Abstract
Transmitted de novo structural chromosomal abnormalities, the majority of which are paternally derived, can lead to abnormal reproductive outcomes as well as genetic diseases in offspring. We developed and validated a new multicolor FISH procedure (sperm ACM, which utilizes DNA probes specific for the alpha [1cen], classical, [1q12], and midi [1p36.3] satellites of chromosome 1) which utilizes DNA probes specific for three regions of chromosome 1 to detect human sperm that carry numerical abnormalities plus two categories of structural aberrations: (1) duplications and deletions of 1pter and 1cen, and (2) chromosomal breaks within the 1cen-1q12 region. In healthy men, the average frequencies of sperm with duplications and deletions were (a) 4.5 ± 0.5 and 4.1 ± 1.3 per 104 involving 1pter and (b) 0.9 ± 0.4 and 0.8 ± 0.3 per 104 involving 1cen, respectively. The frequency of sperm exhibiting breaks within the 1cen-1q12 region was 14.1 ± 1.2 per 104. Structural aberrations accounted for 71% of the abnormalities detected by sperm ACM, which was significantly higher than numerical abnormalities (P=2×10-8). Our findings also suggest that, for healthy men, (a) sperm carrying postmeiotic chromosomal breaks appear to be more prevalent than those carrying products of premeiotic or meiotic breakage or rearrangements, (b) the high frequency of chromosome breaks measured after “fertilization” by the hamster-egg cytogenetic method already appear to be present and detectable within human sperm by FISH, and (c) there are nonrandom and donor-specific distributions of breakpoint locations within 1q12 in sperm. FISH facilitates the analysis of much larger numbers of sperm than was possible when the hamster-egg method was used. Therefore, FISH-based procedures for simultaneously detecting chromosomal breaks, rearrangements, and numerical abnormalities in sperm may have widespread applications in human genetics, genetic toxicology, and reproductive medicine.
Introduction
Chromosomal abnormalities transmitted through gametes are associated with pregnancy loss, infant mortality, birth defects, infertility, and genetic diseases, including cancer (Wyrobek et al., in press). Annually, in the United States, >2 million conceptions are lost before the 20th week of gestation (assuming 4.2 million births per year), and, of these, ∼50% carry chromosomal defects, including aneuploidies and structural aberrations (McFadden and Friedman 1997). Although de novo structural chromosomal abnormalities are less common than aneuploidy at birth (Hassold 1998) (0.25% vs. 0.33%), it is estimated that ∼80% of the cases are paternally derived (Olson and Magenis 1988; Chandley 1991). Despite the health risk to the developing embryo and offspring, little is known about the etiology of paternally derived structural chromosomal abnormalities.
The human-sperm/hamster-egg cytogenetic method (or hamster-egg method), originally developed by Rudak et al. (1978), became the reference method for examining sperm chromosomes by standard cytogenetic staining techniques (Kamiguchi and Mikamo 1986; Martin et al. 1987, 1991; Pellestor et al. 1987; Brandriff and Gordon 1990). With this method, sperm chromosomes were examined at the first metaphase after fusion of capacitated human sperm with hamster oocytes whose zona pellucidae were removed enzymatically. Significant findings with the hamster-egg method have included the following: (a) high frequencies of sperm complements from healthy men carried structural chromosomal abnormalities (5%–13%), compared with aneuploidies (1%–3%); (b) the presence of more breaks and fragments than chromosomal rearrangements; and (c) men exposed to genotoxic agents showed elevated frequencies of sperm with chromosome aberrations (Martin et al. 1986, 1989; Genesca et al. 1990; Jenderny et al. 1992; Brandriff et al. 1994; Tusell et al. 1995; Robbins 1996; Martin 1998).
Of the aberrations detected in sperm from healthy men by the hamster-egg method, nearly 77% were breaks and fragments. Brandriff et al. (1988) and Estop et al. (1995) suggested that these breaks originated sometime between the end of male meiosis and the beginning of the S-phase of the first cell cycle after fertilization but could not determine whether they preexisted in the fertilizing sperm.
