene-silencing machinery such as two Argonaute genes and an RNA-dependent RNA polymerase have been identified and shown to be transcribed (data not shown). Northern blot analyses of RNA extracts enriched for small RNA molecules from silenced and nonsilenced trophozoites did not reveal the presence of any 21- to 25-nt-size molecules that hybridized with probes of the amoebapore ORF. Synthetic oligonucleotides of 22 nt based on the ORF sequence of the ap-a gene were used as markers for detection on the same gels. On the other hand, upon hybridization of blots with a single-stranded, top-strand-oriented probe of the 5′ end of the SINE1 (140 nt) (+1 to +146), RNA molecules of approximately 120 to 140 nt were observed in the extracts of silenced trophozoites (G3, psAP-1, and psAP-11, Fig. 5A) but not in extracts from nonsilenced or ap-a-overexpressing trophozoites. The level of the 140-nt fragments was very low (<1%) in comparison with the level of SINE1 transcripts detected in silenced and nonsilenced RNA extracts, and they had the same orientation. The extracts of small RNA molecules did not hybridize on blots (i) with probes derived from the 3′ end of the SINE1 (+380 to +514), (ii) with a bottom-strand-oriented probe of the 5′ region of the SINE1, (iii) with probes of the T-rich region, or (iv) with a probe of the promoter region or ORF of ap-a (not shown). The RNA fragments as well as the full-length SINE transcripts of 550 nt also strongly hybridized with a synthetic oligonucleotide probe of 39 nt (probe-D; see Materials and Methods) prepared according to the sequence of the 5′ end of the SINE. The single-stranded nature of these molecules was ascertained by testing their sensitivity to predigestion by RNase I, which degrades single-stranded RNA, and their resistance to digestion by RNase III, which degrades double- but not single-stranded RNA (Fig. 5B). A lower band of ∼70 nt which also hybridized with the 5′ SINE probe, or with the synthetic oligonucleotide probe SINE-D, was observed in RNA extracts of all the cultures. The source and sequence of these single-stranded RNA molecules have not yet been confirmed, but since they hybridize with a single-stranded top-strand-oriented probe containing 140 nt of the 5′ SINE and did not hybridize with a probe of the 3′ end of the SINE or with any other region, it is reasonable to conclude that they may represent a naturally occurring truncated SINE 5′ RNA (4) or some other RNA molecules with high homology to this region.
FIG. 5.
(A) Small RNA analysis on a 17% acrylamide gel. Samples are from the indicated trophozoite extracts: lane 1, HM-1:IMSS; lane 2, psAP-1; lane 3, G3; lane 4, psAP-10; lane 5, psAP-11. Blots were hybridized with a radiolabeled, top-strand-oriented probe derived from the 5′ 140 bp of SINE1 (top diagram). (B) The small RNA extracts from parent strain HM-1:IMSS (H) and silenced trophozoites G3 (G) were treated with RNA-modifying enzymes according to manufacturer's instructions before being loaded on the gel, and blots were hybridized as in panel A. (a) Intact samples; (b) RNase I treatment; (c) RNase III treatment.
Analysis of cytosine methylation.
An inverse correlation between gene activity and cytosine methylation, particularly in the promoter and coding regions, has been reported for several eukaryotes (8, 43). Only one type of DNA methyltransferase (Dnmt2) has been identified in the E. histolytica genome (14). The DNA region including the 5′ SINE1 and the ap-a ORF in silenced G3 and in control nontransfected trophozoites was analyzed for cytosine methylation by bisulfite degradation followed by PCR amplification and DNA sequencing of numerous clones (40). A complete conversion of cytosine residues to thymidines occurred in all fragments of the region, except for the segment which includes the 5′ untranslated region of the ap-a gene and the ORF (sense strand), in which some heterogeneity in cytosine modification was observed (data not shown). The absence of a clear difference between the cytosine methylation in the DNA of silenced trophozoites and that in DNA of nonsilenced trophozoites is consistent with our previous finding that cultivation of silenced trophozoites in the presence of the DNA methylation inhibitor (39) 5-azacytidine or zebularine did not restore gene expression (9).
