Abstract
The extracellular human pathogen Trichomonas vaginalis is covered by a dense glycocalyx thought to play a role in host-parasite interactions. The main component of the glycocalyx is lipophosphoglycan (LPG), a polysaccharide anchored in the plasma membrane by inositol phosphoceramide. To study the role of LPG in trichomonads, we produced T. vaginalis LPG mutants by chemical mutagenesis and lectin selection and characterized them using morphological, biochemical, and functional assays. Two independently selected LPG mutants, with growth rates comparable to that of the wild-type (parent) strain, lost the ability to bind the lectins Ricinnus comunis agglutinin I (RCA120) and wheat germ agglutinin, indicating alterations in surface galactose and glucosamine residues. LPG isolated from mutants migrated faster than parent strain LPG on sodium dodecyl sulfate-polyacrylamide gel electrophoresis, suggesting the mutants had shorter LPG molecules. Dionex high-performance anion exchange chromatography with pulsed amperometric detection analyses revealed galactosamine, glucosamine, galactose, glucose, mannose/xylose, and rhamnose as the main monosaccharides of T. vaginalis parent strain LPG. LPG from both mutants showed a reduction of galactose and glucosamine, corresponding with the reduced size of their LPG molecules and inability to bind the lectins RCA120 and wheat germ agglutinin. Mutant parasites were defective in attachment to plastic, a characteristic associated with avirulent strains of T. vaginalis. Moreover, the mutants were less adherent and less cytotoxic to human vaginal ectocervical cells in vitro than the parental strain. Finally, while parent strain LPG could inhibit the attachment of parent strain parasites to vaginal cells, LPG from either mutant could not inhibit attachment. These combined results demonstrate that T. vaginalis adherence to host cells is LPG mediated and that an altered LPG leads to reduced adherence and cytotoxicity of this parasite.
Trichomonas vaginalis is an extracellular aerotolerant flagellated protozoan that causes the most common nonviral sexually transmitted disease in humans (43). Worldwide, over 170 million people are infected with this parasite (57). T. vaginalis colonizes the female and male urogenital tract, and symptoms can vary widely from asymptomatic infections to vaginitis, urethritis, prostatitis (26), low birth weight and preterm delivery (15), premature rupture of membranes, and infertility (9, 40, 44). Infection by this parasite has also been associated with increased susceptibility to human immunodeficiency virus infection (52, 53).
Because T. vaginalis is an extracellular pathogen, adherence to epithelial cells is an important factor for virulence. The various epithelia that this parasite can colonize and the different symptoms it can produce are indicative of a highly promiscuous mechanism for attachment to host cells or the ability to use multiple adhesion factors. Surface proteins are the most extensively studied molecules in trichomonad adhesion, although much controversy remains in the field (2, 10). Previous reports suggest that proteins that bind to extracellular matrix components (4, 14, 16), cysteine proteinases (6, 39, 42), erythrocyte-specific ligands (17, 24, 45), and adhesins (3, 5) are found to be involved in attachment of parasite to cells. Carbohydrates on the surface of trichomonads have received much less attention, but several studies have shown their potential role in adhesion. Exposing T. vaginalis to periodate to oxidize surface polysaccharides (27), enzymes that digest the surface glycocalyx (41), or sugars that compete for binding (30) all result in decreased parasite attachment to mammalian cells.
The main surface polysaccharide in T. vaginalis is lipophosphoglycan (LPG) (47). It is present at high density on the parasite surface (>2.7 × 106 copies/cell) and is a complex molecule with an inositol phosphoceramide anchor. Its phosphorylated glycan core is composed of 50 to 54 monosaccharide residues with a composition distinct from LPG glycan cores of other parasites (47). The detailed polysaccharide structure of T. vaginalis LPG is unknown, but monosaccharide composition analyses have revealed that galactose and glucosamine are the most prevalent monosaccharide residues (48).
The LPG on the parasite Leishmania has been shown to play a role in cell recognition, cell-cell interaction, adherence, resistance to complement and host enzymes, and immune system evasion (31, 46, 54). Thus, it seems likely that LPG in T. vaginalis has similar functions. The role of LPG in adherence has been demonstrated for a related pathogen of cattle, Tritrichomonas foetus, by showing that purified LPG inhibits binding of Tritrichomonas foetus to bovine vaginal epithelial cells in a dose-dependent manner (51). Furthermore, the same group has also shown that treatment of T. vaginalis with periodate abolishes parasite adherence to human vaginal epithelial cells, indirectly suggesting that LPG is involved in adherence (27).
To study the role of T. vaginalis LPG in host-parasite interactions, we have constructed LPG mutants by chemical mutagenesis followed by selection with the galactose-specific lectin RCA120. Here we report the characterization of these T. vaginalis LPG mutants and demonstrate that mutants bearing truncated surface LPG are deficient in binding and lysing host cells.
MATERIALS AND METHODS
Parasites and media.
Strain B7RC2 (ATCC 50167) was selected as it showed the highest adhesion and cytotoxicity to mammalian cells out of several strains tested in preliminary experiments (data not shown). TYM medium supplemented with 10% heat-inactivated horse serum (Invitrogen), 10 U penicillin (Invitrogen), 10 μg streptomycin (Invitrogen), 180 μM ferrous ammonium sulfate, and 28 μM sulfosalicylic acid was used to grow the parasites (12). Parasites were passaged daily (∼106 parasites in 10 ml TYM). As an increased number of in vitro passes has been associated with reduced virulence in T. vaginalis (43), the parent and mutant parasites were passed a comparable number of times.
Chemical mutagenesis and lectin selection.
Two independent mutagenesis experiments were carried out as previously described for Leishmania with slight modifications (35). Briefly, 3 × 108 parasites were grown overnight in complemented TYM and cultures were washed with TYM, resuspended to 3 × 107 cells/ml in medium, and incubated with 9 mg/ml ethyl methanesulfonate (Sigma) shaking (70 rpm) at 37°C for 4 h. No cell death was observed at this time. Mutagenized parasites were washed once with TYM. Approximately 50% cell death was observed for cells exposed to ethyl methanesulfonate after culturing overnight in TYM at 37°C. Cells were then grown for several generations before 107 parasites were selected using Ricinus communis agglutinin I (RCA120).
For lectin selection, parasites were washed in TYM and incubated overnight at 37°C in TYM with 100 μg/ml RCA120 (Sigma). Agglutinated cells were removed by low-speed centrifugation. Lectin selection procedure was repeated three times before cloning of populations in soft agar (32). Parasite colonies that did not label with fluorescein isothiocyanate-RCA120 were cloned three more times by limiting dilution in 96-well tissue culture plates under anaerobic conditions in GasPak jars (Becton Dickinson and Co).