We have adapted multicolor FISH for the simultaneous detection of sperm carrying numerical chromosomal abnormalities as well as two major categories of structural abnormalities: (1) partial chromosomal duplications and deletions (i.e., segmental aneuploidies) which are the sperm products of premeiotic or meiotic chromosomal breakage events or rearrangements, and (2) chromosomal breaks. This new multicolor method integrates three concepts of prior work into a single procedure: (a) the detection of 1p duplications and deletions in sperm (Van Hummelen et al. 1996); (b) the measurement of breaks in 1q12, as developed by Eastmond et al. (1994) for human lymphocytes, and (c) aneuploidy analyses by sperm FISH (Wyrobek et al. 1993). In addition, this new method detects duplications and deletions of 1cen-1q12, provides the distribution of breakpoint locations within the ∼15 Mb 1cen-1q12 region in sperm, and provides data for comparisons of the frequencies of these various chromosomal defects in sperm within a single analysis.
For the human genome, >100 fragile sites have been described (Howard-Peebles 1999). The concurrence between fragile sites and de novo breakpoint locations of chromosomal rearrangements in spontaneous abortions and newborns suggests that certain fragile sites predispose to breakage during gametogenesis (Hecht and Hecht 1984; Warburton 1991). The 1q12 region utilized in our study was identified as a putative fragile site (Sutherland 1991) and found to be sensitive to breakage in mutagen-exposed lymphocytes (Eastmond et al. 1994; Rupa et al. 1995, 1997a, 1997b), buccal mucosal cells (Rupa and Eastmond 1997), cells in amniotic fluid (Dopp et al. 1997b) and cancer cells (Dopp et al. 1997a; Parada et al. 2000).
We recruited healthy men whose semen was evaluated previously in our lab, by means of the hamster-egg method, to address several questions. (i) What are the relative frequencies, in these subjects, of sperm carrying chromosomal breaks versus those carrying partial chromosomal duplications and deletions versus those carrying numerical abnormalities, and are there differences among donors? (ii) What is the breakpoint distribution within the 1cen-1q12 region, and are there differences among donors? (iii) Is there evidence that the chromosomal breaks found in first-cleavage chromosomes with the hamster-egg method are present in the fertilizing human sperm?
Material and Methods
Sperm Donors
Semen samples were provided by four healthy men recruited through the Anonymous Semen Donor Program at Lawrence Livermore National Laboratory (LLNL), which was approved by the LLNL Institutional Review Board. Donors were nonsmokers aged 45–50 years, reported no chronic health or fertility problems, and had no known medical or occupational exposure to genotoxic agents. Donors D1, D2, D3, and D4 correspond to A, C, I, and Z, respectively, in our previous publications (Robbins et al. 1993, 1995, 1997b; Van Hummelen et al. 1996; Baumgartner et al. 1999).
Pretreatment of Sperm, DNA Probes, and FISH
Semen specimens were aliquoted and were stored at −80°C, without preservative. The aliquots were thawed at room temperature (RT) and were gently mixed with a Pasteur pipette. A volume of 5 μl was smeared onto an ethanol-cleaned microscope slide and was air dried for two days. Decondensation of sperm nuclei was performed using DTT and LIS (Wyrobek et al. 1990), according to the method of Robbins et al. (1993), except that sperm nuclei were swollen in LIS for 60 min.
The sperm ACM assay utilized DNA probes for three repetitive-sequence regions on chromosome 1 (fig. 1): D1Z5 (alpha satellite or A), pUC1.77 (classical satellite or C), and D1Z2 (midisatellite or M). D1Z5 was direct-labeled with Texas Red fluorochrome (ONCOR). The pUC1.77 probe was a 1.77-kb cloned EcoRI fragment of human satellite DNA (Cooke and Hindley 1979) labeled with biotin. D1Z2 was a purified plasmid clone (ATCC) labeled with digoxigenin. D1Z2 and pUC1.77 were labeled using the GIBCO BRL Nick Translation System (Life Technologies). Test hybridizations were performed on lymphocyte metaphase spreads to ensure proper localization of each probe.
