Abstract
Stargardt disease (STGD) is a common autosomal recessive maculopathy of early and young-adult onset and is caused by alterations in the gene encoding the photoreceptor-specific ATP-binding cassette (ABC) transporter (ABCA4). We have studied 144 patients with STGD and 220 unaffected individuals ascertained from the German population, to complete a comprehensive, population-specific survey of the sequence variation in the ABCA4 gene. In addition, we have assessed the proposed role for ABCA4 in age-related macular degeneration (AMD), a common cause of late-onset blindness, by studying 200 affected individuals with late-stage disease. Using a screening strategy based primarily on denaturing gradient gel electrophoresis, we have identified in the three study groups a total of 127 unique alterations, of which 90 have not been previously reported, and have classified 72 as probable pathogenic mutations. Of the 288 STGD chromosomes studied, mutations were identified in 166, resulting in a detection rate of ∼58%. Eight different alleles account for 61% of the identified disease alleles, and at least one of these, the L541P-A1038V complex allele, appears to be a founder mutation in the German population. When the group with AMD and the control group were analyzed with the same methodology, 18 patients with AMD and 12 controls were found to harbor possible disease-associated alterations. This represents no significant difference between the two groups; however, for detection of modest effects of rare alleles in complex diseases, the analysis of larger cohorts of patients may be required.
Introduction
Stargardt disease (STGD [MIM 248200]) is an autosomal recessive macular dystrophy causing progressive impairment of central vision, with onset typically in childhood or young adulthood. Affected individuals display atrophic macular lesions, as well as characteristic yellowish flecks in the macular and perimacular region (Stargardt 1909). Recently, the photoreceptor cell–specific ATP-binding cassette transporter (ABCA4, formerly ABCR [MIM 601691]) gene was identified and found to be mutated in patients with STGD (Allikmets et al. 1997b). ABCA4 codes for a 2,273-amino-acid protein, previously characterized as the photoreceptor rim protein (Papermaster et al. 1978) and localizing to the rims of the rod and cone outer-segment disks (Sun and Nathans 1997; Molday et al. 2000). A member of the ABC-transporter superfamily of proteins, which are involved in the energy-dependent transport of substrates across membranes, ABCA4 is thought to flip N-retinylidene-phosphatidylethanolamine from the lumenal to the cytosolic face of the photoreceptor disks (Sun et al. 1999; Weng et al. 1999).
Several series of mutation analyses have confirmed that ABCA4 is the gene underlying STGD and have initiated a characterization of the mutational spectrum (Nasonkin et al. 1998; Rozet et al. 1998; Fishman et al. 1999; Lewis et al. 1999; Maugeri et al. 1999; Papaioannou et al. 2000; Simonelli et al. 2000). Overall, in patients with STGD, standard techniques based primarily on SSCP screening or heteroduplex analysis, followed by direct DNA sequencing of aberrant fragments, identify mutations in ∼60% of ABCA4 alleles (e.g., see Lewis et al. 1999; Maugeri et al. 1999). The majority of mutations are missense, followed by nonsense mutations, small insertions/deletions, and mutations affecting RNA splicing. Certain mutant alleles—for example, G863A, A1038V, and G1961E—appear to be more common and may have altered frequencies in different populations, as a result of founder effect (Maugeri et al. 1999; Simonelli et al. 2000).
ABCA4 has also been evaluated as a possible cause for other diseases with similar pathology in the macula. Gene mutations have been observed in families manifesting cone-rod dystrophy (CRD) and/or atypical retinitis pigmentosa (RP) (Cremers et al. 1998; Martinez-Mir et al. 1998). Of great interest has been an analysis that noted an increased frequency of heterozygous ABCA4 alterations in individuals with age-related macular degeneration (AMD) (Allikmets et al. 1997a), a common multifactorial condition in the elderly population (Bressler et al. 1988). This investigation has been the subject of dispute (Dryja et al. 1998; Klaver et al. 1998), and a subsequent study, comparable in scope, refuted an association (Stone et al. 1998). Taking an alternate approach, Souied et al. (2000) analyzed the segregation of ABCA4 variants in 52 multiplex cases of AMD and concluded that they may be a predisposing factor in ∼4% of cases. Recently, an international consortium screening for two common variants in the ABCA4 gene in >1,200 patients with AMD and as many controls confirmed a significantly increased frequency of both alterations in the group with AMD (Allikmets and The International ABCR Screening Consortium 2000). Given the substantial burden of this disease, with 7.1% of individuals age >75 years affected with late-stage AMD (Klein et al. 1992) and an estimated mutant ABCA4-heterozygote frequency of 2%–3% in the general population (Dean et al. 1998), the clarification of the role of ABCA4 in AMD becomes a critical issue.
We have undertaken a comprehensive analysis of the ABCA4 gene in 144 unrelated patients with STGD, 200 unrelated patients with AMD, and 220 control individuals, all ascertained from the German population. Under an identical screening protocol for each group, we have carefully catalogued the sequence variations both in disease alleles and in normal alleles. This forms a population-specific base of data that will increase the diagnostic and prognostic value of molecular-genetic analysis in STGD and will provide additional information on the issue of ABCA4 involvement in the pathogenesis of AMD.
Subjects and Methods
Subjects
A total of 144 individuals with STGD were included in the study. All patients are unrelated, with the exception of two (STGD139 and STGD139b), a mother-daughter pair demonstrating pseudodominant inheritance because of consanguinity in the family. Each patient has been evaluated by one of the authors (H.P.N.S, E.A.-S., B.L., or B.J.), at the Eye Care Centre, Vancouver (by D. Walker), or at the Eye Clinic Benjamin Franklin, Berlin (by U. Kellner). Examination included ascertainment of personal and family history, measurement of visual acuity, fundus examination, electroretinography, and, in some cases, fluorescein angiography. The diagnosis of STGD was based on the demonstration of bilateral impairment of central vision and the appearance of perimacular and/or peripheral yellow-white flecks, with or without atrophy of the central retinal-pigment epithelium and a normal or only mildly abnormal flash electroretinogram when recorded in early stages of the disease. In addition, for all patients in whom both disease alleles were identified, blood samples from the parents and from other affected family members were obtained, whenever possible.
The AMD study group consists of 200 individuals from two different geographic regions of Germany (Heidelberg and Münster). Diagnosis of AMD was based on an international classification and grading system (Bird et al. 1995). The group represents a broad clinical spectrum, with 100 individuals having geographic atrophy (“dry” AMD) and 100 individuals having exudative (“wet”) AMD, and has been described elsewhere (Krämer et al. 2000). The average age of the group with AMD is 75.6 years (range 55–93 years). We selected as a control group 153 unaffected individuals matched for age (mean 76.2 years, range 34–102 years) and geographic region. All were seen in the same clinics as the patients with AMD, but for nonretinal disease. After examination of the fundus, only individuals with fewer than five hard drusen and no other signs of AMD were included. A further 67 unselected, population-based controls (mean age 51.2 years, range 21–87 years) were included, bringing the total number of individuals in the control group to 220. Each of the three study groups reflects the current ethnic mixture present in the German population, in which the representation by individuals of other nationalities is ∼10% (Statistisches Bundesamt Deutschland).
Mutation Analysis
All 50 exons of the ABCA4 gene were screened using a combination of denaturing gradient gel electrophoresis (DGGE) (Fodde and Losekoot 1994), denaturing high-performance liquid chromatography (dHPLC) (Liu et al. 1998), and SSCP analysis (Orita et al. 1989). For each sample, genomic DNA was isolated from peripheral blood leukocytes, according to standard protocols. The individual coding exons and flanking intron sequences were PCR amplified with the oligonucleotide primers and conditions listed in table 1. Primer sequences published elsewhere (Allikmets et al. 1997b) are identifiable by name. For each exon analyzed by DGGE, GC clamps (5′-CGC CCG CCG CGC CCC GCG CCC GTC CCG CCG CCC CCG CCC G-3′) were applied to either the forward or the reverse primer. Reactions were carried out for 33 cycles in a 1.0- or 1.5-mM MgCl2-containing buffer with or without 4% formamide.
Table 1.