Fluorescent labeling of parasites.
Overnight cultures of parasites were fixed in 5% Formalin at room temperature for 5 min. Fixed parasites were washed once with phosphate-buffered saline (PBS), and pellets were resuspended in a 1:500 dilution of fluorescein isothiocyanate-RCA120 or rhodamine-wheat germ agglutinin (Vector Laboratories). Stained parasites were examined using an Axioscop 2 epifluorescent microscope (Zeiss), and images were recorded with a Axiocam camera and processed with the AxioVision 3.2 program (Zeiss).
LPG isolation and staining.
LPG was isolated by adapting the method for bacterial lipopolysaccharide isolation from Johnson and Perry (33) followed by solvent E extraction as used in Leishmania LPG extraction (34). Briefly, parasites were washed once with PBS and resuspended in 10 mM Tris, pH 8.0. Cells were sonicated on ice for 30 seconds and incubated at 37°C for 2 h with RNase A (2 μg/ml; Sigma) and DNase Q1 (2 μg/ml; Promega), followed by overnight incubation at 4°C with 2.5 μg/ml proteinase K (Roche). Polysaccharides were extracted by adding an equal volume of phenol (equilibrated to pH 8.0), and sonicating on ice for 15 seconds. Phases were separated by centrifugation, and the aqueous phase was dialyzed overnight at 4°C and concentrated. LPG was then extracted with solvent E (H2O, ethanol, diethylether, pyridine, NH4OH, 15:15:5:1:0.02) before dialysis in PBS and further concentration. LPG was visualized by fractionation on sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) and subsequent staining with periodate silver.
Monosaccharide composition.
The monosaccharide composition of purified LPG was determined by hydrolysis in 2 N trifluoroacetic acid at 100°C for 4 h, followed by Dionex high-performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) using a CarboPac PA-1 column at the University of California-San Diego Glycotechnology Core Resource (http://glycotech.ucsd.edu/index.shtml). Preliminary sequence data was obtained from the Institute for Genomic Research through the website at http://www.tigr.org.
Adhesion to plastic.
The parent and LPG mutant parasites were cultured overnight in TYM medium and washed once with warm (37°C) nonsupplemented TYM. Parasites were resuspended in warm TYM to 2.5 × 105 cells/ml, and 2 ml of the suspension were incubated in triplicate in 24-well plates for 4 h at 37°C. After incubation, wells were washed three times with nonsupplemented warm TYM to remove unattached parasites. Wells were then incubated with crystal violet (0.13% crystal violet/5:2 ethanol-formaldehyde) for 5 min and washed three times with PBS. Remaining dye was solubilized with 1% SDS in 50% ethanol, transferred to 96-well plates, and measured in a plate reader at 570 nm. The experiment was repeated three times.
Adherence to human vaginal ectocervical cells.
The human ectocervical cell line Ect1/E6E7 (obtained from Raina Fichorova) was grown as described (23) in keratinocyte-SFM (GIBCO) complemented with bovine pituitary extract (GIBCO), 100 ng/ml recombinant epidermal growth factor (GIBCO), 0.4 mM CaCl2, 100 U/ml penicillin, and 100 μg/ml streptomycin (GIBCO). Ectocervical cells were seeded on 12-mm coverslips in 24-well plates at 5 × 105 cells/well in culture medium and grown to confluence at 37°C and 5% CO2 for 2 days. Cells were washed once with PBS before the addition of parasites. Parasites were labeled with 10 μM CellTracker Blue CMAC as per the manufacturer's instructions (Molecular Probes). Approximately 5 × 105 labeled parasites were washed and added to ectocervical cells in 1 ml PBS. Plates were spun at 500 × g for 5 min and incubated at 37°C and 5% CO2 for 30 min. Coverslips were subsequently washed in PBS, fixed with 4% paraformaldehyde, and mounted on slides. Five 20× magnification fields were analyzed per coverslip. Fluorescent parasites adhered to host cells were counted using Scion Image for Windows, v. Beta 4.0.2 (Scion Corporation). The experiment was repeated at least three independent times.
Adherence inhibition by isolated LPG.
LPG from parent and mutant parasites was quantified by densitometry on polysaccharide-stained SDS-PAGE gels relative to a known concentration of Salmonella lipopolysaccharide using the ImageJ 1.31v program from the National Institutes of Health (http://rsb.info.nih.gov/ij/). Ectocervical cells were incubated with 150 μg of purified LPG in PBS at 37°C and 5% CO2 for 1 h prior to the addition of fluorescently labeled parent parasites. The number of parasites attached to the monolayer was determined as described above.
Cytotoxicity to epithelial cells.
Ectocervical cells were seeded in 48-well plates at 3 × 105 cells/well in culture medium and grown at 37°C and 5% CO2 for 2 days. Cells were washed once with PBS before the addition of parasites. Approximately 1.5 × 106 parasites were washed and added to ectocervical cells in 1 ml PBS. Plates were spun at 500 × g for 5 min and incubated at 37°C and 5% CO2 for 8 h. After incubation, 100 μl of parasite/cell culture supernatant was transferred to 96-well plates and allowed to equilibrate to room temperature for 30 min. Cytotoxicity was measured as a function of lactate dehydrogenase release from ectocervical cells using the CytoTox-ONE homogeneous membrane integrity assay as per the manufacturer's instructions (Promega). Fluorescence was read using an FLx800 microplate fluorescence reader (Bio-Tek Instruments, Inc.). Parasite cytotoxicity was measured against 100% lysis of cells using lysis solution included in the CytoTox-ONE kit. Cytotoxicity values were calculated as follows: % cytotoxicity = 100 × [(fluorescence reading of sample well) -(blank)]/[(fluorescence reading of 100% lysed cells) -(blank)].
Statistical analyses.
The adherence, LPG competition, and cytotoxicity data were analyzed using analysis of variance. Multiple comparisons of all pairs were performed using the method of Tukey-Kramer honestly significant difference (HSD) with an alpha level of 0.05 (Statistical software package JMPIN v3, SAS Institute).
RESULTS AND DISCUSSION
In this study, we have generated mutants of T. vaginalis that express truncated surface LPG with a modified sugar composition. These LPG mutants exhibited reduced adherence and cytotoxicity to human vaginal ectocervical cells. Mutant-derived LPG was incapable of inhibiting binding of the parasite to vaginal cells, in contrast to that observed using parent-derived LPG. These data demonstrate a critical role for LPG in the interaction of this extracellular parasite with host cells.