Evidence for histone modification in silenced trophozoites.
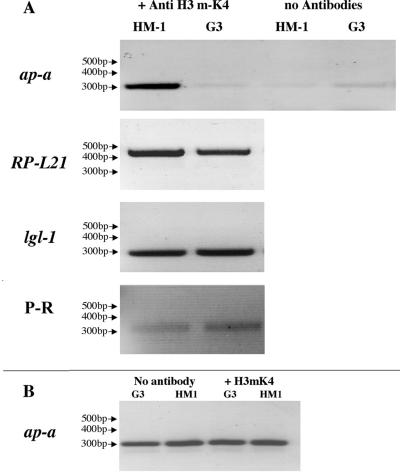
Epigenetic gene silencing has been reported to be maintained by rearrangements in chromatin structure which mainly result from modifications (acetylation, methylation, phosphorylation, ubiquitination, and α-sumoylation) (10, 12, 33, 35) of specific amino acids in the N-terminal tails of various histones. One of the most investigated modifications, which appears to be associated with chromatin domains that are transcriptionally inactive, has been the methylation of lysine 9 in histone H3 of numerous species (5, 17, 24). In contrast, the methylation usually found at lysine 4 has been associated with transcriptionally active euchromatin, and demethylation of lysine 4 is considered an indication of a transcription-inactivated domain. The amino acid sequence of histone H3 of E. histolytica does not have a lysine at position 9 (it has an Arg), but it has sequence homology up to lysine 4 (15) which, as mentioned above, can also be used for determination of transcription-active or inactive domains. An antibody against methylated K4 of histone H3 (Upstate Biotechnology) which reacted with histone H3 of E. histolytica was used to perform ChIP analysis of G3 silenced and nontransfected HM-1:IMSS control trophozoites. DNA fractions that immunoprecipitated from the nonsilenced amoeba clearly showed that the ap-a gene domain was amplified by PCR, but this gene did not amplify in DNA precipitates from the ap-a silenced G3 trophozoites (Fig. 6A). PCR amplification of input samples prior to immunoprecipitation revealed that the genes were present at similar levels (Fig. 6B). In addition, other genes such as ribosomal protein L21 (RPL21) and the gene coding for the light Gal lectin (lgl1) subunit, which are transcriptionally active in both strains, clearly showed up in the chromatin immunoprecipitates from both the control and G3 silenced trophozoites (Fig. 6A). In order to see if other genes that are adjacent to SINEs are affected we examined the expression of a proline-rich gene (locus 1.m00613 in the TIGR database) which is located adjacent to another SINE (42). The proline-rich gene was found to be transcriptionally active in the G3 silenced trophozoites as well as in the parent strain HM-1:IMSS (Fig. 6A). This finding demonstrates that, only at the chromatin domain of the ap-a gene of G3 silenced trophozoites, histone H3 lysine 4 is not methylated, and this provides a possible explanation for the stable suppression of ap-a transcription seen in such amoebas.
FIG. 6.
(A) PCR amplification of genes from DNA fragments following ChIP with antibody against methylated K4 of histone H3 from trophozoites of strain HM-1:IMSS and ap-a gene-silenced G3. Precipitates without antibodies served as negative controls. Genes that were amplified by PCR before and after immunoprecipitation were amoebapore A (ap-a), ribosomal protein L21 (RP-L21), light subunit of Gal lectin (lgl-1), and a proline-rich gene (P-R) which neighbors another SINE (42). (B) DNA samples prior to immunoprecipitation were used as PCR templates and served as input controls. Primers used for PCR amplification of the different genes are shown in Table 1. For details on ChIP procedures see Materials and Methods.