Oligonucleotide Primers and Conditions
|
Forward Primer |
Reverse Primer |
|||||
| AnalysisandExon | Namea | Sequence(5′–3′) | Namea | Sequence(5′–3′) | AnnealingTemperature(°C) | MgCl2(mM)b |
| DGGE: | ||||||
| 1 | ABCR1-For | AATCTGGTCTTCGTGTGGTC | ABCR1-GC | GTTTATTTGCTCCACACCTC | 58 | 1.0+ |
| 2 | ABCR2-GC | AATCTCTTAGCACCACTGAAC | ABCR2-Rev | AGGCCCAGACCAAAGTCTC | 58 | 1.0− |
| 3 | ABCR3-For | CCTGCTTGGTCTCCATGAC | ABCR3-GC | ACGTGAAGGGGTGTGCAAC | 57 | 1.0+ |
| 4 | ABCR4-GC2 | CCTTATTAATGAGGCTTTGTC | ABCR4-Rev2 | ATAGGTGAGGGAAATGATGC | 57 | 1.5+ |
| 5 | ABCR5-GC | CCATTTCCCCTTCAACACCC | ABCR5-Rev | GTGCTTCCCTCCCCTCCAG | 58 | 1.0+ |
| 6 | ABCR6-For | CTACCACAGGGCAGTTTCTA | ABCR6-GC | CAGGAATCACCTTGCAATTG | 58 | 1.0+ |
| 7 | ABCR7-GC | GATCAGACTG TGCCTATGTG | ABCR7-Rev | ATAAGTGGGGTAAATGGTGG | 57 | 1.0+ |
| 9 | ABCR9-GC | AGGTTACAAGCAATGGGGAG | ABCR9-Rev | TCTGGGAGGTCCAGGGTAC | 58 | 1.0− |
| 12 | ABCR12-For | AGTTGAGTCTTTGCAGTTGG | ABCR12-GC | CTGACTTTGGAGAAATGCAG | 58 | 1.5+ |
| 13 | ABCR13-GC | TCGGGAGGTGTGAGTGAGC | ABCR13-Rev | TTAGCGTGTCATGGAGGAGG | 58 | 1.0+ |
| 14 | ABCR14-GC2 | ATTCTGCCTCTACCAGGTAC | ABCR14-rev | AATCCAGGCACATGAACAGG | 57 | 1.5+ |
| 15 | 12-For | AGGCTGGTGGGAGAGAGC | ABCR15-GC | GGACTGCTACGGACCATTC | 56 | 1.5+ |
| 16 | 13-For | CTGTTGCATTGGATAAAAGGC | ABCR16-GC | GATGAATGGAGAGGGCTGG | 56 | 1.5− |
| 17 | Exon J-For | CTGCGGTAAGGTAGGATAGGG | ABCR17-GC | CACACCGTTTACATAGAGGGC | 58 | 1.5+ |
| 18 | Exon K-For | CTCTCCCCTCCTTTCCTG | ABCR18-GC2 | GCCTTTTCCTCGCCTCTG | 56 | 1.0+ |
| 19 | 15-For | TGGGGCCATGTAATTAGGC | ABCR19-GC | TGGGAAAGAGTAGACAGCCG | 57 | 1.5− |
| 19 | ABCR19-For2 | AAGATTTTTGAGCCCTGTGG | ABCR19-GC | TGGGAAAGAGTAGACAGCCG | 57 | 1.5+ |
| 20 | ABCR20-GC | GCCCTCCTAAGGCATGTTG | 2F3-Rev | TATCTCTGCCTGTGCCCAG | 57 | 1.0+ |
| 21 | 2R5N-For | GTAAGATCAGCTGCTGGAAG | ABCR21-GC | GAAGCTCTCCTGCTCCAAGC | 58 | 1.0+ |
| 22 | 2F5R-For2 | AGGTACCCCCACAATGCC | ABCR22-GC | AGCCCAGCCCAGGAGACT | 56 | 1.0+ |
| 23 | ABCR23-GC | TTTTTGCAACTATATAGCCAGG | 2F6-Rev | AGCCTGTGTGAGTAGCCATG | 58 | 1.5− |
| 24 | 2F7R-For | GCATCAGGGAGAGGCTGTC | ABCR24-GC | CCAGACGGAACCCAAGTATG | 59 | 1.0+ |
| 25 | ABCR25-GC | GGTAACCTCACAGTCTTCC | 23F1-Rev | GGGAACGATGGCTTTTTGC | 56 | 1.5− |
| 26 | ABCR26-GC2 | TCCCATTATGAAGCAATACC | ABCR26-Rev | CCTTAGACTTTCGAGATGG | 48 | 1.5+ |
| 28 | ABCR28-GC | ACGTGTGACATCTCCATGCC | ABCR28-Rev | CCCTTCTAAGCAGCATGTGA | 58 | 1.0+ |
| 29 | ABCR29-GC | AGGCTCTGAGTTGCATGATG | ABCR29-Rev | CTGCCATCTTGAACCCACC | 59 | 1.0+ |
| 31 | ABCR31-For | TATAAGTCCTCAAGTTCCAAG | ABCR31-GC | AATATCTTCTACAGGGAGCC | 56 | 1.5+ |
| 32 | ABCR32-For | TAACGGCACTGCTGTACTTG | ABCR32-GC | TCATGGCTGTGAGGTGTGC | 58 | 1.0+ |
| 33 | 33G1-For | TTCATGTTTCCCTACAAAACCC | ABCR33-GC | AAAATCCTACTCAAATCTCCAG | 58 | 1.5− |
| 34 | ExA-For | GCTTAACTACCATGAATGAG | ABCR34-GC | TCAGCAGGAGGAGGGATG | 56 | 1.0− |
| 35 | ABCR35-GC | TAACTAGCTGTTAATGCAGCG | ExB-Rev | AAGAGTGGAGAAGGTGACAA | 58 | 1.5− |
| 36 | ABCR36-GC | GTATCTTCTCCTCCTTCTGC | ABCR36-Rev | CACACAAGCTCCACCTTGG | 58 | 1.5+ |
| 37 | ABCR37-GC | CAGGTCTGAGAGGTTAAGTG | ABCR37-Rev | CCACCAGGCTTCTCTTCAG | 58 | 1.0+ |
| 39 | ABCR39-For2 | GGTTTGCCCCGTTTCCAAC | ABCR39-GC | TCCCAGCTTTGGACCCAG | 56 | 1.0+ |
| 40 | ABCR40-GC | AGGTCTGTGGGGTGAGCTG | ABCR40-Rev | TCTGGATGCCCTGAGCTGC | 58 | 1.0+ |
| 41 | ABCR41-For | GAAAGGACAGTGCCAAGGAC | ABCR41-GC | TCTAACCAGCACCTCCAAAC | 58 | 1.5+ |
| 42 | ABCR42-GC | CCGTCTCAGTTCTCAGTCC | ABCR42-Rev | AGAGCTGATGTTCGGAAGCC | 57 | 1.0+ |
| 43 | 4RX-For | CTTACCCTGGGGCCTGAC | ABCR43-GC | TCAGAGCCACCCTACTATAG | 56 | 1.5+ |
| 45 | ABCR45-GC | CTTGTCTTCTCCAAATGGCA | ABCR45-Rev | TTTAAGCCCTTGGTGCGGC | 51 | 1.0+ |
| 46 | E1R-For | GAAGCAGTAATCAGAAGGGC | ABCR46-GC | CCTCACATTCTTCCATGCTG | 57 | 1.0+ |
| 47 | ABCR47-For | CACATCCCACAGGCAAGAG | ABCR47-GC | ATCCACAGAAGGCAACAAGG | 57 | 1.0+ |
| 48 | ABCR48-GC | AGGCCCAACCACTAACAGAG | E3F-Rev | ACACTGGGTGTTCTGGACC | 57 | 1.0+ |
| 50 | ABCR50-GC | AAACCAAGATGACGCGAGTC | ABCR50-Rev | GGAACGAGCGGTGTGAAAG | 57 | 1.0+ |
| dHPLC: | ||||||
| 8 | ABCR8F2 | GAGCATTGGCCTCACAGCAG | ABCR8R2 | CCCCAGGTTTGGTTTCACC | 54 | 1.0− |
| 27 | ABCR27F2 | GAGATCCAGACCTTATAGGC | 23F3R | GTTATAACCCATGCCTGAAG | 54 | 1.5− |
| 30 | ABCR30F2 | ACTTTGAGGCTGATTATGGAA | ABCR30R | CCCCGTTGTTTGGAGGTC | 54 | 1.5− |
| 38 | ABCR38F2 | GGAATGGAATGTGGAACTCC | ABCR38R2 | ACACATACTCTACTATCCTAC | 54 | 1.5− |
| 44 | 62r4F | GAAGCTTCTCCAGCCCTAGC | 62r3R | TGCACTCTCATGAAACAGGC | 54 | 1.0− |
| 49 | ABCR49F | GTGTAGGGTGCTGTTTTCC | ABCR49R | CAAGCTGTGGACTGCATAAG | 54 | 1.5− |
| SSCP: | ||||||
| 10 | ABCR10F4 | ATCTTTGTCTGGTTTTAGGC | ABCR10R4 | CCCCCCTTACTCTGATCAT | 50 | 1.5− |
| 11 | ABCR11F2 | GAATTTCTAAGCAGAGCAGTG | ABCR11R | AGCTCTGGCCCCACTCATG | 54 | 1.5− |
GC = GC clamp at the 5′ end of the respective primer.