T. vaginalis chemical mutants do not bind RCA120 or wheat germ agglutinin lectins.
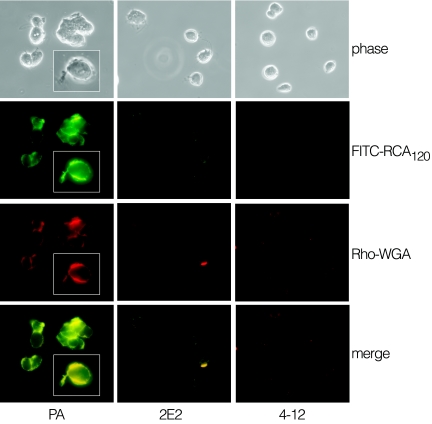
Mutants of T. vaginalis strain B7RC2 (PA strain) were obtained by chemical mutagenesis with ethyl methanesulfonate and selection with the galactose-specific lectin RCA120. Two clonal populations (2E2 and 4-12), selected from independent mutagenesis experiments and used throughout the study, showed no surface reactivity when probed with fluorescein isothiocyanate-RCA120 or rhodamine-wheat germ agglutinin (Fig. 1). Mutant parasites also did not agglutinate in the presence of fluorescein isothiocyanate-RCA120 or rhodamine-wheat germ agglutinin, whereas strong agglutination of parent parasites was observed. The surface of all three parasite lines was found to be positively labeled with the mannose-specific lectin concanavalin A (data not shown).
FIG. 1.
Fluorescent lectin labeling of parent (PA) and LPG mutant parasites (2E2 and 4-12). Parasites were fixed with 5% formalin, washed with PBS, and labeled with the fluorescently labeled lectins fluorescein isothiocyanate-RCA120 (FITC-RCA120) or rhodamine-wheat germ agglutinin (Rho-WGA), as indicated.
The lack of reactivity with RCA120 and wheat germ agglutinin suggested that mutant parasites had lost surface terminal galactosyl and glucosamine residues, the main two monosaccharides in T. vaginalis LPG (47). Lectin-specific binding to the surface of the parent strain was demonstrated by showing that surface labeling with RCA120 and wheat germ agglutinin lectins can be blocked by competition with galactose and glucosamine, respectively (data not shown). Interestingly, the fluorescent lectins also labeled flagella of the parent strain with the same intensity as the cell membrane (inset, Fig. 1), indicating that T. vaginalis flagella may be covered by LPG, as reported for Leishmania flagella (46). This observation is noteworthy as previous studies have described adherence of trichomonads to host cells by flagella (13), raising the possibility that flagellum-mediated adherence may involve LPG.
A small percentage (1 to 4%) of lectin-positive cells were occasionally observed in the 2E2 and 4-12 mutants. Mutant parasites, however, remained negative for lectin binding after prolonged culture (>1 month of daily passage) and did not revert to the wild-type phenotype. Complementation studies to rescue lectin binding phenotype and uncover the mutated gene responsible for this phenotype were attempted without success. This is likely due to the combination of low transfection efficiency and the atypically large genome size (151 to 177 Mb) (36) of T. vaginalis, as the cosmid libraries used to attempt complementation were found to represent only half of the genome (data not shown).
T. vaginalis mutants do not have impaired growth in vitro.
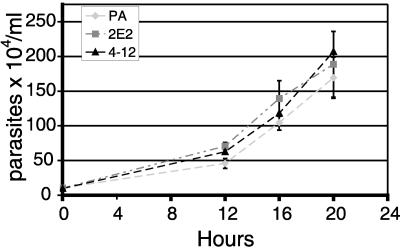
Chemical mutagenesis can simultaneously affect more than one gene, and altered parasite growth rates might influence adherence and cytotoxicity experiments. It was therefore important to demonstrate that the mutagenesis did not negatively affect the growth and morphological characteristics of the selected mutants. Light microscopic examination of the parasites did not reveal any morphological differences between the parent and the mutants. Analyses of growth rates over a 20-h period also showed no significant difference between the mutant and parent strains (Fig. 2). Based on growth rates, exponentially dividing cells were used to further characterize the adherent and cytotoxic properties of LPG mutants.
FIG. 2.
Growth curve comparing T. vaginalis parent strain with LPG chemical mutants 2E2 and 4-12. Parasites were resuspended to 104 parasites/ml and suspensions were aliquoted in 15-ml conical tubes at 37°C. At the points indicated, triplicates of each sample were incubated on ice for 10 min to release parasites that had adhered to culture tube and cells were counted in a hemacytometer. The graph represents data from three independent experiments (mean ± standard deviation).
T. vaginalis chemical mutants have truncated LPG molecules.
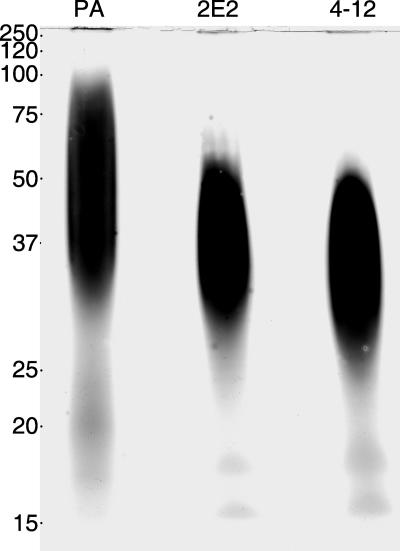
To determine if the lack of surface reactivity of mutant parasites for RCA120 and wheat germ agglutinin lectins was due to LPG loss, LPG was isolated from the parent and mutant parasites and subjected to SDS-PAGE analysis. Polysaccharide-specific silver staining of the gel revealed that the LPG of the two mutants migrated faster than that of parent cells, suggesting truncations in the polysaccharide chain (Fig. 3). Isolated LPG migrated as a broad band as previously described (48), with the parent LPG band migrating between 30 and 100 kDa, and the mutant LPG bands running between 25 and 50 kDa. Small shifts in mobility were observed between the two mutant lines, suggesting that they carry mutations in different LPG synthesis or assembly genes.
FIG. 3.
SDS-PAGE of extracted LPG from parent (PA) and mutant parasites. LPG was prepared by sonication of parasites, DNase and RNase treatment, followed by proteinase K digestion and phenol extraction. The aqueous phase was concentrated and extracted with solvent E (H2O, ethanol, diethylether, pyridine, NH4OH, 15:15:5:1:0.02) as previously described (48). Purified LPG was run on a 12% SDS-PAGE gel under reducing conditions and stained with polysaccharide-specific silver stain.