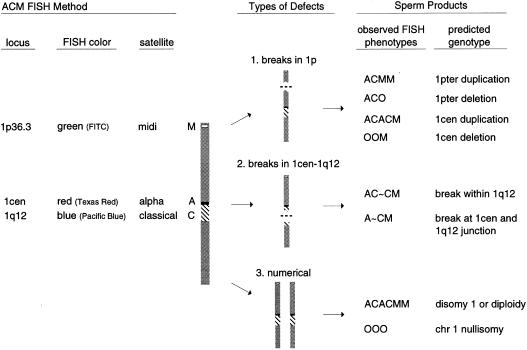
Figure 1.
ACM FISH methodology for human sperm. DNA probes were hybridized to three target regions on chromosome 1 (1cen, 1q12, and 1p36.3) to detect three major types of abnormalities: 1, breakage or rearrangements involving 1p in stem cells, spermatogonia, or spermatocytes can produce multiple sperm carrying partial chromosomal duplications or deletions of 1pter (fig. 2B) or 1cen; 2, chromosome breaks at various locations within 1cen-1q12 (fig. 2C–E); and 3, meiotic nondisjunction of chromosome 1 (fig. 2A).
In situ hybridization was performed according to the method of Van Hummelen et al. (1996), with the following modifications. The probe mix for each slide contained 20 ng each of D1Z2 and pUC1.77 probe, 30 ng of D1Z5 probe, and 10 μg of herring sperm DNA (carrier molecule) in a final concentration of 55% formamide/2 × SSC and 10% dextran sulfate. Hybridization was carried out over 2 nights. Prior to washing, the cover slips were removed in 2 × SSC at RT. Slides were washed in 60% formamide/2 × SSC at 45°C for 5 min, followed by two 10 min washes in 2 × SSC (pH 7.0) at RT. The biotinylated and digoxigenin-labeled probes were detected using a 1:100 dilution in PNM buffer of streptavidin–Pacific Blue (stock concentration 2.5 μg/ml; Molecular Probes) and sheep anti–digoxigenin-fluorescein isothiocyanate (FITC; stock concentration 0.2 mg/ml; Boehringer Mannheim). Immunofluorescence was carried out for 30 min at RT in a humidity chamber, followed by two washes in 2 × SSC for 3 min each. 4,6-diamidino-2-phenylindole (DAPI), diluted to 0.01 μg/ml in Vectashield antifade medium (Vector), was used as counterstain. FISH analysis was performed on a Zeiss Axioplan fluorescence microscope (Carl Zeiss), using a 100× Plan-NEOFLUAR Ph3 objective and equipped as reported by Van Hummelen et al. (1996). Photomicrographs were taken using the SmartCapture VP version 1.4 imaging system (Digital Scientific).
Assay Nomenclature and Scoring Criteria
New microscope-scoring criteria were developed for the sperm ACM assay. Briefly, sperm carrying an abnormal number of same-color domains were scored as abnormal only if the domains were of similar size and intensity (except for breaks within 1q12) and clearly separated (see below). Each fluorescence domain had to be located within the boundary of an intact sperm nucleus. Overlapping sperm nuclei were not scored. Only cells with a flagellum (or tail-attachment site) under bright-field microscopy were scored. Cells outside the normal size limits for decondensed sperm (5–10 μm length) by a microscope-eyepiece reticle were not scored.
The following describes the nomenclature and scoring criteria developed for the ACM assay. One-letter abbreviations were used to denote the presence of each fluorescence domain: A (alpha satellite, 1cen); C (classical satellite, 1q12); and M (midisatellite, 1p36.3). The A and C regions are contiguous on chromosome 1, and the fluorescent domains are adjoined in normal sperm (fig. 1). A normal sperm was scored as ACM; addition or removal of letters denoted duplications or deletions. An “O” was used to indicate the absence of an expected domain.
Duplications and deletions
Sperm containing a duplication or deletion of M were represented by ACMM (fig. 2B) and ACO, respectively. The two M domains had to be separated by the distance of at least one normal M domain. ACACM and OOM represented centromeric duplications and deletions, respectively, of only the AC region.