PCR reaction with (+) or without (−) 4% formamide.
Exons 1–7, 9, 12–26, 28, 29, 31–37, 39–43, 45–48, and 50 were analyzed by DGGE. Between 30 and 50 μl of each GC-clamped PCR product was loaded onto a 6% polyacrylamide gel with a 20%–70% or 0%–70% gradient of urea and formamide. The gels were run for 6 h at 150 V in TAE buffer (40 mM Tris base, 20 mM glacial acetic acid, and 1 mM EDTA, pH 8.0) at 60°C, before being stained with ethidium bromide and photographed under UV transillumination.
Several exons resolved poorly with DGGE and were analyzed by other methods. dHPLC with the WAVE DNA Fragment Analysis System (Transgenomic) was used to analyze exons 8, 27, 30, 38, 44, and 49. PCR products (4–8 μl) were injected and eluted from the column with a linear acetonitrile gradient at a constant flow of 0.9 ml/min. The optimal temperature for resolution of homoduplex and heteroduplex DNA was determined by injecting the PCR product for each exon at increasing temperatures until the sample retention time decreased. Exons 10 and 11 required the use of SSCP analysis. Exon 10 was analyzed as a single fragment, and exon 11 was analyzed as two fragments, cleaved with restriction enzyme AluI prior to gel loading. In brief, 3 μl of a 1:5 mixture of [32P]-dCTP radiolabeled PCR product and loading buffer (95% formamide, 0.1% xylene cyanol, and 0.1% bromophenol blue) were denatured for 3 min and were run on a 6% nondenaturing polyacrylamide gel in 0.5× TBE (45 mM Tris-HCl, 45 mM boric acid, and 1 mM Na2EDTA, pH 8.3) buffer with 5% glycerol at 4°C and 25 W for 3–6 h. After drying of the gel, detection was achieved by autoradiography.
For each of the techniques applied, all aberrant fragments were directly DNA sequenced with the Big-Dye Terminator Cycle Sequencing Ready Reaction Kit (PE Biosystems) and an ABI 310 automated sequencer. Similarly, for two additional patients with STGD (STGD40/163 and STGD47/164), the 50 exonic PCR products of ABCA4 were sequenced using the respective forward and reverse oligonucleotide primers.
Analysis of Splicing Mutations
The functional consequences of five identified splice-site alterations—IVS20+5G→A, IVS23+10T→G, IVS28+5G→A, IVS38−10T→C, and IVS40+5 G→A—were studied with the Exon Trapping System pSPL3b (Gibco Life Technology). In each case, the region encompassing the affected splice site and the adjacent exon(s) was PCR amplified from the patient’s genomic DNA by the following oligonucleotide primer pairs: for IVS20, ABCR20EcoRI-linkF (5′-CCG GAA TTC GCC CTC CTA AGG CAT GTT G-3′) and ABCR20BamHI-linkR (5′-CGC GGA TCC TAT CTC TGC CTG TGC CCA G-3′); for IVS23, ABCR23EcoRI-linkF (5′-CCG GAA TTC TTT TTG CAA CTA TAT AGC CAG G-3′) and ABCR24BamHI-linkR (5′-CGC GGA TCC CCA GAC GGA ACC CAA GTA TG-3′); for IVS28, ABCR28EcoRI-linkF (5′-CCG GAA TTC ACG TGT GAC ATC TCC ATG CC-3′) and ABCR28BamHI-linkR (5′-CGC GGA TCC CCC TTC TAA GCA GCA TGT GA-3′); for IVS 38, ABCR39EcoRI-linkF (5′-CCG GAA TTC GCC CCA CCT GCT GAA GAG-3′) and ABCR39BamHI-linkR (5′-CGC GGA TCC TCC CAG CTT TGG ACC CAG G-3′); and for IVS40, ABCR39EcoRI-linkF and ABCR40BamHI-linkR (5′-CGC GGA TCC TCT GGA TGC CCT GAG CTG C-3′). EcoRI and BamHI recognition sequences were coupled to the forward and reverse primers, respectively, to ensure directional insertion into the pSPL3b vector. Wild-type and mutant clones were selected for transformation into COS7 cells. After 48 h, mRNA was isolated from the COS7 cells and was analyzed by reverse transcription (RT)–PCR using either vector primer SA2 (5′-ATC TCA GTG CTA TTT GTG AGC-3′) and nested primer SA4 (5′-CAC CTG AGG AGT GAA TTG GTC G-3′) or SD6 (5′-TCT GAG TCA CCT GGA CAA CC-3′) and nested primer SD2 (5′-GTG AAC TGC ACT GTG ACA AGC-3′), as well as exon-specific primers. All RT-PCR products were electrophoresed on a 1% agarose gel, and their sequences were determined after excision of the respective fragments.
Results
Using a combination of DGGE, dHPLC, and SSCP screening, we studied 288 ABCA4 alleles from patients with STGD, 400 alleles from patients with AMD, and 440 alleles from the control group, and we identified 2,365 sequence changes (on average, 5.77 alterations per patient with STGD, 3.52 per patient with AMD, and 3.77 per control individual).
Classification of Sequence Alterations
Definition of a “disease-associated” mutation is a difficult task, particularly if functional assays to determine phenotypic effects of specific variations are not readily available (Cotton and Scriver 1998). For the purposes of this study, we have used the following defining criteria. ABCA4 sequence alterations whose predicted consequence is premature truncation of the protein—that is, nonsense mutations, small insertions, or deletions causing a frameshift—and alterations affecting splicing were classified as disease-associated mutations. Also considered pathological were missense mutations causing nonconservative amino acid changes—for example, A60T and A60E (table 2)—with the exception of those that were found at similar frequencies in the three study groups—for example, R152Q, N1868I, and V1921M (tables 3 and 4). Nucleotide alterations occurring in similar frequencies and found in >1% of the control alleles were regarded to be polymorphic. All remaining changes were categorized as rare sequence variants with unclassifiable pathogenicity.
Table 2.
ABCA4 Mutations Found in Patients with STGD and AMD and in Controls
|
No. of Alleles |
|||||
| Exon andNucleotideChange | Effect | STGD(288) | AMD(400) | Control(440) | Reference(s) |
| 3: | |||||
| 178G→A | A60T | 1 | 0 | 0 | This study |
| 179C→T | A60E | 1 | 0 | 0 | This study |
| 194G→A | G65E | 1 | 0 | 0 | Fishman et al. (1999) |
| 203C→T | P68L | 1 | 0 | 0 | This study |
| 214G→A | G72R | 1 | 0 | 0 | This study |
| 296insA | Frameshift | 2 | 0 | 0 | This study |
| 5: | |||||
| 454C→T | R152X | 1 | 0 | 0 | This study |
| 6: | |||||
| 634C→T | R212C | 1 | 0 | 0 | Lewis et al. (1999) |
| 688T→A | C230S | 1 | 0 | 0 | This study |
| 730delCT | Frameshift | 1 | 0 | 0 | This study |
| 740A→G | N247S | 1 | 0 | 0 | This study |
| 768G→T | Splice | 2 | 0 | 0 | Maugeri et al. (1999) |
| 8: | |||||
| 983A→T | E328V | 1a | 0 | 0 | This study |
| 1086T→A | Y362X | 1 | 0 | 0 | This study |
| 10: | |||||
| 1317G→A | W438X | 1 | 0 | 0 | This study |
| 11: | |||||
| 1411G→A | E471K | 1 | 0 | 0 | Lewis et al. (1999) |