The polysaccharide nature of the isolated LPG was confirmed by staining LPG gels with the polysaccharide-specific periodic acid Schiff stain (data not shown). In contrast to the Leishmania donovani LPG chemical mutant R2D2 (35), which does not synthesize appreciable amounts of LPG, our T. vaginalis LPG mutants express LPG with apparent modification in polysaccharide content and/or structure. However, other L. donovani mutants that generate truncated LPG have also been produced (8).
LPG from mutant parasites has reduced levels of galactose and glucosamine.
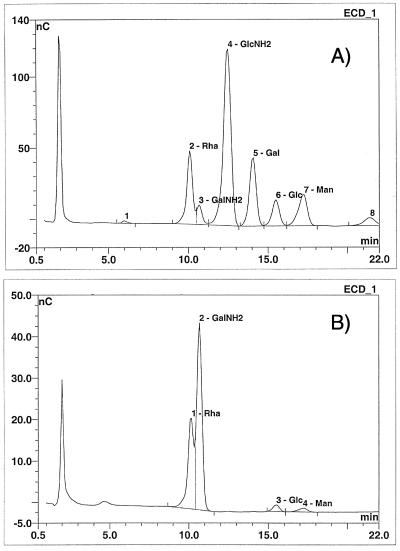
To further investigate the LPG from mutant parasites, the monosaccharide composition of isolated LPGs was determined by Dionex HPAEC-PAD (Table 1). The main monosaccharides resolved from the T. vaginalis parent strain LPG were galactosamine, glucosamine, galactose, glucose, mannose/xylose, and rhamnose (Table 1). The reduced relative levels of galactose and glucosamine in the mutant LPG are consistent with their lack of reactivity with RCA120 and wheat germ agglutinin (Fig. 1). Because the mutant parasite LPGs appear to be truncated (Fig. 3), the large reduction of glucosamine and galactose in the mutant LPGs (>50%) indicates that the majority of these monosaccharides are located within the side chains and/or the terminal cap of the LPG. The reduction of these monosaccharides is accompanied by an increase in galactosamine and glucose. Galactosamine, along with glucosamine is the main component of the glycan core of T. vaginalis LPG (49). The relative increased concentration of galactosamine in the LPG mutants is consistent with its presence primarily in the glycan core and further reinforces that the LPG of the mutants has sustained losses in the LPG side chains or cap.
TABLE 1.
Monosaccharide composition of LPG from parent and mutant parasites expressed as a percentage of total monosaccharides
| Monosaccharidea | % of total monosaccharides
|
||
|---|---|---|---|
| Parent | 2E2 | 4-12 | |
| Rha | 23.4 | 23.9 | 20.0 |
| GalNH2 | 2.3 | 22.0 | 10.9 |
| GlcNH2 | 33.0 | 15.5 | 9.9 |
| Gal | 18.9 | 5.1 | 3.2 |
| Glc | 6.0 | 20.4 | 43.8 |
| Man/Xyl | 16.5 | 13.1 | 12.1 |
Rha, rhamnose; GalNH2, galactosamine; GlcNH2, glucosamine; Gal, galactose; Glc, glucose; Man, mannose; Xyl, xylose.
Although glucose appears to be increased in the mutant LPGs, it is worth noting that glucose is a common environmental contaminant, and therefore this result should be interpreted with caution. An apparent difference in the relative concentration of monosaccharides also exists between the LPG mutants. Mutant 4-12, for example, has about half the amount of galactosamine of mutant 2E2. These differences between the mutants reflect the fact that the mutants were independently selected and suggest that these two strains have mutations in different steps of LPG biosynthesis.
The LPG monosaccharide composition of the parent strain shown in Table 1 differs in two aspects from that previously reported for another T. vaginalis strain (47, 48). One is the higher percentage of mannose/xylose, and second and most notable is the presence of rhamnose, which was not previously reported. Mannose and xylose frequently comigrate on Dionex HPAEC-PAD. Using different conditions to resolve the mannose/xylose peak, we determined that xylose is about 75% and 40% of the combined mannose/xylose peak for the parent and mutant LPGs, respectively. The difference between our results and those published previously for mannose can therefore be accounted for by the presence of xylose, another common contaminant in polysaccharide preparations. Whether the presence of rhamnose in T. vaginalis LPG reflects strain differences is unclear. The high percentage (>20%) of rhamnose strongly argues against its being a contaminant. Furthermore, rhamnose has recently been described in Tritrichomonas foetus LPG (50). Because rhamnose runs adjacent to galactosamine in Dionex HPAEC-PAD, we ran a rhamnose standard ramp and confirmed its presence and abundance in T. vaginalis parent and mutant strain LPGs (Fig. 4).
FIG. 4.
Dionex HPAEC-PAD rhamnose ramp. Chromatogram from HPLC analysis of monosaccharides using CarboPac PA-1 column. Monosaccharides from purified parent LPG (a) or standards (b) were resolved by Dionex HPAEC-PAD to confirm the presence of rhamnose in T. vaginalis LPG. The retention time for rhamnose in the standards coincides with the rhamnose peak in the LPG sample.
Rhamnose is a monosaccharide found in bacteria, some protists, and plants but is absent in mammals (1, 20, 28). It is notable that rhamnose is also not present in Leishmania LPG, the only well-characterized protist LPG (19, 38, 55). Analyses of the T. vaginalis genome database (www.tigr.org/tdb/tgi/tvgi) have revealed putative homologues for the genes encoding the four enzymes required for production and activation of dTDP-l-rhamnose: rmlA, rmlB, rmlC, and rmlD (data not shown), consistent with the presence of rhamnose in T. vaginalis LPG. Unlike RmlA and RmlB, which are shared by different sugar metabolic pathways, RmlC and RmlD, are unique to the synthesis of dTDP-l-rhamnose and dTDP-6-deoxytalose and dTDP-l-rhamnose, respectively. In mycobacteria, both RmlC and RmlD are being exploited as possible targets for drug therapy (7, 37). The uniformity of rhamnose in LPG of both the parent strain and the mutants suggests this monosaccharide is likely to be located in the main backbone or the glycan core, raising the possibility that drug-mediated interference of l-rhamnose synthesis or assembly might disrupt the surface of these parasites, rendering them avirulent (see results below). In addition, disruption of this pathway in bacteria produces less-virulent organisms with truncated or rough lipopolysaccharide, the functional and structural analog to LPG in bacteria (11, 25).
LPG mutants show reduced adherence to vaginal epithelial cells in vitro.