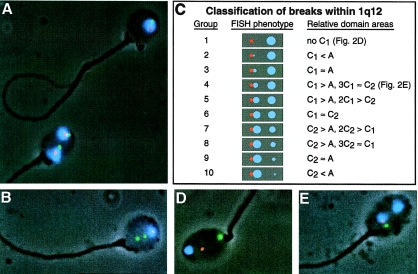
Figure 2.
Photomicrographs and classification scheme for ACM FISH methodology. A, Sperm containing two copies of chromosome 1 (bottom) can represent sperm diploidy or chromosome 1 disomy. A normal sperm is shown on top. B, Sperm carrying a terminal duplication of 1p. C, Fluorescence phenotypes and scoring criteria used to classify sperm according to the relative location of chromosomal breaks within 1q12 (see Material and Methods section and fig. 3). D, Example of a sperm carrying a chromosomal break directly between 1cen and 1q12 (i.e., group 1). E, Example of a sperm carrying a chromosomal break within 1q12 (i.e., group 4). Sperm were imaged at 1,000× under fluorescence and phase-contrast microscopy.
Chromosomal breaks
Sperm carrying breaks within the 1cen-1q12 region were divided into ten groups based on their fluorescence phenotype (fig. 2C). Group 1 included sperm containing a separation directly between the A and C regions and were denoted by A∼CM (fig. 2D). The A and C domains had to be separated by at least half the diameter of the C domain. Sperm classified into groups 2-10 carried two C domains and were represented by AC1∼C2M (fig. 2C and 2E). The C1 and C2 domains had to be separated by at least half the diameter of a normal C domain. C1 represented the domain attached to A and varied proportionally in size with C2. For example, the C1 domain of sperm in group 4 (fig. 2E) was larger than the A domain and would easily fit within the C2 domain area 2–3 times. In comparison, the C1 domain of group 5 would not fit more than once within the C2 area. The scoring criteria for classifying sperm with breaks in 1q12 are shown in figure 2C. These criteria were used to assign a breakpoint location relative to the AC junction for each group of sperm.
Numerical abnormalities
Sperm containing two copies of each domain represented chromosome 1 disomy or sperm diploidy (fig. 2A). Each same-color domain had to be separated by the distance of at least one full domain width. The large C domain required a half-domain separation. The absence of all three fluorescent domains was denoted by “OOO” (i.e., nullisomy 1). This phenotype could also represent lack of hybridization for technical reasons.
Scoring Procedure and Statistical Analyses
A group of slides was coded by a person not involved in scoring. At least 5,000 sperm were scored from the top half of each slide. Then the slides were recoded, and a second set of 5,000 sperm was analyzed from the bottom half of each slide, for a total of ∼10,000 sperm per slide. The CytoScore software program developed at LLNL was adapted for sperm ACM to provide more detailed information regarding each abnormal sperm (i.e., breakpoint location).
The slides were decoded by the original encoder, and the 1st and 2nd scoring analyses of each slide were compared using χ2 analysis. Inter- and intradonor variation in the frequencies of abnormal sperm was evaluated using contingency table analysis. A χ2 statistic was also used to measure inter- and intradonor variation in the distribution of the ten breakpoint locations measured within 1q12.
Results
Duplications or Deletions of 1p and 1cen
The frequencies of sperm containing partial chromosomal duplications or deletions of chromosome 1 were determined for 50,421 sperm from healthy men (table 1). Sperm with terminal duplications or deletions of 1p were detected at average frequencies of 4.5 ± 0.5 and 4.1 ± 1.3 per 104 sperm, respectively. Sperm carrying terminal duplications or deletions of 1p occurred more frequently than centromeric duplications or deletions, which occurred with frequencies of 0.9 ± 0.4 and 0.8 ± 0.3 per 104 sperm, respectively. In both cases, the frequencies of sperm carrying duplications and deletions did not differ significantly from a 1:1 ratio. There was no significant interdonor variation for these types of sperm defects.
Table 1.