| 12: | |||||
| 1622T→C | L541P | 21a | 1a | 0 | Rozet et al. (1998), Fishman et al. (1999), Lewis et al. (1999), Maugeri et al. (1999) |
| 1715G→A | R572Q | 1a | 0 | 0 | Lewis et al. (1999) |
| 13: | |||||
| 1819G→A | G607R | 1 | 0 | 0 | This study |
| 1903C→A | Q635K | 2a | 0 | 0 | This study |
| 1903C→T | Q635X | 1 | 0 | 0 | This study |
| IVS13+1G→A | Splice | 2 | 0 | 0 | This study |
| 14: | |||||
| 1957C→T | R653C | 1 | 0 | 0 | This study |
| 1988G→A | W663X | 1 | 0 | 0 | This study |
| 2041C→T | R681X | 4 | 0 | 0 | Maugeri et al. (1999) |
| 15: | |||||
| 2291G→A | C764Y | 1 | 0 | 0 | This study |
| 2292delT | Frameshift | 1a | 0 | 0 | This study |
| 2295T→G | S765R | 1a | 0 | 0 | This study |
| 16: | |||||
| 2564G→A | W855X | 1 | 0 | 0 | Nasonkin et al. (1998) |
| 17: | |||||
| 2588G→C | Spliceb | 17a | 6 | 5 | Allikmets et al. (1997a), Cremers et al. (1998), Lewis et al. (1999), Maugeri et al. (1999), Papaioannou et al. (2000) |
| 18: | |||||
| 2701A→G | T901A | 0 | 2 | 0 | This study |
| 2741A→G | H914A | 0 | 0 | 1 | This study |
| 19: | |||||
| 2876C→T | T959I | 1 | 0 | 0 | This study |
| 20: | |||||
| IVS20+5G→A | Splice | 1 | 0 | 0 | This study |
| 21: | |||||
| 3106G→A | E1036K | 1a | 0 | 0 | Nasonkin et al. (1998) |
| 3113C→T | A1038V | 26a | 4a | 1 | Allikmets et al. (1997a), Cremers et al. (1998), Rozet et al. (1998), Fishman et al. (1999), Lewis et al. (1999), Maugeri et al. (1999) |
| T3187T→C | S1063P | 1 | 0 | 0 | This study |
| 22: | |||||
| 3292C→T | R1097C | 1 | 0 | 0 | This study |
| 3322C→T | R1108C | 4 | 0 | 0 | Rozet et al. (1998), Fishman et al. (1999), Lewis et al. (1999) |
| 24: | |||||
| 3528insTGCA | Frameshift | 1 | 0 | 0 | This study |
| 25: | |||||
| 3808G→T | E1270X | 1 | 0 | 0 | This study |
| 27: | |||||
| 3898C→T | R1300X | 1 | 0 | 0 | This study |
| 28: | |||||
| IVS28+5G→A | Splice | 1 | 0 | 0 | This study |
| 4139C→T | P1380L | 1 | 0 | 0 | Lewis et al. (1999) |
| 4195G→A | E1399K | 2 | 0 | 0 | This study |
| 4234C→T | Q1412X | 4 | 0 | 0 | Maugeri et al. (1999) |
| 29: | |||||
| 4289T→C | L1430P | 2 | 0 | 0 | This study |
| 4318T→G | F1440V | 1 | 0 | 0 | This study |
| 4328G→A | R1443H | 1 | 0 | 0 | This study |
| 30: | |||||
| 4457C→T | P1486L | 1 | 0 | 0 | Lewis et al. (1999) |
| 4463G→A | C1488Y | 1 | 0 | 0 | This study |
| 31: | |||||
| 4610C→T | T1537M | 1 | 0 | 0 | This study |
| 35: | |||||
| IVS35+2T→A | Splice | 1 | 0 | 0 | This study |
| 36: | |||||
| 5065T→C | S1689P | 1 | 0 | 0 | This study |
| 5114G→T | R1705L | 1 | 0 | 0 | This study |
| IVS36+1G→A | Splice | 1 | 0 | 0 | This study |
| 37: | |||||
| 5198T→C | M1733T | 0 | 0 | 1 | This study |
| 5242G→A | G1748R | 1 | 0 | 0 | This study |
| 5248C→T | Q1750X | 1 | 0 | 0 | This study |
| 5288T→C | L1763P | 1 | 0 | 0 | This study |
| 38: | |||||
| IVS38+1G→A | Splice | 1 | 0 | 0 | This study |
| 40: | |||||
| 5653G→A | E1885K | 1 | 0 | 0 | This study |
| 5693G→A | R1898H | 5 | 2 | 1 | Allikmets et al. (1997b), Lewis et al. (1999) |
| IVS40+5G→A | Splice | 8a | 0 | 0 | Cremers et al. (1998), Lewis et al. (1999), Maugeri et al. (1999) |
| 42: | |||||
| 5882G→A | G1961E | 34 | 4 | 2 | Allikmets et al. (1997b), Fishman et al. (1999), Lewis et al. (1999), Maugeri et al. (1999) |
| 43: | |||||
| 5917delG | Frameshift | 3 | 0 | 0 | This study |
| 5923G→C | G1975R | 1 | 0 | 0 | This study |
| 5929G→A | G1977S | 1 | 0 | 0 | Rozet et al. (1998), Lewis et al. (1999) |
| 45: | |||||
| 6229C→G | R2077G | 1 | 0 | 0 | This study |
| 6229C→T | R2077W | 1 | 0 | 0 | Allikmets et al. (1997a), Fishman et al. (1999), Lewis et al. (1999) |
| 48: | |||||
| 6609C→A | Y2203X | 2 | 0 | 0 | This study |
| 6647G→T | A2216V | 0 | 0 | 1 | This study |
Mutation pairs occurring on a single haplotype.
Effect is missense mutation (G863A) and in-frame deletion (delG863), according to Maugeri et al. (1999).
Table 3.
Rare Sequence Variants in the ABCA4 Gene
|
No. of Alleles |
|||||
| Exon andNucleotideChange | Effect | STGD(288) | AMD(400) | Control(440) | Reference(s) |
| 5: | |||||
| 455G→A | R152Q | 3 | 1 | 3 | This study |
| 8: | |||||
| IVS8+38A→T | Unknown | 0 | 1 | 0 | This study |
| 12: | |||||
| 1654G→A | V552I | 0 | 0 | 2 | This study |
| IVS11−6C→G | Unknown | 0 | 4 | 2 | This study |
| 13: | |||||
| 1932C→T | D644D | 2 | 0 | 0 | This study |
| 17: | |||||
| IVS16−12C→G | Unknown | 0 | 0 | 8 | This study |
| 18: | |||||
| IVS17−56C→G | Unknown | 3 | 0 | 0 | This study |
| IVS17−36C→T | Unknown | 0 | 2 | 1 | This study |
| 22: | |||||
| 3261A→C | E1087D | 1 | 0 | 0 | This study |
| 3264C→T | P1088P | 0 | 0 | 1 | This study |
| IVS21−20C→T | Unknown | 1 | 0 | 0 | This study |
| 23: | |||||
| IVS23+10T→G | Unknown | 1 | 0 | 0 | This study |
| IVS23+17G→C | Unknown | 1 | 0 | 0 | This study |
| 24: | |||||
| IVS23−28T→C | Unknown | 2 | 4 | 1 | This study |
| 25: | |||||
| 3759G→A | T1253T | 1 | 0 | 0 | This study |
| 28: | |||||
| 4140G→A | P1380P | 2 | 0 | 0 | This study |
| IVS28+43G→A | Unknown | 4 | 3 | 1 | This study |
| 29: | |||||
| IVS29+13G→A | Unknown | 0 | 1 | 0 | This study |
| IVS29+32A→G | Unknown | 1 | 0 | 0 | This study |
| 31: | |||||
| 4578G→A | T1526T | 0 | 1 | 0 | This study |
| 32: | |||||
| IVS32+45T→C | Unknown | 1 | 0 | 0 | This study |
| 33: | |||||
| IVS32−57T→G | Unknown | 0 | 0 | 1 | This study |
| 4685T→C | I1562T | 0 | 0 | 6 | Allikmets et al. (1997b) |
| 36: | |||||
| IVS36+20G→A | Unknown | 1 | 0 | 0 | This study |
| 39: | |||||
| 5487G→T | L1829L | 0 | 0 | 1 | This study |
| IVS38−10T→C | Unknown | 9 | 0 | 0 | Maugeri et al. (1999) |
| 41: | |||||
| 5761G→A | V1921M | 1 | 1 | 1 | This study |
| 43: | |||||
| 5908C→T | L1970F | 1 | 0 | 1 | Allikmets et al. (1997b), Rozet et al. (1998), Lewis et al. (1999) |
| IVS43+7A→C | Unknown | 1 | 0 | 0 | This study |
| 44: | |||||
| 6027C→T | I2023I | 1 | 0 | 0 | Allikmets et al. (1997a), Nasonkin et al. (1998) |
| 45: | |||||
| 6176G→C | G2059A | 0 | 0 | 1 | This study |
| 46: | |||||
| IVS46+27G→A | Unknown | 0 | 0 | 1 | This study |
| 47: | |||||
| IVS46−46T→A | Unknown | 1 | 0 | 0 | This study |
| 48: | |||||
| IVS48+21C→T | Unknown | 18a | 2a | 0 | Allikmets et al. (1997b), Nasonkin et al. (1998), Papaioannou et al. (2000) |
| 6529G→A | D2177N | 2 | 3 | 4 | Allikmets et al. (1997b) |
| 6721C→G | L2241V | 1 | 0 | 0 | This study |
Occurs together with G1961E in 17/18 and 2/2 instances.
Table 4.