DISCUSSION
We have recently shown that the transfection of a plasmid containing 473 bp of the 5′ upstream regulatory sequences of the amoebapore A gene (ap-a) triggered the total suppression of transcription of the chromosomal copy of the ap-a gene (9). Gene silencing has persisted even after excision of the plasmid from the trophozoites, and all our attempts to date to reactivate transcription of the ap-a gene have failed. Sequence analysis of the 473-bp upstream DNA fragment used in the plasmid construct which triggered the silencing phenomenon revealed that, in addition to the 5′ regulatory elements of the ap-a gene, it includes a T-rich region of 48 bp as well as 140 bp of the 5′ end of a SINE1 which is transcribed in the opposite orientation (42). Our present results show that plasmids whose construct (i) did not include the T-rich region which precedes the SINE1 (psAP-10); (ii) contained the full-length SINE1 including its 3′ regulatory sequences (pEhapa); (iii) included a heterologous gene, p5AP3:CAT, which contains the 3′ regulatory element of actin fused to the truncated SINE; (iv) included the 3′ regulatory region of SINE fused to the truncated SINE (p5AP3:SINE); or (v) contained a short segment (<80 bp) of SINE (psAP-15 or psAP-23) did not induce gene silencing and in most cases enabled transfectants to overexpress the ap-a gene. On the other hand, transfection with plasmids containing a truncated SINE1 like psAP-11, which included 516 bp of the SINE1 without its 3′ regulatory element, or psAP-1, which contained only 140 bp of SINE1, leads to silencing. These results indicate that the downstream silencing of the ap-a gene requires the insertion of a plasmid which contains both T-rich and truncated SINE sequences (>80 bp) flanking the promoter region of the ap-a gene. Most of the noncoding SINE1 retroposon elements (>400) that are dispersed in the genome of E. histolytica have been shown to have at their 5′ end a T-rich stretch of approximately 50 bp (25, 42). Interestingly, however, we have found that there is considerable sequence divergence among the different T-rich regions which precede the SINE1 repetitive elements, and it is not yet clear which of the SINE1s that are present in the genome are transcribed. Using a chimeric plasmid containing the CAT reporter gene fused to different 5′ putative promoter regions of the SINE1, we have found that the particular SINE1 which is present upstream of the ap-a gene is transcribed. Removal of the T-rich stretch from the CAT-containing plasmid or of the 5′ SINE1 sequences abolished the transcription of CAT, indicating that both regions are essential for the transcription of this particular SINE. Primer extension of the CAT chimeric transcript revealed that the transcription initiation sites of SINE1 are 6 and 9 bases downstream of the T-rich stretch. The significance of the T-rich region as well as the 5′-end SINE sequences for transcription of other SINE1s is currently being investigated. Another interesting observation is that, in the G3 silenced (plasmid-less) trophozoites, there was approximately a fivefold-higher level of transcriptional activity of the SINE1 neighboring the silenced ap-a gene. In plants it has been shown that the transcriptional activity of some non-LTR transposable elements is sensitive to the presence of homologous transgenes, suggesting the involvement of homology-dependent gene-silencing mechanisms in their regulation. It has also been shown that transcriptional activation of retrotransposons can alter the expression of adjacent genes in wheat as well as in mice (23, 41). The reason why transfections of other isolates of E. histolytica (avirulent strain Rahman and another isolate of strain HM-1:IMSS) with plasmid psAP-2 did not result in silencing of the ap-a gene is not yet clear. Genomic sequence analysis of the analogous 5′ upstream regions of the ap-a gene in the above strains found them to be identical to the sequences of the two virulent isolates of strain HM-1:IMSS which were capable of becoming silenced, both at our lab and independently at the lab of S. Stanley in St. Louis, Mo. (44). Recently it was reported (34) that small variations appear to exist in the genomic organization of SINE retroposon elements in different strains of E. histolytica. Such differences may have an effect on the ability of some strains to trigger the gene-silencing process. The mapping and transcription capabilities and levels of the SINE1s present in the two strains which were incapable of gene silencing are currently under investigation.