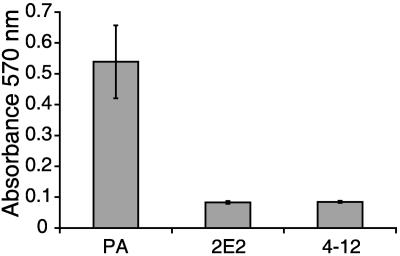
To gain insight into the role of LPG in T. vaginalis pathogenesis, we focused on functional analyses of the LPG mutant parasites. Attachment and cytotoxicity to vaginal epithelial cells are critical for the virulence of this extracellular parasite (21, 43). Moreover, changes in LPG have also been associated with reduced virulence in Leishmania spp. (18, 55, 56). Because in vitro adhesion to plastic has been associated with in vivo virulence of T. vaginalis strains (29), we first tested if the parent and LPG mutants differed in attachment to plastic surfaces. The parasites showed significant differences in attachment to polystyrene surfaces (P < 0.0001; mean ± standard deviation: parent, 0.54 ± 0.118; 2E2, 0.08 ± 0.005; 4-12, 0.08 ± 0.003), with the adherence of the parent strain being > 6x that of the mutants after four hours incubation (Fig. 5). Attachment differences between mutants were minor and not statistically significantly different.
FIG. 5.
Adherence to plastic by parent and LPG mutant parasites. Parent (PA) and LPG mutant parasites were grown to mid-log phase, washed, and resuspended in TYM medium, and 5 × 105 cells were distributed in triplicate in the wells of 24-well tissue culture plates. After 4 h incubation at 37°C and 5% CO2, wells were washed three times and attached parasites were stained with crystal violet. Absorbance was then measured at 570 nm after dissolving the dye in SDS-ethanol. The graph depicts data from a representative experiment with standard deviations.
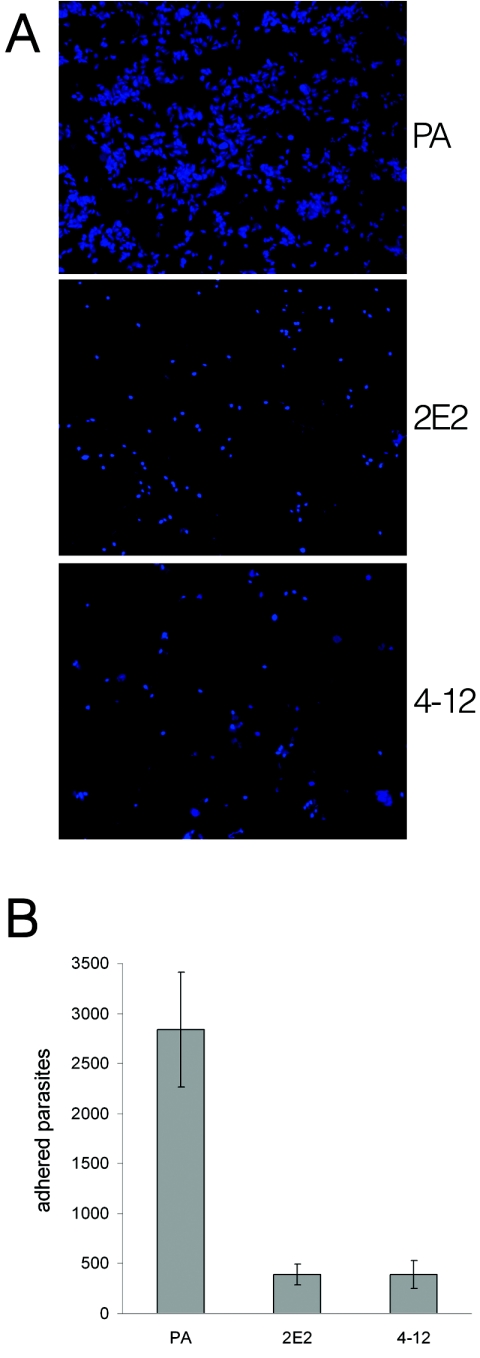
We then tested our T. vaginalis LPG mutants for in vitro attachment to recently established human ectocervical epithelial cells Ect1/E6E7 (23). After 30 min incubation of fluorescence labeled parasites on ectocervical cell monolayers, the parent strain showed a strikingly better attachment to ectocervical cells than the LPG mutants (Fig. 6a). The average number of parent parasites attached to the ectocervical cells per coverslip was seven fold higher than that of the LPG mutants (parent 2,841 ± 570.3 versus 2E2 391 ± 107.7 and 4-12 393 ± 138.5) (Fig. 6b). No significant differences in adhesion to ectocervical cells were observed between mutant parasites. These data indicate that the truncation in the LPG molecules of the mutant strains is responsible for reduced adherence to ectocervical cells, and provides the most direct evidence to date for a role for T. vaginalis LPG in host cell adherence. The human ectocervical epithelial cell line used for these studies, although immortalized, has been shown to express epithelial differentiation proteins and immune mediators characteristic of primary cells from the human ectocervix (22, 23). These results therefore are likely to reflect properties of adherence in vivo (27). It is interesting to note the strong correlation between the ability to adhere to plastic and to ectocervical cells, as previously reported (29).
FIG. 6.
Adherence of parent (PA) and LPG mutant parasites to human ectocervical cell monolayers. Mid-log-phase parasites were labeled with Cell Tracker Blue (Molecular Probes). Labeled parasites were then incubated for 30 min with ectocervical cell monolayers grown on coverslips in 24-well plates at 37°C and 5% CO2. Coverslips were washed to remove nonadherent parasites, mounted, and visualized by fluorescence microscopy (a). Data are from three experiments showing the average number of parasites counted per coverslip with standard deviations (b).
LPG from the parent strain but not the mutant strains inhibits adherence to ectocervical cells.
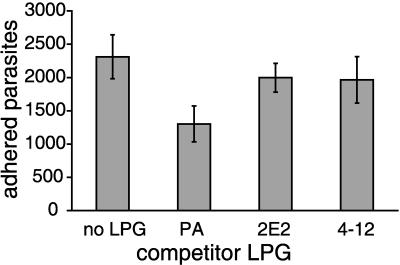
To further demonstrate that LPG plays a critical role in T. vaginalis adherence to host cells, and to determine whether the reduced adherence of the LPG mutant strains to host cells is directly attributable to their modified LPG, we performed competitive inhibition adherence assays. For these experiments, we analyzed the binding of parent parasites to ectocervical cells in the presence of LPG isolated from parent or mutant parasites. Preliminary experiments showed that inhibition of attachment with parent LPG was dose dependent and reached saturation at 150 μg/ml (data not shown). Parent LPG (150 μg/ml) inhibited parasite attachment to ectocervical cells significantly better than 2E2 or 4-12 LPG (Fig. 7). The number of attached parasites was reduced by 44% in the presence of parent LPG compared to the control untreated ectocervical cells. In contrast, 2E2 and 4-12 LPG only reduced parasite binding by 14% and 15%, respectively. These data show that T. vaginalis adherence to ectocervical cells is mediated partially by LPG and that mutation of the LPG renders it incapable of mediating this host-parasite interaction.