Polymorphisms in the ABCA4 Gene
|
No. of Alleles |
|||||
| Exon and NucleotideChange | Effect | STGD(n = 288) | AMD(n = 400) | Control(n = 440) | Reference(s) |
| 6: | |||||
| 635G→A | R212H | 8 | 8 | 32 | This study |
| 7: | |||||
| IVS6−32T→C | Unknown | 53 | 115 | 130 | This study |
| 10: | |||||
| 1267A→G | H423R | 52 | 79 | 101 | This study |
| 1268C→T | H423H | 11 | 17 | 17 | This study |
| 14: | |||||
| IVS14+50T→Ca | Unknown | 22 | 18 | 9 | This study |
| 19: | |||||
| 2828G→Aa | R943Q | 23 | 14 | 10 | Allikmets et al. (1997a, 1997b), Maugeri et al. (1999), Papaioannou et al. (2000) |
| 28: | |||||
| 4203C→A | P1401P | 29 | 13 | 20 | Maugeri et al. (1999) |
| 33: | |||||
| IVS32−38C→T | Unknown | 1 | 4 | 12 | This study |
| 34: | |||||
| IVS33−16delGT | Unknown | 24 | 8 | 12 | This study |
| 40: | |||||
| 5603A→T | N1868I | 37 | 40 | 46 | Maugeri et al. (1999) |
| 5682G→C | L1894L | 73 | 52 | 91 | Maugeri et al. (1999), Papaioannou et al. (2000) |
| 41: | |||||
| 5814A→G | L1938L | 50 | 68 | 70 | This study |
| 42: | |||||
| IVS41−11G→A | Unknown | 46 | 56 | 55 | Maugeri et al. (1999) |
| 5844A→G | P1948P | 40 | 40 | 39 | Maugeri et al. (1999), Papaioannou et al. (2000) |
| 5843CA→TG | P1948L | 5 | 14 | 13 | Maugeri et al. (1999) |
| 44: | |||||
| IVS43−16G→A | Unknown | 46 | 48 | 55 | Papaioannou et al. (2000) |
| 45: | |||||
| IVS45+7G→A | Unknown | 10 | 15 | 11 | Papaioannou et al. (2000) |
| 6249C→T | I2083I | 13 | 17 | 27 | Allikmets et al. (1997a), Maugeri et al. (1999) |
| 46: | |||||
| 6285T→C | D2095D | 38 | 36 | 33 | Maugeri et al. (1999) |
2828G→A and IVS14+50T→C occur on the same haplotype together with 2588G→C.
ABCA4 Mutations in STGD, AMD, and Controls
In 288 STGD chromosomes, we identified 191 disease-associated mutations (table 2table 2). Missense mutations make up the majority (76.4%), followed by nonsense (9.9%), splice (9.4%), and frameshift (4.2%) mutations. Fifty mutations occurred as part of 25 complex alleles, resulting in a total of 166 disease chromosomes and an overall detection rate of 57.6%. Both alleles were detected in 59 patients (40.9%), a single disease allele was found in 48 patients (33.3%), and no disease allele was identifiable in 37 patients (25.7%).
In the group with AMD, 400 chromosomes were studied and 19 mutations were identified, with L541P and A1038V occurring on a single haplotype in one patient (AMD43). In the control group, 440 chromosomes were analyzed, and 12 mutations were detected. Missense variants were the only mutation type found in the group with AMD and in the control group (table 2table 2).
ABCA4 Rare Sequence Variants and Polymorphisms in Patients with Either STGD or AMD and in Controls
Thirty-six different alterations detected in either the group with STGD or the group with AMD or the control group were considered to be rare sequence variants of unknown pathogenicity (table 3). More than half of these alterations were detected in the intervening sequences, and, although they are less likely to be pathogenic, a possible effect on RNA splicing cannot be ruled out. One such alteration, IVS48+21C→T, appears to be present at a significantly higher frequency in the population with STGD; however, the frequency is increased because of linkage disequilibrium with the mutation G1961E (data not shown). Nineteen different alterations were present in >1% of the control alleles and were classified as polymorphisms (table 4); these include five nonconservative amino acid substitutions (R212H, H423R, R943Q, N1868I, and P1948L).
Evaluation of Splice-Site Alterations IVS20+5G→A, IVS23+10T→G, IVS28+5G→A, IVS38−10T→C, and IVS40+5G→A
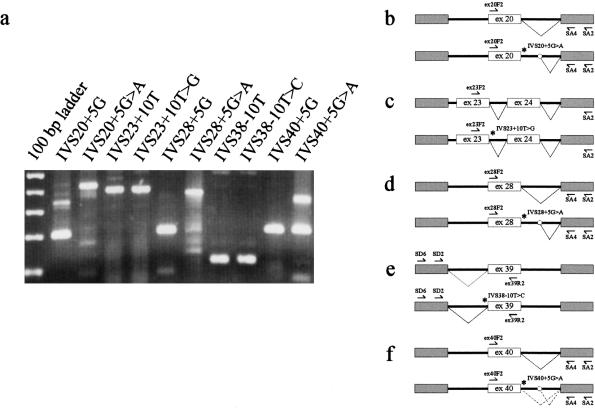
Five different intronic alterations found in patients with STGD but not in either patients with AMD or in controls were evaluated for their effects on RNA splicing. Three alterations (IVS20+5G→A, IVS28+5G→A, and IVS40+5G→A) affect the highly conserved G at the +5 position of splice donor sites. In this series, IVS20+5G→A and IVS28+5G→A were each found in one patient with STGD, and IVS40+5G→A was found in eight patients with STGD. Another alteration, at the +10 position of the intervening sequence-23 splice donor site (IVS23+10T→G), was found in a single patient with STGD. The fifth alteration affects the −10 position of a splice-acceptor site (IVS38−10T→C) and was observed in nine patients with STGD. We used an exon-trapping system to introduce DNA fragments encompassing each affected splice site and adjacent exon(s) into COS7 cells. For both wild-type and mutant sequences, the resulting splicing of the RNA products was then studied by nested RT-PCR analysis and direct sequencing (fig. 1).
Figure 1 .
Evaluation of consequences of intervening sequence alterations: results of RT-PCR analyses (a) and schematic representations (b–f), indicating abnormal splicing at mutant splice site IVS20+5G→A (b and d) and normal splicing at mutant splice sites IVS23+10T→G and IVS38−10T→C (c and e). Clone IVS40+5G→A reveals both normal and abnormal splicing products, suggesting partial activity at the mutant site (a and f). The relative positions of the forward (⇀)and reverse (↼) RT-PCR primers, mutant sites (*), and the cryptic splice site in the pSPL3b vector sequence (○) are indicated. For sizing of RT-PCR products, the 100-bp ladder is given, and spans range 100–500 bp.
The RT-PCR product amplified from COS7 cell RNA containing the IVS20+5G→A alteration results in a fragment of 412 bp and was confirmed, by DNA sequencing, to include exon 20 and sequence from intron 20, indicating that the mutation abolishes correct splicing at the normal donor site and, instead, leads to the activation of a cryptic splice site within the pSPL3b vector sequence (fig. 1a and b). In contrast, an RT-PCR product of 204 bp was obtained from an appropriately spliced RNA derived from the corresponding wild-type clone (fig. 1a and b). Likewise, for the intron 28 alteration, a spliced product (200 bp) was produced from the wild-type clone, whereas a larger abnormally spliced product (369 bp) was obtained from the IVS28+5G→A mutant clone (fig. 1a and d). The demonstration, in this system, of abnormal splicing in both mutant clones, compared with normal splicing in the wild-type clones, allowed us to classify both alterations, IVS20+5G→A and IVS28+5G→A, as disease-associated mutations. The IVS40+5G→A mutant clone yielded two different fragments, a larger abnormally spliced product (350 bp) and a smaller, correctly spliced product (200 bp) identical to that obtained from the wild-type clone (fig. 1a and f). This provides experimental evidence for the suggestion by Cremers et al. (1998) that IVS 40+5G→A does not represent a true null allele but, because of correct splicing at the IVS40+5G→A site in a percentage of transcripts, results in residual activity of the ABCA4 transporter protein.
RT-PCR amplification of COS7 cell RNAs yielded 391-bp products (exons 23 and 24) from both the wild-type and IVS23+10T→G clones (fig. 1a and c) and yielded 105-bp products (exon 39) from both the wild-type and IVS38-10T→C clones (fig. 1a and e). DNA sequencing confirmed that all fragments represent correctly spliced exons. In agreement with these findings, additional RT-PCR analysis with RNA derived from an Epstein-Barr virus–transformed lymphoblastoid cell line from a patient (STGD169) heterozygous for the IVS38−10T→C alteration did not detect any aberrant splicing (data not shown). These results led us to classify the IVS23+10T→G and IVS38−10T→C alterations as rare sequence variants.
Patients with STGD Who Have Two Identified Disease Chromosomes
Fifty-nine patients with STGD were found to be homozygotes or compound heterozygotes for ABCA4 disease alleles (table 5). Correct segregation of disease alleles was demonstrated in all 39 cases in which family samples were available for study (table 5). In 11 of these families, segregation was confirmed in multiple affected members, whereas in the remaining cases the study was limited to the demonstration of correct parental transmission of disease alleles.