The most commonly reported form of gene silencing, or gene quelling as it is termed in Neurospora, is the posttranscriptional (PTGS) type, which requires the insertion of homologous transcribed sequences (10). Such silencing of gene expression is currently understood to be the consequence of mRNA destruction which appears to be triggered by the formation of double-stranded RNA and siRNA (6, 20, 27, 38). Most of the PTGS gene-silencing events have reported the finding of small (21- to 25-nt) siRNA molecules which hybridize with probes consisting of the ORF of the gene which has been silenced. TGS has been shown to occur in a number of systems including plants following the insertion of multiple homologous repeats of a transgene promoter region (26, 29, 43). Such silencing has been shown to be inheritable in the progeny, and it can persist even when the silencer sequence is excised (46). In Saccharomyces cerevisiae an epigenetic formation of a heterochromatin-like structure results in a transcriptional gene silencing which is inherited for many mitotic divisions (45). Transformation of the plant pathogen Phytophthora infestans with constructs containing the elicitin gene inf1 resulted in the specific transcriptional silencing of the transgene as well as the endogenous gene, and the silencing status remained in effect also in nontransgenic progeny (37). Silencing was not due to gene disruption, and it was not based on high turnover of inf1 mRNA. In some TGS cases, small siRNA molecules have not been found. In E. histolytica in spite of exhaustive efforts we have not found such siRNA molecules in extracts of silenced trophozoites but we did find longer (∼140-nt), single-stranded RNA molecules that hybridized only with a top-strand-oriented probe prepared from the 5′ end of the SINE1, similarly to the hybridization of the SINE1 transcripts. At present we don't know the role that these SINE-encoded RNA molecules may play. It is possible that their accumulation in the nuclei may trigger a signal for the epigenetic silencing of the downstream gene, as has recently been proposed for small heterochromatic RNA molecules (16).
In plants it has been established that introduction of homologous, duplicate DNA sequences can sometimes lead to epigenetic gene silencing, and this can manifest itself in modifications such as DNA methylation, histone deacetylation, or methylation and changes in chromatin arrangements (5, 27). We have not detected any DNA rearrangements or changes in the restriction pattern of genomic DNA of the silenced trophozoites using 5′-methylated cytosine-dependent restriction enzymes. Furthermore treatment of DNA with bisulfite followed by PCR amplification and sequence comparisons between the treated DNA of silenced and parent cultures revealed very low if any 5′-cytosine methylation and no significant difference in the methylation levels of the DNA of the silenced and of the nonsilenced trophozoites. In addition, treatment of silenced trophozoite cultures with inhibitors of DNA methylation, such as 5-azacytidine (39), or of histone deacetylation, such as trichostatin A (32), failed to restore transcription of the ap-a gene. Although our current data do not imply that DNA methylation is involved in triggering or maintaining the silencing of the ap-a gene, we still cannot rule out a possible involvement of a very low level of specific cytosine methylation which could be resistant to 5-azacytidine.
A number of possible epigenetic mechanisms have been proposed for locking a gene into a silenced state, and these seem to be quite unique for each of the different species in which they were discovered. In some cases the silencing mechanisms involve DNA or protein modifications which trigger the assembly of a repressed chromatin structure. Well-documented modifications include in addition to the above-mentioned ones DNA methylation (8, 43); histone deacetylation (17, 30); histone methylation at specific lysine or arginine residues (5, 24); and histone phosphorylation, ubiquitination, or α-sumoylation (33, 35). As has been shown in many cases, methylation of lysine 4 of histone H3 is an indication of a transcriptionally active domain whereas a nonmethylated lysine 4 has been associated with a transcriptionally inactive domain (5, 24). Using an antibody against methylated K4 of histone H3 for ChIP analysis, we were able to show that the chromatin precipitates of nontransfected strain HM-1:IMSS contained the ap-a gene whereas immunoprecipitates of chromatin from the silenced G3 trophozoites did not contain the ap-a gene. The DNA of other transcribed genes was detected in the immunoprecipitates at the same level as in the control. Moreover, a proline-rich gene which is located adjacent to another SINE (42) was found to be transcribed, and its DNA domain also precipitated with the antibody against methylated H3K4 in both the silenced G3 trophozoites and the parent strain HM-1:IMSS. These results demonstrate that in the G3 silenced trophozoites the demethylation occurred only in the histone H3 lysine 4 residue at the chromatin domain of the ap-a gene. Unfortunately, due to significant amino acid sequence divergence of other regions of the histone molecules of E. histolytica (15), other commercially available antibodies which are regularly used in mammalian and plant species for ChIP analysis to identify transcriptionally active or inactive domains such as methylation at H3 K9 had no cross-reactivity and could not be applied. Thus, our indication of transcriptionally inactive ap-a domain from the demethylation of lysine 4 is not yet definitive. New specific antibodies against putative methylated lysine and arginine residues of E. histolytica histones are currently being prepared.