Structural Chromosomal Abnormalities in Sperm of Four Healthy Men Evaluated by the ACM Assay
|
Frequencies Per 104 Sperm for Donor |
|||||
| Genotype | D1a | D2b | D3c | D4d | Mean ± SE |
| Segmental aneuploidies: | |||||
| 1pter duplication | 5.3 | 4.6 | 3.0 | 4.9 | 4.5 ± .5 |
| 1pter deletion | 7.3 | 4.0 | 1.0 | 3.9 | 4.1 ± 1.3 |
| Total | 12.6 | 8.6 | 4.0 | 8.8 | 8.5 ± 1.8 |
| 1cen-1q12 duplication | .7 | 0 | 2.0 | 1.0 | .9 ± .4 |
| 1cen-1q12 deletion | 1.3 | .7 | 1.0 | 0 | .8 ± .3 |
| Total | 2.0 | .7 | 3.0 | 1.0 | 1.7 ± .5 |
| Chromosomal breaks: | |||||
| Between 1cen and 1q12e | 2.7 | .7 | 4.0 | 1.0 | 2.1 ± .8 |
| Within 1q12 regionf | 11.3 | 16.5 | 9.0 | 11.0 | 12.0 ± 1.6 |
| Total | 14.0 | 17.2 | 13.0 | 12.0 | 14.1 ± 1.2 |
Frequency and Distribution of Breakpoints within 1cen-1q12
The average frequency of sperm carrying a break within the 1cen-1q12 region was 14.1 ± 1.2 per 104 sperm (table 1; figs. 1 and 2). Breaks directly between 1cen and 1q12 were observed in 2.1 ± 0.8 per 104 sperm. Breaks within 1q12 occurred in 12.0 ± 1.6 per 104 sperm. There was no significant interdonor variation in the total frequency of breaks within the 1cen-1q12 region among the four healthy men studied.
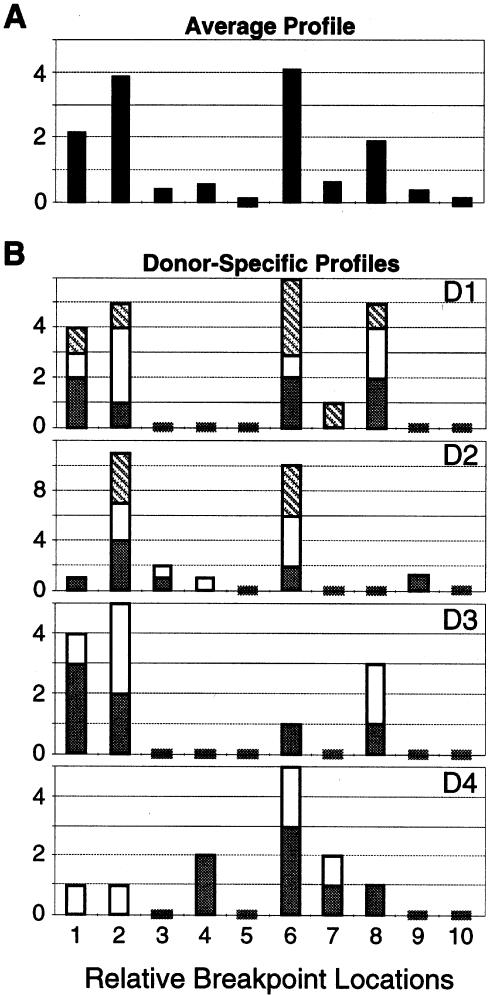
As is illustrated in figure 3A, the average distribution of break sites within 1q12 was strikingly nonrandom (P=1.9×10-10). Overall, the average distribution of breakpoints appeared to be bimodally distributed, with a significantly higher frequency of sperm exhibiting break sites at or near the AC junction (P=3×10-7) and near the middle of 1q12 (P=2×10-6) than elsewhere. Furthermore, there were significant and repeatable differences among donors (fig. 3B). The interdonor distributions of break sites were more variable than was predicted by Poisson analyses (P=.049). For example, the break sites for donor D4 appeared to cluster around the middle of 1q12 (group 6) (fig. 2C) with very few near the centromere (groups 1 and 2), while donor D3 showed a contrasting pattern. These donor differences were confirmed in 2–3 replicated scorings of coded samples for each donor (fig. 3B).
