Abstract
Multinodular goiter (MNG) is a common disorder characterized by a nodular enlargement of the thyroid gland and occurring with a female:male ratio of 5:1. This article reports the analysis of an Italian three-generation pedigree MNG, including 10 affected females and 2 affected males. After linkage to candidate regions previously implicated in various forms of goiter was excluded, a novel MNG locus was searched. Because no male-to-male transmission was present in the study pedigree, an X-linked autosomal dominant pattern of inheritance was hypothesized. Therefore, 18 markers spaced at 10-cM intervals on the X chromosome were examined. A significant LOD score was observed in the Xp22 region, where marker DXS1226 generated a maximum LOD score of 4.73 at a recombination fraction of 0. Analysis of six flanking microsatellites confirmed these data, and haplotype inspection delimited a 9.6-cM interval lying between DXS1052 and DXS8039.
Nontoxic thyroid goiter is a common disorder characterized by a diffuse or nodular enlargement of the thyroid gland (multinodular goiter [MNG] [MIM 138800]), an enlargement that is not the result of an inflammatory or neoplastic process. It is generally agreed that the disease represents a compensatory response to the impairment of thyroid hormonogenesis: as a consequence of the increases in thyroid mass and unit functional activity, a normal rate of hormonal secretion is restored, so that the patient is eumetabolic but goitrous (Ingbar and Woeber, 1968). The incidence of MNG is dependent on sex (female:male ratio 5:1) and on the iodine intake of the population (World Health Organization 1993, p. 1). Although MNG incidence may be >10% in endemic areas, the persistence of MNG in regions with sufficient iodine intake has been documented in several reports, which supports the notion of a genetic basis for the disease (Greig et al. 1967). Moreover, several authors have reported goiter families with vertical and/or male-to-male transmission, suggesting an autosomal dominant susceptibility (Murray et al. 1966; Bignell et al. 1997; Neumann et al. 1999). Actually, the genetic analysis of one Canadian pedigree, showing no skewed female:male ratio, has allowed the assignment of the MNG1 locus to chromosome 14q (Bignell et al. 1997). This location has subsequently been confirmed in a German pedigree with similar features (Neumann et al. 1999). In addition, the molecular characterization of many forms of goiter has revealed the presence of mutations in several genes involved in thyroid hormonogenesis. In particular, mutations have been detected in thyroid peroxidase, thyroglobulin, sodium iodide symporter member 5, thyrotropin receptor, and pendrin genes (Ieiri et al. 1991; Abramowicz et al. 1992; Corral et al. 1993; van Sande et al. 1995; Everett et al. 1997; Fujiwara et al. 1997).
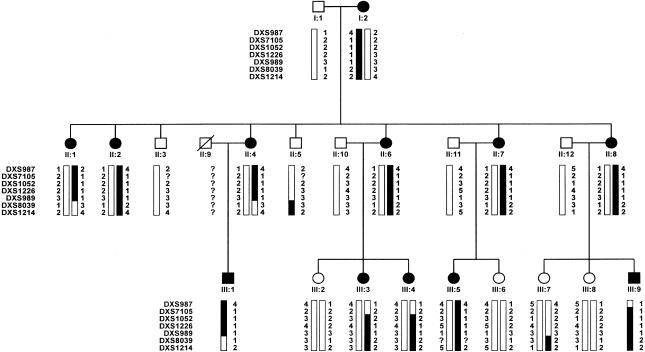
We report the linkage analysis of a three-generation sex-biased Italian pedigree with MNG, leading to the assignment of a novel MNG locus on chromosome Xp22. The examined pedigree included 22 individuals (fig. 1), whose clinical classification was based on both palpation and ultrasonographic examination. Thyroid ultrasound examination was performed using real-time instruments (model AU5 Harmonic; ESAOTE) and a 7.5-MHz linear transducer. Thyroid volume was calculated according to the formula of the ellipsoid model (width × length × thickness × .52) for each lobe. The propositus (subject II:1 in fig. 1) was a 41-year-old woman living in the mildly endemic goiter area of Montalbano Jonico (Matera, Italy). She carried an MNG (three nodules) and was euthyroid at diagnosis. No thyroid autoantibodies were detected. The parents of the propositus were nonconsanguineous; the mother and all five sisters were affected by either MNG or diffuse goiter (only one individual), whereas both brothers were unaffected. In the third generation, five of nine subjects were affected. One of them (III:4) displayed low positivity for antithyroperoxidase antibodies. A total of 12 affected subjects (10 females and 2 males) were identified. The mean age at onset was 20.6 years (range 6–40 years). Thyroid volume was 22–66 ml in adult patients. In adolescent patients, the thyroid volume was 14–16 ml. Thyroid hormone levels were within the normal range in all members of the family. In addition, all family members had normal somatic and intellectual development and showed no signs of hypothyroidism.