FIG. 7.
Adherence of T. vaginalis parent strain to human vaginal ectocervical inhibited by parent (PA) but not mutant LPG. Adherence assays were performed as described in Materials and Methods except ectocervical cells were incubated with 150 μg of purified LPG for 1 h before addition of parasites. The average number of parasites counted per coverslip is shown with standard deviations.
The inability of parent LPG to completely abrogate adherence of the parent strain to epithelial cells indicates that other factors in T. vaginalis are also involved in adherence to epithelial cells. This saturation of binding inhibition with LPG has previously been described for Tritrichomonas foetus (51), where a maximum 46% reduction in parasite binding to bovine vaginal epithelial cells was observed with 100 μg/ml of purified Tritrichomonas foetus LPG. Our binding inhibition data for T. vaginalis LPG closely mirrors the results obtained for Tritrichomonas foetus.
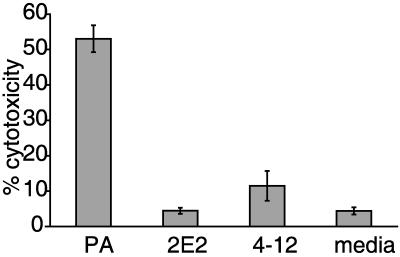
LPG mutants are less cytotoxic to ectocervical cells.
To determine the relative cytotoxicity of the parent strain and LPG mutants to ectocervical cells, an in vitro assay that quantifies the release of lactate dehydrogenase as a measure of host cell intactness was used (Promega). As shown in Fig. 8, parent parasites were over 5 times more cytotoxic to ectocervical cells than mutant parasites. Differences in cytoxicity between the LPG mutants were observed, with mutant 4-12 being twice as cytotoxic as mutant 2E2. This difference is statistically significant and was not expected since the adherence of the mutants to ectocervical cells was comparable (Fig. 6b). This difference in cytotoxicity may reflect limitations of the lactate dehydrogenase assay. The maximum incubation time recommended for this assay by the manufacturer is 9 h, whereas microscopic evaluation of ectocervical cell monolayers coincubated with the parasites overnight show that parent strain parasites completely destroy and ingest the epithelial cell monolayer, while incubation with LPG mutant parasites resulted in almost fully intact monolayers (data not shown). Nonetheless, results from both assays illustrate that LPG plays a critical role in the interaction of T. vaginalis with host cells, as LPG mutants are significantly less cytotoxic to epithelial cells of the reproductive tract than the parent strain.
FIG. 8.
Cytotoxicity of parent (PA) and LPG mutant parasites to ectocervical cells. Parasites were washed and incubated in 48-well tissue culture plates with human ectocervical cell monolayers for 8 h at 37°C and 5% CO2. Release of lactate dehydrogenase in the supernatants from the mammalian cells was determined with the Cytotox ONE kit (Promega), following the manufacturer's instructions. Data from a representative experiment are shown, expressed as percent cytotoxicity calculated from the maximum release of lactate dehydrogenase after total lysis of ectocervical cells.
In summary, these studies comparing parental and mutant LPG on T. vaginalis demonstrate a direct role for LPG in the host cell adherence and cytotoxicity of this extracellular parasite in vitro. Future efforts towards determining the structures of parent and mutant LPGs should shed light on the critical properties of this parasite glycoconjugate required for host cell recognition and binding.
Acknowledgments
This work was supported by a Burroughs Wellcome Scholar Award for Molecular Parasitology (to P.J.J.) and a Microbial Pathogenesis Training Grant (NIAID-T32-AI07323) and NRSA grant (NIAID-F31-AI056659) (to C.Y.O.).
We thank Julio-Cesar Carrero and Dara Chang for technical support, Bradley K. Hayes from the UCSD Glycotechnology Core Resource, San Diego, California, for advice on monosaccharide analyses, Raina Fichorova from Brigham and Women's Hospital, Boston, Massachusetts, for the human ectocervical cells, and Kent Hill for use of the fluorescent microscope. Also, we thank our lab members for helpful discussions.
REFERENCES
- 1.Abreu Filho, B. A., B. P. Dias Filho, A. B. Vermelho, S. I. Jankevicius, J. V. Jankevicius, and R. L. dos Santos. 2001. Surface component characterization as taxonomic tools for Phytomonas spp identification. Parasitol. Res. 87:138-144. [DOI] [PubMed] [Google Scholar]
- 2.Addis, M. F., P. Rappelli, and P. L. Fiori. 2000. Host and tissue specificity of Trichomonas vaginalis is not mediated by its known adhesion proteins. Infect. Immun. 68:4358-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alderete, J. F., R. Arroyo, and M. W. Lehker. 1995. Analysis for adhesins and specific cytoadhesion of Trichomonas vaginalis. Methods Enzymol. 253:407-414. [DOI] [PubMed] [Google Scholar]
- 4.Alderete, J. F., M. Benchimol, M. W. Lehker, and M. L. Crouch. 2002. The complex fibronectin-Trichomonas vaginalis interactions and trichomonosis. Parasitol. Int. 51:285-292. [DOI] [PubMed] [Google Scholar]
- 5.Alderete, J. F., J. Nguyen, V. Mundodi, and M. W. Lehker. 2004. Heme-iron increases levels of AP65-mediated adherence by Trichomonas vaginalis. Microb. Pathog. 36:263-271. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Sanchez, M. E., L. Avila-Gonzalez, C. Becerril-Garcia, L. V. Fattel-Facenda, J. Ortega-Lopez, and R. Arroyo. 2000. A novel cysteine proteinase (CP65) of Trichomonas vaginalis involved in cytotoxicity. Microb. Pathog. 28:193-202. [DOI] [PubMed] [Google Scholar]
- 7.Babaoglu, K., M. A. Page, V. C. Jones, M. R. McNeil, C. Dong, J. H. Naismith, and R. E. Lee. 2003. Novel inhibitors of an emerging target in Mycobacterium tuberculosis; substituted thiazolidinones as inhibitors of dTDP-rhamnose synthesis. Bioorg. Med. Chem. Lett. 13:3227-3230. [DOI] [PubMed] [Google Scholar]