Our current working hypothesis is that, in E. histolytica, the transfection of multiple copies of a truncated SINE1 which included its promoter region as well as the promoter region of the adjacent ap-a gene enabled the transcription of a single-stranded and relatively short RNA molecule from the 5′ end of the SINE1. The accumulation of such truncated RNA molecules may somehow guide and trigger the silencing machinery that also causes histone modifications as shown by the demethylation of lysine 4 of histone H3 at the chromatin domain of ap-a, resulting in the suppression of transcription of the gene. A major goal of our ongoing research is to dissect the mechanism of this pathway and to identify the enzymes and targets that participate in this process. These studies will hopefully help us to better understand the molecular elements and events which cause and maintain the transcriptional gene silencing.
Acknowledgments
This investigation was supported by grants from the Drake family and from Henry H. Meyer, Jr.
We thank M. Leippe, Kiel University, Germany, for the anti-amoebapore A antibodies; E. Tannich from BNI, Germany, for the genomic clone Ehap; and S. Michaeli from BIU, Israel, for her generous help and advice.
REFERENCES
- 1.Abed, M., and S. Ankri. 2005. Molecular characterization of Entamoeba histolytica RNase III and AGO2, two RNA interference hallmark proteins. Exp. Parasitol. 110:265-269. [DOI] [PubMed] [Google Scholar]
- 2.Ainbinder, E., M. Revach, O. Wolstein, S. Moshonov, N. Diamant, and R. Dikstein. 2002. Mechanism of rapid transcriptional induction of tumor necrosis factor alpha-responsive genes by NF-κB. Mol. Cell. Biol. 22:6354-6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alon, R. N., R. Bracha, and D. Mirelman. 1997. Inhibition of expression of the lysine-rich 30 kDa surface antigen of Entamoeba dispar by the transcription of its antisense RNA. Mol. Biochem. Parasitol. 90:193-201. [DOI] [PubMed] [Google Scholar]
- 4.Bakrea, A. A., K. Rawal, R. Ramaswamy, A. Bhattacharya, and S. Bhattacharya. 2005. The LINEs and SINEs of Entamoeba histolytica: comparative analysis and genomic distribution. Exp. Parasitol. 110:207-213. [DOI] [PubMed] [Google Scholar]
- 5.Bannister, A. J., R. Schneider, and T. Kouzarides. 2002. Histone methylation: dynamic or static? Cell 109:801-806. [DOI] [PubMed] [Google Scholar]
- 6.Baulcombe, D. 2004. RNA silencing in plants. Nature 431:356-363. [DOI] [PubMed] [Google Scholar]
- 7.Bhattacharya, S., A. Bakre, and A. Bhattacharya. 2002. Mobile genetic elements in protozoan parasites. J. Genet. 81:73-86. [DOI] [PubMed] [Google Scholar]
- 8.Bird, A. 2002. DNA methylation patterns and epigenetic memory. Genes Dev. 16:6-21. [DOI] [PubMed] [Google Scholar]
- 9.Bracha, R., Y. Nuchamowitz, and D. Mirelman. 2003. Transcriptional silencing of an amoebapore gene in Entamoeba histolytica: molecular analysis and effect on pathogenicity. Eukaryot. Cell 2:295-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cogoni, C. 2001. Homology-dependent gene silencing mechanisms in fungi. Annu. Rev. Microbiol. 55:381-406. [DOI] [PubMed] [Google Scholar]
- 11.Cruz-Reyes, J., T. Ur-Rehman, W. M. Spice, and J. P. Ackers. 1995. A novel transcribed repeat element from Entamoeba histolytica. Gene 166:183-184. [DOI] [PubMed] [Google Scholar]
- 12.Csink, A. K., A. Bounoutas, M. L. Griffith, J. F. Sabi, and B. T. Sage. 2002. Differential gene silencing by trans-heterochromatin in Drosophila melanogaster. Genetics 160:257-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diamond, L. S., D. R. Harlow, and C. C. Cunnick. 1978. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 72:431-432. [DOI] [PubMed] [Google Scholar]