Table 5.
Patients with STGD Who Have Two Identified Disease Alleles
|
Mutation |
|||
| Age at Onsetand Patient | Allele 1 | Allele 2 | Segregation in Familya |
| 5–9 years: | |||
| STGD17 | Q1412X | R2077W | Yes |
| STGD88 | G65E | G1961E | NA |
| STGD93 | G1961E | G1961E | Yes |
| STGD99 | L541P-A1038V | G1961E | Yes |
| STGD100 | L541P-A1038V | IVS40+5G→A | Yes |
| STGD108 | Y362X | IVS40+5G→A | Yes |
| STGD109 | L541P-A1038V | W855X | Yes |
| STGD139b | 5917delG | 5917delG | Yes |
| STGD167 | C1488Y | IVS40+5G→A | Yes |
| 10–14 years: | |||
| STGD21 | R681X | R1898H | NA |
| STGD37 | L541P-A1038V | L541P-A1038V | Yes |
| STGD47/164 | IVS13+1G→A | 2588G→C | Yes |
| STGD50 | 2588G→C | A1038V | NA |
| STGD70 | 2588G→C | IVS40+5G→A | NA |
| STGD82 | L541P-A1038V | S1063P | Yes |
| STGD87 | 2588G→C | Q1750X | Yes |
| STGD98 | R212C | T959I | Yes |
| STGD102 | R572Q-2588G→C | IVS35+2T→A | Yes |
| STGD107 | C764Y | 3528ins4 | Yes |
| STGD120 | L1430P | L1430P | NA |
| STGD121 | R1300X | IVS40+5G→A | Yes |
| STGD156 | R1108C | G1961E | NA |
| STGD159 | R1108C | Q1412X | Yes |
| STGD171 | L541P-A1038V | G1961E | NA |
| 15–19 years: | |||
| STGD34 | G768T | G1961E | Yes |
| STGD39 | L541P-A1038V | R1443H | NA |
| STGD40/163 | 2588G→C | E1885K | Yes |
| STGD45 | E1399K | G1977S | Yes |
| STGD59 | R1898H | G1975R | NA |
| STGD67 | P68L | S1689P | Yes |
| STGD75 | Q635K | IVS40+5G→A | Yes |
| STGD111 | 2292delT-S765R | G1961E | Yes |
| STGD114 | Y2203X | G1961E | Yes |
| STGD138 | IVS13+1GA | 2588G→C | Yes |
| 20–24 years: | |||
| STGD41 | R681X | G1961E | Yes |
| STGD63 | A60T | R1898H | NA |
| STGD86 | 296insA | G1961E | Yes |
| STGD91 | L541P-A1038V | A1038V | NA |
| STGD113 | L541P-A1038V | 2588G→C | Yes |
| STGD118b | IVS20+5G→A | G1961E | Yes |
| STGD119 | L541P-A1038V | G1961E | Yes |
| STGD122 | L541P-A1038V | G1961E | Yes |
| STGD135 | W663X | G1961E | NA |
| STGD147 | IVS36+1G→A | G1961E | Yes |
| STGD168 | L541P-A1038V | G1961E | NA |
| 25–29 years: | |||
| STGD62 | G607R | G1961E | NA |
| STGD71 | 296insA | A1038V | Yes |
| STGD78 | 2588G→C | Q1412X | Yes |
| STGD103 | 2588G→C | IVS20+5G→A | Yes |
| STGD116 | L541P-A1038V | G1961E | Yes |
| STGD139bb | G1961E | 5917delG | Yes |
| ⩾30 years: | |||
| STGD38 | E471K | G1961E | Yes |
| STGD68 | E1399K | G1961E | Yes |
| STGD69 | L541P-A1038V | 2588G→C | NA |
| STGD95 | F1440V | G1748R | Yes |
| STGD134 | C230S | G1961E | NA |
| STGD144 | 2588G→C | R1705L | NA |
| STGD148 | R1097C | Y2203X | NA |
| STGD170 | L541P-A1038V | 2588G→C | NA |
NA = not applicable.
139b and 139 are a mother-daughter pair.
The majority of patients are compound heterozygotes for two missense mutations (31/59 patients ) or one truncating and one missense mutation (25/59 patients). Two patients, STGD108 and STGD121, are heterozygous for both an alteration in the donor splice site of intron 40 (IVS40+5G→A) and a nonsense mutation (STGD108, Y362X and STGD121, R1300X). STGD108 had disease onset at age 9 years, and at last examination (age 13 years), significant visual impairment (OD/OS 0.08/0.08), moderate fundus changes, and perimacular and peripheral flecks. STGD121 had disease onset at age 10 years and, at age 27 years, visual acuity of 0.07/0.1 (OD/OS) and moderate fundus changes with perimacular and peripheral flecks and pigment clumping nasal to the optic nerve. Both eyes exhibited extensive central retinal pigment epithelium atrophy. Both patients have normal electrodiagnostic values. One patient (STGD139) is a homozygote for a 1-bp deletion, 5917delG. She had onset of disease at age 5 years and, on examination at age 10 years, visual acuity of 0.8/0.8 (OD/OS), mild fundus changes, and abnormal electroradiography values suggestive of both rod and cone involvement (rod response [OU] and 30-Hz flicker [OD] amplitudes <50% of the 5th percentile; 30-Hz flicker latency [OU] >150% of the 95th percentile).
Discussion
We have provided the results of a comprehensive mutation analysis of the 50 exons of the ABCA4 gene in 144 patients with STGD, 200 individuals diagnosed with AMD, and 220 controls, all ascertained from the German population. This study has addressed a number of important issues. First, since, in the studies conducted to date, the detection rate of ABCA4 alterations has been below expectations, we aimed to develop an improved and efficient screening protocol with maximal sensitivity. Second, by describing a large number of sequence alterations in the ABCA4 gene in affected individuals and controls, we provided a survey of alterations that facilitates the categorizing of disease-associated mutations versus benign polymorphisms or rare sequence variants with unclear pathogenicity and that adds to the growing amount of data correlating genotype with phenotype. Third, we established a mutation profile for the German population that will aid in ABCA4 gene testing and risk assessment for STGD. Finally, we compare the sequence alterations found in each of the three sample groups, to further evaluate the much debated role of ABCA4 in the pathogenesis of AMD.
A total of 127 unique alterations in the ABCA4 gene were identified, of which 90 have not been described elsewhere; 110 distinct changes were present in patients with STGD, 36 in patients with AMD, and 42 in control individuals. We have classified 72 of these alterations as probable pathogenic mutations, because of their predicted deleterious effects on the protein. Nineteen alterations were defined as common polymorphisms, whereas 36 distinct alterations were classified as rare sequence variants, since their contribution to disease is uncertain and remains to be clarified. As well as changes not affecting the specificity of amino acid residues and conservative amino acid substitutions, included in the latter category are some rare nonconservative changes found in similar frequencies in the three study groups—for example, R152Q, V1921M, and L1970F. Under our definitions, the conservative amino acid alteration D2177N, which has been described elsewhere as associated with AMD (Allikmets et al. 1997a; Allikmets and the International ABCR Screening Consortium 2000), was also classified as a rare sequence variant. In the present study, however, it was found at similar frequencies in patients with either STGD or AMD and in controls (2/288, 3/400, and 4/440 alleles, respectively), which neither supports nor refutes a role for this mutation in disease. A notable finding is the high frequencies in the control group of two independent alterations, IVS16-12C→G (1.8%) and I1562T (1.4%), in contrast to their complete absence in the group with STGD as well as in the group with AMD. Although IVS16-12C→G has not been reported elsewhere, I1562T has been found two times in patients with AMD but not in those with STGD or in 220 controls (Allikmets et al. 1997a). Larger study numbers may be required in order to determine the significance of these findings.
Of the 72 distinct pathogenic mutations, 68 were found in the group with STGD and account for 166 disease chromosomes. Although the majority of mutations are rare, found in only one or two families, three disease alleles (2588G→C, L541P-A1038V, and G1961E) are present at high frequencies in the German population. The most frequent is G1961E, which represents 34 (20.5%) of 166 identified alleles, a frequency that is significantly higher than the 4%–9% reported in other studies (Allikmets 1997a; Lewis et al. 1999; Maugeri et al. 1999; Simonelli et al. 2000). Notable is the presence of a patient with STGD (STGD93) who is homozygous for this mutation, a combination of alleles that has been speculated not to result in a STGD phenotype (Simonelli et al. 2000). The second-most-frequent allele is a complex allele, with A1038V occurring on the same haplotype as L541P (21/166 [12.7%]). Although the A1038V mutation is commonly reported in the literature, the L541P-A1038V complex allele has been reported only five times (Rozet et al. 1998; Fishman et al. 1999; Lewis et al. 1999; Maugeri et al. 1999). Given the relatively high frequency in the population that we studied, it is likely to represent a German founder allele. The third-most-frequent allele is 2588G→C, representing 10.2% (17/166) of identified disease chromosomes. These three alterations, in combination with five others (R681X, A1038V as noncomplex allele, R1108C, Q1412X, R1898H, and IVS40+5G→A), account for 61.4% of the detectable disease chromosomes in the German patients with STGD.