Figure 1 .
Pedigree of family with multinodular goiter (MNG). Blackened bars denote at-risk haplotypes.
Genomic DNA was obtained from peripheral blood lymphocytes, by use of standard phenol-chloroform extraction methods. In a first phase, we tested linkage at the MNG1 locus and at several regions containing genes implicated in the biosynthesis of thyroid hormone. The latter candidate loci included the deiodinase I gene on chromosome 1p32 (Mandel et al. 1992), the thyroperoxidase gene on 2p24 (Abramowicz et al. 1992), the pendrin gene on 7q31 (Everett et al. 1997), the thyroglobulin gene on 8q24 (Ieiri et al. 1991), the genes for thyrotropin receptor (van Sande et al. 1995) and deiodinase III (Salvatore et al. 1995) on 14q31, the deiodinase II gene (Croteau et al. 1996) on 14q24, the thyroid transcription factor 1 gene on 14q13 (Ikeda et al. 1995), and the sodium iodide symporter gene on chromosome 19p13 (Fujiwara et al. 1997). Pairs of microsatellite markers flanking each candidate locus were selected for genotyping, on the basis of the map locations reported at the GeneCard Web site. Markers D14S62 and D14S68 were used to test linkage at the MNG1 locus. Two-point LOD scores were calculated using the MLINK program from the LINKAGE (version 5.2) package (Lathrop and Laouel 1984). The model used assumed an autosomal dominant trait (frequency .02) segregating with .95 penetrance. This analysis generated LOD scores <−3.00 at recombination fraction (θ) 0, for all loci examined (data not shown but available, on request, from the corresponding author).
At this stage, we undertook the search for a novel MNG locus. Because the pedigree showed an excess of affected females and presented no instance of male-to-male transmission, we hypothesized an X-linked autosomal dominant pattern of inheritance. Therefore, we did not analyze any additional autosomal region but, instead, typed 18 markers spaced at ∼10-cM intervals on the X chromosome, with the use of primers from the ABI-Prism linkage mapping set (P-E Biosystems). We used the MLINK and LINKMAP programs to compute two-point and multipoint LOD scores, under the assumption of X-linked autosomal dominant inheritance with .95 penetrance. A frequency of 1/n was used for each marker allele, where n is the number of alleles at the examined locus. Although this assumption is unlikely to hold true in the examined population, a misspecification of allele frequencies is not expected to cause errors in the linkage analysis of the pedigree, in which genotypes are available for all the affected and disease-transmitting individuals. Indeed, we simulated a set of various marker-allele frequencies and always obtained the same two-point LOD scores. Because of the constraints imposed by the amount of computer memory available, only genotypes of loci DXS1052, DXS1226, DXS989 and DXS8039 were used for multipoint analysis.
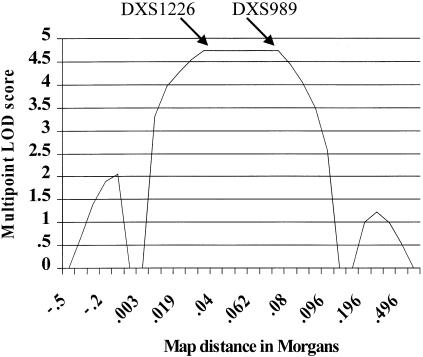
A significant two-point LOD score was observed in the Xp22 region, where marker DXS1226 generated a maximum LOD score (Zmax) of 4.73 at θ = 0 (table 1). The analysis of six flanking microsatellites confirmed these data (table 1). Haplotype inspection disclosed key recombination events between DXS1052 and DXS1226, in individuals III:3 and III:4, thereby defining the telomeric boundary of the disease interval (fig. 1). The centromeric limit is determined by a crossover, between DXS989 and DXS8039, that was observed in subjects II:4 and III:1 (fig. 1). Therefore, the MNG locus lies within the 9.6-cM region delimited by the DXS1052 and DX8039 markers. As expected, the multipoint LOD-score curve peaks within this interval (fig. 2), further confirming the disease-locus position.