- 14.Fisher, O., R. Siman-Tov, and S. Ankri. 2004. Characterization of cytosine methylated regions and 5-cytosine DNA methyltransferase (Ehmeth) in the protozoan parasite Entamoeba histolytica. Nucleic Acids Res. 32:287-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fodinger, M., S. Ortner, B. Plaimauer, G. Wiedermann, O. Scheiner, and M. Duchene. 1993. Pathogenic Entamoeba histolytica: cDNA cloning of a histone H3 with a divergent primary structure. Mol. Bichem. Parasitol. 59:315-322. [DOI] [PubMed] [Google Scholar]
- 16.Grewal, S. I., and D. Moazed. 2003. Heterochromatin and epigenetic control of gene expression. Science 301:798-802. [DOI] [PubMed] [Google Scholar]
- 17.Grunstein, M. 1997. Histone acetylation in chromatin structure and transcription. Nature 389:349-352. [DOI] [PubMed] [Google Scholar]
- 18.Hamann, L., H. Buss, and E. Tannich. 1997. Tetracycline-controlled gene expression in Entamoeba histolytica. Mol. Biochem. Parasitol. 84:83-91. [DOI] [PubMed] [Google Scholar]
- 19.Hamann, L., R. Nickel, and E. Tannich. 1995. Transfection and continuous expression of heterologous genes in the protozoan parasite Entamoeba histolytica. Proc. Natl. Acad. Sci. USA 92:8975-8979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannon, G. J. 2002. RNA interference. Nature 418:244-251. [DOI] [PubMed] [Google Scholar]
- 21.Jensen, S., M. P. Gassama, X. Dramard, and T. Heidmann. 2002. Regulation of I-transposon activity in Drosophila: evidence for cosuppression of nonhomologous transgenes and possible role of ancestral I-related pericentromeric elements. Genetics 162:1197-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong, B. R., D. Wu-Scharf, C. Zhang, and H. Cerutti. 2002. Suppressors of transcriptional transgenic silencing in Chlamydomonas are sensitive to DNA-damaging agents and reactivate transposable elements. Proc. Natl. Acad. Sci. USA 99:1076-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kashkush, K., M. Feldman, and A. A. Levy. 2003. Transcriptional activation of retrotransposons alters the expression of adjacent genes in wheat. Nat. Genet. 33:102-106. [DOI] [PubMed] [Google Scholar]
- 24.Kouzarides, T. 2002. Histone methylation in transcriptional control. Curr. Opin. Genet. Dev. 12:198-209. [DOI] [PubMed] [Google Scholar]
- 25.Mandal, P. K., A. Bagchi, A. Bhattacharya, and S. Bhattacharya. 2004. An Entamoeba histolytica LINE/SINE pair inserts at common target sites cleaved by the restriction enzyme-like LINE-encoded endonuclease. Eukaryot. Cell 3:170-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matzke, M. A., M. F. Mette, and A. J. Matzke. 2000. Transgene silencing by the host genome defense: implications for the evolution of epigenetic control mechanisms in plants and vertebrates. Plant Mol. Biol. 43:401-415. [DOI] [PubMed] [Google Scholar]
- 27.Moazed, D. 2001. Common themes in mechanisms of gene silencing. Mol. Cell 8:489-498. [DOI] [PubMed] [Google Scholar]
- 28.Moshitch-Moshkovitch, S., T. Stolarsky, D. Mirelman, and R. N. Alon. 1997. Stable episomal transfection and gene expression in Entamoeba dispar. Mol. Biochem. Parasitol. 83:257-261. [DOI] [PubMed] [Google Scholar]
- 29.Muskens, M. W. M., A. P. A. Vissers, J. N. M. Mol, and J. M. Kooter. 2000. Role of inverted DNA repeats in transcriptional and post-transcriptional gene silencing. Plant Mol. Biol. 43:243-260. [DOI] [PubMed] [Google Scholar]
- 30.Ramakrishnan, G., C. A. Gilchrist, H. Musa, and M. S. Torok. 2004. Histone acetyltransferases and deacetylase in Entamoeba histolytica. Mol. Biochem. Parasitol. 138:205-216. [DOI] [PubMed] [Google Scholar]