- 8.Beverley, S. M., and S. J. Turco. 1998. Lipophosphoglycan (LPG) and the identification of virulence genes in the protozoan parasite Leishmania. Trends Microbiol. 6:35-40. [DOI] [PubMed] [Google Scholar]
- 9.Bowden, F. J., and G. P. Garnett. 2000. Trichomonas vaginalis epidemiology: parameterising and analysing a model of treatment interventions. Sex Transm. Infect. 76:248-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brugerolle, G., G. Bricheux, and G. Coffe. 2000. Immunolocalization of two hydrogenosomal enzymes of Trichomonas vaginalis. Parasitol. Res. 86: 30-35. [DOI] [PubMed] [Google Scholar]
- 11.Burns, S. M., and S. I. Hull. 1998. Comparison of loss of serum resistance by defined lipopolysaccharide mutants and an acapsular mutant of uropathogenic Escherichia coli O75:K5. Infect. Immun. 66:4244-4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clark, C. G., and L. S. Diamond. 2002. Methods for cultivation of luminal parasitic protists of clinical importance. Clin. Microbiol. Rev. 15:329-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corbeil, L. B., J. L. Hodgson, D. W. Jones, R. R. Corbeil, P. R. Widders, and L. R. Stephens. 1989. Adherence of Tritrichomonas foetus to bovine vaginal epithelial cells. Infect. Immun. 57:2158-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa e Silva Filho, F., W. de Souza, and J. D. Lopes. 1988. Presence of laminin-binding proteins in trichomonads and their role in adhesion. Proc. Natl. Acad. Sci. USA 85:8042-8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cotch, M. F., J. G. Pastorek, R. P. Nugent, S. L. Hillier, R. S. Gibbs, D. H. Martin, D. A. Eschenbach, R. Edelman, J. C. Carey, J. A. Regan, M. A. Krohn, M. A. Klebanoff, A. V. Rao, and G. G. Rhoads. 1997. Trichomonas vaginalis associated with low birth weight and preterm delivery. The Vaginal Infections and Prematurity Study Group. Sex. Transm. Dis. 24:353-360. [DOI] [PubMed] [Google Scholar]
- 16.Crouch, M. L., and J. F. Alderete. 1999. Trichomonas vaginalis interactions with fibronectin and laminin. Microbiology 145:2835-2843. [DOI] [PubMed] [Google Scholar]
- 17.Demirezen, S., Z. Safi, and S. Beksac. 2000. The interaction of trichomonas vaginalis with epithelial cells, polymorphonuclear leucocytes and erythrocytes on vaginal smears: light microscopic observation. Cytopathology 11:326-332. [DOI] [PubMed] [Google Scholar]
- 18.Descoteaux, A., H. A. Avila, K. Zhang, S. J. Turco, and S. M. Beverley. 2002. Leishmania LPG3 encodes a GRP94 homolog required for phosphoglycan synthesis implicated in parasite virulence but not viability. EMBO J. 21:4458-4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Descoteaux, A., Y. Luo, S. J. Turco, and S. M. Beverley. 1995. A specialized pathway affecting virulence glycoconjugates of Leishmania. Science 269:1869-1872. [DOI] [PubMed] [Google Scholar]
- 20.Dong, C., K. Beis, M. F. Giraud, W. Blankenfeldt, S. Allard, L. L. Major, I. D. Kerr, C. Whitfield, and J. H. Naismith. 2003. A structural perspective on the enzymes that convert dTDP-d-glucose into dTDP-l-rhamnose. Biochem. Soc. Trans. 31:532-536. [DOI] [PubMed] [Google Scholar]
- 21.Escario, J. A., B. A. Gomez, and A. R. Martinez Fernandez. 1995. The relationship of experimental pathogenicity in vivo with in vitro cytoadherence and cytotoxicity of 6 different isolates of Trichomonas vaginalis. Int. J. Parasitol. 25:999-1000. [DOI] [PubMed] [Google Scholar]
- 22.Fichorova, R. N., and D. J. Anderson. 1999. Differential expression of immunobiological mediators by immortalized human cervical and vaginal epithelial cells. Biol. Reprod. 60:508-514. [DOI] [PubMed] [Google Scholar]
- 23.Fichorova, R. N., J. G. Rheinwald, and D. J. Anderson. 1997. Generation of papillomavirus-immortalized cell lines from normal human ectocervical, endocervical, and vaginal epithelium that maintain expression of tissue-specific differentiation proteins. Biol. Reprod. 57:847-855. [DOI] [PubMed] [Google Scholar]
- 24.Fiori, P. L., P. Rappelli, and M. F. Addis. 1999. The flagellated parasite Trichomonas vaginalis: new insights into cytopathogenicity mechanisms. Microbes. Infect. 1:149-156. [DOI] [PubMed] [Google Scholar]
- 25.Gao, M., W. D'Haeze, R. De Rycke, B. Wolucka, and M. Holsters. 2001. Knockout of an azorhizobial dTDP-l-rhamnose synthase affects lipopolysaccharide and extracellular polysaccharide production and disables symbiosis with Sesbania rostrata. Mol. Plant-Microbe Interact. 14:857-866. [DOI] [PubMed] [Google Scholar]
- 26.Gardner, W. A., Jr., D. E. Culberson, and B. D. Bennett. 1986. Trichomonas vaginalis in the prostate gland. Arch. Pathol. Lab. Med. 110:430-432. [PubMed] [Google Scholar]
- 27.Gilbert, R. O., G. Elia, D. H. Beach, S. Klaessig, and B. N. Singh. 2000. Cytopathogenic effect of Trichomonas vaginalis on human vaginal epithelial cells cultured in vitro. Infect. Immun. 68:4200-4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giraud, M. F., and J. H. Naismith. 2000. The rhamnose pathway. Curr. Opin. Struct. Biol. 10:687-696. [DOI] [PubMed] [Google Scholar]
- 29.Gold, D. 1993. Trichomonas vaginalis: strain differences in adhesion to plastic and virulence in vitro and in vivo. Parasitol. Res. 79:309-315. [DOI] [PubMed] [Google Scholar]
- 30.Gold, D., and I. Ofek. 1992. Adhesion of Trichomonas vaginalis to plastic surfaces: requirement for energy and serum constituents. Parasitology 105:55-62. [DOI] [PubMed] [Google Scholar]
- 31.Guha-Niyogi, A., D. R. Sullivan, and S. J. Turco. 2001. Glycoconjugate structures of parasitic protozoa. Glycobiology 11:45R-59R. [DOI] [PubMed] [Google Scholar]
- 32.Hollander, D. H. 1976. Colonial morphology of Trichomonas vaginalis in agar. J. Parasitol. 62:826-828. [PubMed] [Google Scholar]
- 33.Johnson, K. G., and M. B. Perry. 1976. Improved techniques for the preparation of bacterial lipopolysaccharides. Can. J. Microbiol. 22:29-34. [DOI] [PubMed] [Google Scholar]