Table 1.
Two-Point LOD Scores between the MNG Disease Locus and Seven Markers Mapping to Chromosome Xp22
| LOD Score at θ = |
|||||
| Marker | .00 | .05 | .1 | .2 | .3 |
| DXS987 | −∞ | −1.74 | −.65 | .17 | .34 |
| DXS7105 | −∞ | 1.24 | 1.54 | 1.50 | 1.11 |
| DXS1052 | −∞ | 1.80 | 2.05 | 1.90 | 1.40 |
| DXS1226 | 4.73 | 4.36 | 3.97 | 3.10 | 2.13 |
| DXS989 | 4.73 | 4.36 | 3.97 | 3.10 | 2.13 |
| DXS8039 | −∞ | −1.54 | −.45 | .07 | .14 |
| DXS1214 | −∞ | −3.88 | −2.47 | −1.17 | −.52 |
Figure 2 .
Multipoint LOD-score analysis of four markers spanning the MNG locus on chromosome Xp22. Arrows indicate the position of DXS1226 and DXS989.
Genes in the disease region include those encoding dystrophin (DMD), sperimidin acetyl transferase (SAT), pyruvate dehydrogenase kinase-3 (PDK3), DNA polymerase α (POLA), and zinc-finger protein X-linked (ZFX). However, none seems an obvious candidate gene for the MNG phenotype. On the other hand, the disease region also harbors several mapped expressed sequence tags (ESTs), whose characterization may identify more-attractive candidate genes.
Our data indicate that the MNG phenotype observed in this pedigree is not due to the impairment of any gene known to be directly involved in thyroid hormonogenesis. This finding is consistent both with the fact that this biochemical pathway has not been fully elucidated and with the general agreement that additional proteins are likely to be involved. In this context, the genetic characterization of extended pedigrees with goiter can become a powerful tool for the identification of such proteins. This approach has already proved successful, since its application to pedigrees with Pendred syndrome has led to the isolation of the pendrin gene (PDS), which encodes a transmembrane protein that transports iodide and chloride (Everett et al. 1997; Scott et al. 1999). Therefore, we are endeavoring to recruit additional extended pedigrees with X-linked MNG, which may allow us to refine the disease-locus position. Although MNG has proved to be a genetically heterogeneous disease, the exclusion of families showing male-to-male transmission and no sex bias among the affected group is expected to eliminate most non–X-linked pedigrees from our sample. In this context, the isolation of the MNG gene, mapping to the Xp22 locus, may become feasible by a combination of positional cloning (further refinement of disease locus) and positional-candidate strategies (characterization of Xp22 ESTs).
Acknowledgments
We thank the following colleagues for providing continuous help and advice on study design and organization: M. Andreoli, A. Pontecorvi, F. Orlandi, D. Meringolo, and E. Papini (Goiter Genetics Group). This work was funded by Italian Telethon grant E1031 and the Ministry of Health.