- 31.Robin, S., S. Chambeyron, A. Bucheton, and I. Busseau. 2003. Gene silencing triggered by non-LTR retrotransposons in the female germline of Drosophila melanogaster. Genetics 164:521-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selker, E. U. 1998. Trichostatin A causes selective loss of DNA methylation in Neurospora. Proc. Natl. Acad. Sci. USA 95:9430-9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiio, Y., and R. N. Eisenman. 2003. Histone sumoylation is associated with transcriptional repression. Proc. Natl. Acad. Sci. USA 100:13225-13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Srivastava, S., S. Bhattacharya, and J. Paul. 2005. Species and strain-specific probes derived from repetitive DNA for distinguishing Entamoeba histolytica and Entamoeba dispar. Exp. Parasitol. 110:303-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Strahl, B. D., and C. D. Allis. 2000. The language of covalent histone modifications. Nature 403:41-45. [DOI] [PubMed] [Google Scholar]
- 36.VanDellen, K., J. Field, Z. Wang, B. Loftus, and J. Samuelson. 2002. LINEs and SINE-like elements of the protist Entamoeba histolytica. Gene 297:229-239. [DOI] [PubMed] [Google Scholar]
- 37.van West, P., S. Kamoun, J. W. van't Klooster, and F. Govers. 1999. Inter-nuclear gene silencing in Phytophthora infestans. Mol. Cell 3:339-348. [DOI] [PubMed] [Google Scholar]
- 38.Vaucheret, H., and M. Fagard. 2001. Transcriptional gene silencing in plants: targets, inducers and regulators. Trends Genet. 17:29-35. [DOI] [PubMed] [Google Scholar]
- 39.Venkatasubbarao, K., S. Ammanamanchi, M. G. Brattain, D. Mimari, and J. W. Freeman. 2001. Reversion of transcriptional repression of Sp1 by 5-aza-2-deoxycytidine restores TGF-β type II receptor expression in the pancreatic cancer cell line MIA PaCa-21. Cancer Res. 61:6239-6247. [PubMed] [Google Scholar]
- 40.Warnecke, P. M., C. Stirzaker, J. Song, C. Grunau, J. R. Melki, and S. J. Clark. 2002. Identification and resolution of artifacts in bisulfite sequencing. Methods 27:101-107. [DOI] [PubMed] [Google Scholar]
- 41.Whitelaw, E., and D. I. K. Martin. 2001. Retrotransposons as epigenetic mediators of phenotypic variation in mammals. Nat. Genet. 27:361-365. [DOI] [PubMed] [Google Scholar]
- 42.Wilhoeft, U., H. Bub, and E. Tannich. 2002. The abundant polyadenylated transcript 2 DNA sequence of the pathogenic protozoan parasite Entamoeba histolytica represents a nonautonomous non-long-terminal-repeat retrotransposon-like element which is absent in the closely related nonpathogenic species Entamoeba dispar. Infect. Immun. 70:6798-6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolffe, A. P., and M. A. Matzke. 1999. Epigenetics: regulation through repression. Science 286:481-486. [DOI] [PubMed] [Google Scholar]
- 44.Zhang, X., Z. Zhang, D. Alexander, R. Brancha, D. Mirelman, and S. L. Stanley, Jr. 2004. Expression of amoebapores is required for full expression of Entamoeba histolytica virulence in amebic liver abscess but is not necessary for the induction of inflammation or tissue damage in amebic colitis. Infect. Immun. 72:678-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang, Z., K.-I. Shibahara, and B. Stillman. 2000. PCNA connects DNA replication to epigenetic inheritance in yeast. Nature 408:221-225. [DOI] [PubMed] [Google Scholar]
- 46.Zou, Y. R., J.-J. Sunshine, I. Taniuchi, F. Hatam, N. Killeen, and D. R. Littman. 2001. Epigenetic silencing of CD4 in T cells committed to the cytotoxic lineage. Nat. Genet. 29:332-336. [DOI] [PubMed] [Google Scholar]