- 34.King, D. L., Y. D. Chang, and S. J. Turco. 1987. Cell surface lipophosphoglycan of Leishmania donovani. Mol. Biochem. Parasitol. 24:47-53. [DOI] [PubMed] [Google Scholar]
- 35.King, D. L., and S. J. Turco. 1988. A ricin agglutinin-resistant clone of Leishmania donovani deficient in lipophosphoglycan. Mol. Biochem. Parasitol. 28:285-293. [DOI] [PubMed] [Google Scholar]
- 36.Lyons, E. J., and J. M. Carlton. 2004. Mind the gap: bridging the divide between clinical and molecular studies of the trichomonads. Trends Parasitol. 20:204-207. [DOI] [PubMed] [Google Scholar]
- 37.Ma, Y., R. J. Stern, M. S. Scherman, V. D. Vissa, W. Yan, V. C. Jones, F. Zhang, S. G. Franzblau, W. H. Lewis, and M. R. McNeil. 2001. Drug targeting Mycobacterium tuberculosis cell wall synthesis: genetics of dTDP-rhamnose synthetic enzymes and development of a microtiter plate-based screen for inhibitors of conversion of dTDP-glucose to dTDP-rhamnose. Antimicrob. Agents Chemother. 45:1407-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McConville, M. J., L. F. Schnur, C. Jaffe, and P. Schneider. 1995. Structure of Leishmania lipophosphoglycan: inter- and intra-specific polymorphism in Old World species. Biochem. J. 310:807-818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mendoza-Lopez, M. R., C. Becerril-Garcia, L. V. Fattel-Facenda, L. Avila-Gonzalez, M. E. Ruiz-Tachiquin, J. Ortega-Lopez, and R. Arroyo. 2000. CP30, a cysteine proteinase involved in Trichomonas vaginalis cytoadherence. Infect. Immun. 68:4907-4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Minkoff, H., A. N. Grunebaum, R. H. Schwarz, J. Feldman, M. Cummings, W. Crombleholme, L. Clark, G. Pringle, and W. M. McCormack. 1984. Risk factors for prematurity and premature rupture of membranes: a prospective study of the vaginal flora in pregnancy. Am. J. Obstet. Gynecol. 150:965-972. [DOI] [PubMed] [Google Scholar]
- 41.Mirhaghani, A., and A. Warton. 1998. Involvement of Trichomonas vaginalis surface-associated glycoconjugates in the parasite/target cell interaction. A quantitative electron microscopy study. Parasitol. Res. 84:374-381. [DOI] [PubMed] [Google Scholar]
- 42.North, M. J. 1994. Cysteine endopeptidases of parasitic protozoa. Methods Enzymol. 244:523-539. [DOI] [PubMed] [Google Scholar]
- 43.Petrin, D., K. Delgaty, R. Bhatt, and G. Garber. 1998. Clinical and microbiological aspects of Trichomonas vaginalis. Clin. Microbiol. Rev. 11:300-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reimer, T., N. Ulfig, and K. Friese. 1999. Antibiotics: treatment of preterm labor. J. Perinat. Med. 27:35-40. [DOI] [PubMed] [Google Scholar]
- 45.Rosset, I., T. Tasca, P. M. Tessele, and G. A. De Carli. 2002. Scanning electron microscopy in the investigation of the in vitro hemolytic activity of Trichomonas vaginalis. Parasitol. Res. 88:356-359. [DOI] [PubMed] [Google Scholar]
- 46.Sacks, D. L., G. Modi, E. Rowton, G. Spath, L. Epstein, S. J. Turco, and S. M. Beverley. 2000. The role of phosphoglycans in Leishmania-sand fly interactions. Proc. Natl. Acad. Sci. USA 97:406-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh, B. N. 1994. The existence of lipophosphoglycanlike molecules in Trichomonads. Parasitol. Today 10:152-154. [DOI] [PubMed] [Google Scholar]
- 48.Singh, B. N. 1993. Lipophosphoglycan-like glycoconjugate of Tritrichomonas foetus and Trichomonas vaginalis. Mol. Biochem. Parasitol. 57:281-294. [DOI] [PubMed] [Google Scholar]
- 49.Singh, B. N., D. H. Beach, D. G. Lindmark, and C. E. Costello. 1994. Identification of the lipid moiety and further characterization of the novel lipophosphoglycan-like glycoconjugates of Trichomonas vaginalis and Trichomonas foetus. Arch. Biochem. Biophys. 309:273-280. [DOI] [PubMed] [Google Scholar]
- 50.Singh, B. N., R. H. BonDurant, C. M. Campero, and L. B. Corbeil. 2001. Immunological and biochemical analysis of glycosylated surface antigens and lipophosphoglycan of Tritrichomonas foetus. J. Parasitol. 87:770-777. [DOI] [PubMed] [Google Scholar]
- 51.Singh, B. N., J. J. Lucas, D. H. Beach, S. T. Shin, and R. O. Gilbert. 1999. Adhesion of Tritrichomonas foetus to bovine vaginal epithelial cells. Infect. Immun. 67:3847-3854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sorvillo, F., A. Kovacs, P. Kerndt, A. Stek, L. Muderspach, and L. Sanchez-Keeland. 1998. Risk factors for trichomoniasis among women with human immunodeficiency virus (HIV) infection at a public clinic in Los Angeles County, California: implications for HIV prevention. Am. J. Trop. Med. Hyg. 58:495-500. [DOI] [PubMed] [Google Scholar]
- 53.Sorvillo, F., L. Smith, P. Kerndt, and L. Ash. 2001. Trichomonas vaginalis, HIV, and African-Americans. Emerg. Infect. Dis. 7:927-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spath, G. F., L. Epstein, B. Leader, S. M. Singer, H. A. Avila, S. J. Turco, and S. M. Beverley. 2000. Lipophosphoglycan is a virulence factor distinct from related glycoconjugates in the protozoan parasite Leishmania major. Proc. Natl. Acad. Sci. USA 97:9258-9263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spath, G. F., L. A. Garraway, S. J. Turco, and S. M. Beverley. 2003. The role(s) of lipophosphoglycan (LPG) in the establishment of Leishmania major infections in mammalian hosts. Proc. Natl. Acad. Sci. USA 100:9536-9541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turco, S. J., G. F. Spath, and S. M. Beverley. 2001. Is lipophosphoglycan a virulence factor? A surprising diversity between Leishmania species. Trends Parasitol. 17:223-226. [DOI] [PubMed] [Google Scholar]
- 57.World Health Organization. 1996. Sexually transmitted diseases fact sheet no. 110. World Health Organization, Geneva, Switzerland.