Electronic-Database Information
The accession number and URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for multinodular goiter [MIM 138800])
- UDB, The Unified Database for Human Genome Mapping, http://bioinformatics.weizmann.ac.il/udb/
References
- Abramowicz MJ, Tarkovnik HM, Varela V, Cochaux P, Krawiec L, Pisarev MA, Propato FVE, Juvenal G, Chester HA, Vassart G (1992) Identification of a mutation in the coding sequence of the human thyroid peroxidase gene causing congenital goiter. J Clin Invest 90:1200–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignell GR, Canzian F, Shayeghi M, Stark M, Shugart YY, Biggs P, Mangion J, Hamoudi R, Rosenblatt J, Buu P, Sun S, Stoffer SS, Goldgar DE, Romeo G, Houlston RS, Narod SA, Stratton MR, Foulkes WD (1997) Familial nontoxic multinodular thyroid goiter locus maps to chromosome 14q but does not account for familial nonmedullary thyroid cancer. Am J Hum Genet 61:1123–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corral J, Martin C, Perez R, Sanchez I, Mories MT, San Millan JL, Miralles JM, Gonzalez-Sarmiento R (1993) Thyroglobulin gene point mutation associated with non-endemic simple goitre. Lancet 341: 462–464 [DOI] [PubMed] [Google Scholar]
- Croteau W, Davey JC, Galton VA, St German DL (1996) Cloning of the mammalian type II iodothyronine deiodinase: a selenoprotein differentially expressed and regulated in human and rat brain and other tissues. J Clin Invest 98:405–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett LA, Glaser B, Beck JC, Idol JR, Buchs A, Heyman M, Adawi F, Hazani E, Nassir E, Baxevanis AD, Sheffield VC, Green ED (1997) Pendred syndrome is caused by mutations in a putative sulphate transporter gene (PDS). Nat Genet 17:411–422 [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Tatsumi K, Miki K, Harada T, Miyai K, Takai S, Amino N (1997) Congenital hypothyroidism caused by a mutation in the Na+/I− symporter. Nat Genet 16:124–125 [DOI] [PubMed] [Google Scholar]
- Greig WR, Boyle JA, Duncan A (1967) Genetic and non-genetic factors in simple goitre formation: evidence from a twin study. Q J Med 36:175–188 [PubMed] [Google Scholar]
- Ieiri T, Cocheaux P, Targovnik HM, Suzuki M, Shimoda S, Perret J, Vassart G (1991) A 3′ splice site mutation in the thyroglobulin gene responsible for congenital goiter with hypothyroidism. J Clin Invest 88:1901–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Clark JC, Shaw-White JR, Stahlman MT, Boutell CJ, Whitsett JA (1995) Gene structure and expression of human thyroid transcription factor 1 in respiratory epithelial cells. J Biol Chem 270:8108–8114 [DOI] [PubMed] [Google Scholar]
- Ingbar SH, Woeber KA (1968) The thyroid gland. In: Williams RH (ed) Textbook of endocrinology. WB Saunders, Philadelphia, pp 105–279 [Google Scholar]
- Lathrop GM, Lalouel JM (1984) Easy calculations of LOD scores and genetic risks on small computers. Am J Hum Genet 36:460–465 [PMC free article] [PubMed] [Google Scholar]
- Mandel SJ, Berry MJ, Kieffer JD, Harney JW, Warne RL, Larsen PR (1992) Cloning and in vitro expression of the human selenoprotein, type I iodothyronine deiodinase. J Clin Endocrinol Metab 75:1133–1139 [DOI] [PubMed] [Google Scholar]
- Murray IP, Thomson JA, McGirr EM, MacDonald EM, Kennedy JS, McLennan I (1966) Unusual familial goiter associated with intrathyroidal calcification. J Clin Endocrinol Metab 26:1039–1049 [DOI] [PubMed] [Google Scholar]
- Neumann S, Willgerodt H, Ackermann F, Reske A, Jung M, Reis A, Paschke R (1999) Linkage of familial euthyroid goiter–1 locus and exclusion of the candidate genes thyroglobulin, thyroperoxidase, and Na+/I− symporter. J Clin Endocrinol Metab 84:3750–3756 [DOI] [PubMed] [Google Scholar]
- Salvatore D, Low SC, Berry M, Maia AL, Harney JW, Croteau W, St German DL, Larsen PR (1995) Type 3 iodothyronine deiodinase: cloning, in vitro expression, and functional analysis of the placental selenoenzyme. J Clin Invest 96:2421–2430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott DA, Wang R, Kreman TM, Sheffield VC, Karnishki LP (1999) The Pendred syndrome gene encodes a chloride-iodide transport protein. Nat Genet 21:440–443 [DOI] [PubMed] [Google Scholar]
- van Sande J, Parma J, Tonacchera M, Swillens S, Dumont J, Vassart G (1995) Genetic basis of endocrine disease: somatic and germline mutations of the TSH receptor gene in thyroid diseases. J Clin Endocrinol Metab 80:2577–2585 [DOI] [PubMed] [Google Scholar]
- World Health Organization (1993) Global prevalence of iodine deficiency disorders. Micronutrient Deficiency Information System working paper 1. WHO-Nutrition Unit, Geneva [Google Scholar]