The 2588G→C transversion is a relatively common mutation, with an allele frequency of 1/17 in the patients with STGD whom we studied but also with a surprisingly high allele frequency, 1/88, in control individuals. Other authors have also remarked on this phenomenon, and they have calculated that the predicted homozygote frequency for this allele alone is greater than the estimated 1/10,000 incidence of STGD (Maugeri et al. 1999). Taking this, as well as the observed scarcity of 2588G→C homozygotes, into account, Maugeri et al. (1999) concluded that 2588G→C is a mild mutation, causing disease only in combination with a severe allele. We have tested an alternate hypothesis—that this allele is not a disease-causing mutation but rather a variant that is frequent in the general population and, because of linkage disequilibrium with another, as-yet undetected, mutation in ABCA4, has an increased frequency in the population with STGD. We sequenced both DNA strands of all 50 exons of the ABCA4 gene in two patients with the 2588G→C alteration, in whom the second disease allele was also known. No additional alterations were detected in these patients, a finding that provides no further clarification for this remarkable observation.
Unlike previous studies, which have mostly applied SSCP, the present study has used a combination of the highly sensitive DGGE and dHPLC techniques to screen the ABCA4 gene, using SSCP analysis only for exons 10 and 11. Applying these techniques, we have achieved an overall detection rate of close to 58%, which is comparable to the results of previous large studies, which have used primarily SSCP (e.g., see Lewis et al. 1999; Maugeri et al. 1999). The undetected mutations have been attributed to possible large DNA rearrangements or alterations of the promoter or intronic sequences, all undetectable by PCR-based methodologies; however, one study addressing this issue by use of Southern blot analysis revealed only a single additional DNA deletion (Maugeri et al. 1999). To determine whether methodological limitations are the reason for the low detection rate, the complete sequence analysis of the ABCA4 gene in patients screening negative under the aforementioned protocols will be required. It is also possible that some cases without an ABCA4 mutation either represent phenocopies or may be associated with different genes—for example, as de novo mutations in autosomal dominant loci. Several families have been described that show dominant inheritance of a Stargardt-like phenotype (Cibis et al. 1980; Lopez et al. 1990; Mansour 1992; Stone et al. 1994; Zhang et al. 1994), and at least three loci have been mapped, by genetic linkage analysis, to chromosomal regions 4p (Kniazeva et al. 1999), 6q (Stone et al. 1994), and 13q (Zhang et al. 1994). Unfortunately, until these genes are cloned, it may be difficult to assess their contribution, since a large number of cases of STGD are single occurrences without a history of the disease in the family.
In an attempt to correlate ABCA4 genotype to phenotype, it has been suggested that there is an association between the location of ABCA4 gene mutations and clinical severity, as defined by the age at onset of visual impairment (Lewis et al. 1999). We have not observed this trend for either missense or truncating mutations. On the contrary, specific mutations are associated with highly variable ages at onset; for example, compound heterozygosity for the complex allele L541P-A1038V and G1961E was found in patients with age at onset of 9–25 years (table 5). Age at onset is an easily quantifiable measurement, but it also appears to be highly subjective and has been demonstrated to vary considerably within families, independent of disease severity (Lois et al. 1999).
Previous documentation of ABCA4 mutations in individuals with either AMD, STGD, CRD, or RP led to the suggestion of a model in which ABCA4 mutations can cause a spectrum of retinal disease, with the clinical phenotype determined by the level of residual ABCA4-protein activity (Rozet et al. 1997; Cremers et al. 1998; Martinez-Mir et al. 1998; Maugeri et al. 1999). Supporting this is the presence, in the cohort of patients whom we studied, of patient STGD139, who is a carrier of a homozygous frameshift mutation (5917delG) and has, at a young age, a relatively severe phenotype, with features suggestive of cone-rod dystrophy. Two additional patients, STGD108 and STGD121, each have a truncating mutation (Y362X and R1300X) in combination with the splice-site mutation IVS40+5G→A. Although both patients had an early onset of visual impairment, their phenotypes appear to be somewhat milder than that of patient STGD139. This is consistent with previous clinical observations (Cremers et al. 1998), as well as with the findings in our exon-trapping system that point to the retention, because of the normal splicing at the mutant intervening sequence-40 donor site in a percentage of transcripts, of residual activity of the ABCA4 protein.
By the reasoning proposed under the residual activity model, the mild end of the ABCA4-associated phenotypic spectrum would be AMD, resulting from either homozygosity for a very mild mutation or heterozygosity for a moderate or severe alteration. In this study, comparing the frequency of the probable disease chromosomes in the group with AMD versus that in the control ,no significant difference between the number of patients with AMD (18/200) and control individuals (12/220) who harbor ABCA4 mutations (Fisher’s exact test, two-tailed; P=.19). Inclusion of alterations classified as rare sequence variants similarly results in comparable numbers of ABCA4 alterations in the two groups (P=.72). This stands in contrast to a recent study, involving 15 research groups from the United States and Europe, that specifically investigated the frequency of two common ABCA4 variants, G1961E and D2177N, in a large cohort (>1,200 individuals each) of patients with AMD and of controls (Allikmets and The International ABCR Screening Consortium 2000). These two variants were found at significantly different frequencies in the two study populations (3.4% in the group with AMD vs. 0.95% in controls). The numbers of patients and controls included in our study may be large enough to rule out a major contribution of mutant ABCA4 alleles in predisposition to AMD; however, they may not be of the size required to allow more-modest effects to be discerned. This makes it extremely difficult to determine the significance of individual mutant ABCA4 alleles in the predisposition to AMD, particularly those which are present in low frequency in the general population. Given the current technology, the analysis, in its entirety, of a large gene such as ABCA4 still represents a challenging task. At this juncture, it may therefore be more reasonable to follow the approach that the consortium has chosen for analysis of the more common variants for their contributions to susceptibility to AMD. The findings in this study point to additional variants (2588G→C, A1038V, and R1898H) that are present in reasonable frequencies in the German population and that may be worthwhile candidates for further extended analyses in large-scale international efforts.
Acknowledgments
We are grateful to M. Andrassi and C. Gerth (Regensburg) for examining the patients with STGD, to D. Besch (Tübingen), D. Walker (Eye Care Centre Vancouver), and U. Kellner (Berlin) for their referral of patients with STGD, and to the many individuals and families who participated in this project. This work was supported by Pro Retina Deutschland, Deutsche Forschungsgemeinschaft We 1259/10-1 and Ap 57/3-1 grants, and Fortüne Grant 707-0-0.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for STGD1 [MIM 248200] and ABCA4 [MIM 601691])
- Statistisches Bundesamt Deutschland, http://www.statistik-bund.de
References
- Allikmets R, The International ABCR Screening Consortium (2000) Further evidence for an association of ABCR alleles with age-related macular degeneration. Am J Hum Genet 67:487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allikmets R, Shroyer NF, Singh N, Seddon JM, Lewis RA, Bernstein PS, Peiffer A, Zabriskie NA, Li Y, Hutchinson A, Dean M, Lupski JR, Leppert M (1997a) Mutation of the Stargardt disease gene (ABCR) in age-related macular degeneration. Science 277:1805–1807 [DOI] [PubMed] [Google Scholar]
- Allikmets R, Singh N, Sun H, Shroyer NF, Hutchinson A, Chidambaram A, Gerrard B, Baird L, Stauffer D, Peiffer A, Rattner A, Smallwood P, Li Y, Anderson KL, Lewis RA, Nathans J, Leppert M, Dean M, Lupski JR (1997b) A photoreceptor cell-specific ATP-binding transporter gene (ABCR) is mutated in recessive Stargardt macular dystrophy. Nat Genet 15:236–246 [DOI] [PubMed] [Google Scholar]
- Bird AC, Bressler NM, Bressler SB, Chisholm IH, Coscas G, Davis MD, de Jong PT, Klaver CC, Klein BE, Klein R, Mitchell P, Sarks JP, Sarks SH, Soubrane G, Taylor HP, Vingerling JR (1995) An international classification and grading system for age-related maculopathy and age-related macular degeneration. The International ARM Epidemiology Study Group. Surv Ophthalmol 39:367–374 [DOI] [PubMed]
- Bressler NM, Bressler SB, Fine SL (1988) Age-related macular degeneration. Surv Ophthalmol 32:375–413 [DOI] [PubMed] [Google Scholar]
- Cibis GW, Morey M, Harris DJ (1980) Dominantly inherited macular dystrophy with flecks (Stargardt). Arch Ophthalmol 98:1785–1789 [DOI] [PubMed] [Google Scholar]
- Cotton RGH, Scriver CR (1998) Proof of “disease causing” mutation. Hum Mutat 12:1–3 [DOI] [PubMed] [Google Scholar]
- Cremers FP, van de Pol DJ, van Driel M, den Hollander AI, van Haren FJ, Knoers NV, Tijmes N, Bergen AA, Rohrschneider K, Blankenagel A, Pinckers AJ, Deutman AF, Hoyng CB (1998) Autosomal recessive retinitis pigmentosa and cone-rod dystrophy caused by splice site mutations in the Stargardt's disease gene ABCR. Hum Mol Genet 7:355–362 [DOI] [PubMed] [Google Scholar]
- Dean M, Allikmets R, Shroyer NF, Lupski JR, Lewis RA, Leppert M, Bernstein PS, Seddon JM (1998) ABCR gene and age-related macular degeneration. Science 279:1107a [DOI] [PubMed] [Google Scholar]
- Dryja TP, Briggs CE, Berson EL (1998) ABCR gene and age-related macular degeneration. Science 279:1107a [Google Scholar]
- Fishman GA, Stone EM, Grover S, Derlacki DJ, Haines HL, Hockey RR (1999) Variation of clinical expression in patients with Stargardt dystrophy and sequence variations in the ABCR gene. Arch Ophthalmol 117:504–510 [DOI] [PubMed] [Google Scholar]
- Fodde R, Losekoot M (1994) Mutation detection by denaturing gradient gel electrophoresis (DGGE). Hum Mutat 3:83–94 [DOI] [PubMed] [Google Scholar]
- Klaver CCW, Assink JJM, Bergen AAB, van Duijn CM (1998) ABCR gene and age-related macular degeneration. Science 279:1107a [Google Scholar]
- Klein BE, Klein R, Linton KL (1992) Prevalence of age-related maculopathy. The Beaver Dam Eye Study. Ophthalmology 99:933–943 [DOI] [PubMed] [Google Scholar]
- Kniazeva M, Chiang MF, Morgan B, Anduze AL, Zack DJ, Han M, Zhang K (1999) A new locus for autosomal dominant Stargardt-like disease maps to chromosome 4. Am J Hum Genet 64:1394–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krämer F, White K, Pauleikhoff D, Gehrig A, Passmore L, Rivera A, Rudolph G, Kellner U, Andrassi M, Lorenz B, Rohrschneider K, Blankenagel A, Jurklies B, Schilling H, Schutt F, Holz FG, Weber BHF (2000) Mutations in the VMD2 gene are associated with juvenile-onset vitelliform macular dystrophy (Best disease) and adult vitelliform macular dystrophy but not age-related macular degeneration. Eur J Hum Genet. 8:286–292 [DOI] [PubMed]
- Lewis RA, Shroyer NF, Singh N, Allikmets R, Hutchinson A, Li Y, Lupski JR, Leppert M, Dean M (1999) Genotype/phenotype analysis of a photoreceptor-specific ATP-binding cassette transporter gene, ABCR in Stargardt disease. Am J Hum Genet 64:422–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Smith DI, Rechtizigel KJ, Thibodeau SN, James CD (1998) Denaturing high performance liquid chromatography (DHPLC) used in the detection of germline and somatic mutations. Nucleic Acids Res 26:1396–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lois N, Holder GE, Fitzke FW, Plant C, Bird AC (1999) Intrafamilial variation of phenotype in Stargardt macular dystrophy—fundus flavimaculatus. Invest Ophthalmol Vis Sci 40:2668–2675 [PubMed] [Google Scholar]
- Lopez PF, Maumenee IH, de la Cruz Z, Green WR (1990) Autosomal-dominant fundus flavimaculatus: clinicopathologic correlation. Ophthalmology 97:798–809 [DOI] [PubMed] [Google Scholar]
- Mansour AM (1992) Long-term follow-up of dominant macular dystrophy with flecks (Stargardt). Ophthalmologica 205:138–143 [DOI] [PubMed]
- Martinez-Mir A, Paloma E, Allikmets R, Ayuso C, del Rio T, Dean M, Vilageliu L, Gonzalez-Duarte R, Balcells S (1998) Retinitis pigmentosa caused by a homozygous mutation in the Stargardt disease gene ABCR. Nat Genet 18:11–12 [DOI] [PubMed] [Google Scholar]
- Maugeri A, van Driel MA, van de Pol DJ, Klevering BJ, van Haren FJ, Tijmes N, Bergen AA, Rohrschneider K, Blankenagel A, Pinckers AJ, Dahl N, Brunner HG, Deutman AF, Hoyng CB, Cremers FP (1999) The 2588G→C mutation in the ABCR gene is a mild frequent founder mutation in the Western European population and allows the classification of ABCR mutations in patients with Stargardt disease. Am J Hum Genet 64:1024–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molday LL, Rabin AR, Molday RS (2000) ABCR expression in foveal cone photoreceptors and its role in Stargardt macular dystrophy. Nat Genet 25: 257–258 [DOI] [PubMed] [Google Scholar]
- Nasonkin I, Illing M, Koehler MR, Schmid M, Molday RS, Weber BHF (1998) Mapping of the rod photoreceptor ABC transporter (ABCR) to 1p21-p22.1 and identification of novel mutations in Stargardt's disease. Hum Genet 102:21–26 [DOI] [PubMed] [Google Scholar]
- Orita M, Iwahana H, Kanazawa H, Hayashi K, Sekiya T (1989) Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci USA 86:2766–2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou M, Ocaka L, Bessant D, Lois N, Bird A, Payne A, Bhattacharya S (2000) An analysis of ABCR mutations in British patients with recessive retinal dystrophies. Invest Ophthalmol Vis Sci 41:16–19 [PubMed] [Google Scholar]
- Papermaster DS, Schneider BG, Zorn MA, Kraehenbuhl JP (1978) Immunocytochemical localization of a large intrinsic membrane protein to the incisures and margins of frog rod outer segment disks. J Cell Biol 78:415–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozet JM, Gerber S, Souied E, Perrault I, Chatelin S, Ghazi I, Leowski C, Dufier JL, Munnich A, Kaplan J (1998) Spectrum of ABCR gene mutations in autosomal recessive macular dystrophies. Eur J Hum Genet 6:291–295 [DOI] [PubMed] [Google Scholar]
- Simonelli F, Testa F, de Crecchio G, Rinaldi E, Hutchinson A, Atkinson A, Dean M, D'Urso M, Allikmets R (2000) New ABCR mutations and clinical phenotype in Italian patients with Stargardt disease. Invest Ophthalmol Vis Sci 41:892–897 [PubMed] [Google Scholar]
- Souied EH, Ducroq D, Rozet JM, Gerber S, Perrault I, Munnich A, Coscas G, Soubrane G, Kaplan J (2000) ABCR gene analysis in familial exudative age-related macular degeneration. Invest Ophthalmol Vis Sci 41:244–247 [PubMed] [Google Scholar]
- Stargardt K (1909) Über familiäre, progressive Degeneration in der Maculagegend des Auges. Graefes Arch Ophthalmol 71:534–550 [Google Scholar]
- Stone EM, Nichols BE, Kimura AE, Weingeist TA, Drack A, Sheffield VC (1994) Clinical features of a Stargardt-like dominant progressive macular dystrophy with genetic linkage to chromosome 6q. Arch Ophthalmol 112:765–772 [DOI] [PubMed] [Google Scholar]
- Stone EM, Webster AR, Vandenburgh K, Streb LM, Hockey RR, Lotery AJ, Sheffield VC (1998) Allelic variation in ABCR associated with Stargardt disease but not age-related macular degeneration. Nat Genet 20:328–329 [DOI] [PubMed] [Google Scholar]
- Sun H, Molday RS, Nathans J (1999) Retinal stimulates ATP hydrolysis by purified and reconstituted ABCR, the photoreceptor-specific ATP-binding cassette transporter responsible for Stargardt disease. J Biol Chem 274:8269–8281 [DOI] [PubMed] [Google Scholar]
- Sun H, Nathans J (1997) Stargardt's ABCR is localized to the disc membrane of retinal rod outer segments. Nat Genet 17:15–16 [DOI] [PubMed] [Google Scholar]
- Weng J, Mata NL, Azarian SM, Tzekov RT, Birch DG, Travis GH (1999) Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in abcr knockout mice. Cell 98:13–23 [DOI] [PubMed] [Google Scholar]
- Zhang K, Bither PP, Park R, Donoso LA, Seidman JG, Seidman CE (1994) A dominant Stargardt's macular dystrophy locus maps to chromosome 13q34. Arch Ophthalmol 112:759–764 [DOI] [PubMed] [Google Scholar]