Figure 3.
Distribution of breakpoint locations within 1cen-1q12 of sperm (see fig. 2C). A, Average profile of the frequencies of sperm (per 104) in each breakpoint group across donors. B, Profiles of the raw number of sperm in each breakpoint group per donor (D1–D4). Stacked bars indicate the number of breaks observed during the first (gray), second (white), and third (hatched) coded scoring analyses for each donor. Short bars that cross the X-axis represent zero values.
Structural Aberrations versus Numerical Abnormalities in Sperm
Sperm carrying two copies of all three target regions indicated the presence of an extra copy of chromosome 1 (fig. 2A). However, chromosome 1 disomy cannot be distinguished from diploidy by the ACM assay. As shown in table 2, the average baseline frequency of sperm containing two copies of chromosome 1 was 8.9±0.7 per 104 sperm. This was significantly higher (P=.015) than the 1.2±0.8 per 104 sperm missing all three hybridization domains (6 sperm of 50,421; hybridization efficiency >99.99%), which represents an upper estimate of the frequency of nullisomy 1. This difference presumably represents the fraction of diploid sperm. There was no significant variation among our donors for sperm carrying two copies of chromosome 1. Of the 176 chromosomally abnormal sperm detected by the ACM assay, 29% (51/176) contained numerical abnormalities. This was significantly lower than the 71% carrying structural chromosomal abnormalities of chromosome 1 (P=2×10-8).
Table 2.
Numerical Chromosomal Abnormalities in Sperm of Four Healthy Men Evaluated by the ACM Assay
|
Frequencies per 104 Sperm |
||
| Donor ID (No. ofSpermAnalyzed) | Disomy 1 or Diploidya | Nullisomy 1b |
| D1 (15,073) | 10.6 | 1.9 |
| D2 (15,145) | 7.9 | 0 |
| D3 (10,087) | 8.0 | 0 |
| D4 (10,116) | 9.0 | 3.0 |
| Mean ± SE | 8.9 ± .7 | 1.2 ± .8 |
Characterizations of disomy and diploidy were based on the presence of two copies each of all three domains in a cell. The sperm ACM assay cannot distinguish between disomy 1 and diploidy.
Characterization of nullisomy was based on the absence of all three domains from a cell.
Discussion
Our study of sperm from healthy men provides direct evidence that (a) the spontaneous frequencies of structural chromosomal abnormalities are higher than those of numerical abnormalities, (b) chromosomal breaks are more prevalent than partial duplications and deletions, (c) duplications and deletions of 1pter are symmetrical and occur five times more frequently than those of 1cen, and (d) there are reproducible donor differences in breakpoint locations within 1q12. The following establishes the validity of the sperm ACM assay against the hamster-egg reference method and discusses the etiology of chromosomal breaks and rearrangements in sperm, donor variability, and clinical relevance of this new sperm FISH methodology. The ACM method relies on a high hybridization efficiency in sperm (>99.99%), high probe sensitivities (>99.94% for M and >99.98% for A and C, under the assumption of the extreme case that all missing domains were due to technical reasons), and rigorous scoring criteria (see Material and Methods section).
Validation of Sperm ACM Assay against the Hamster-Egg Method
Genomewide comparison
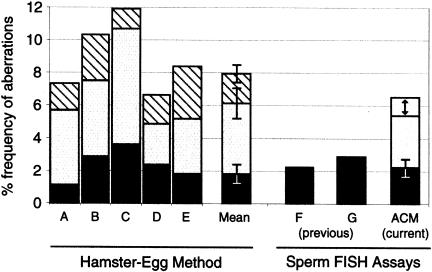
Data obtained by the sperm ACM assay were compared against several hamster-egg studies that evaluated healthy donors (Martin et al. 1987; Estop et al. 1991, 1995; Benet et al. 1992; Jenderny et al. 1992), including the studies of Brandriff et al. (1984, 1985, 1988), from our laboratory, who evaluated the same men used in the current FISH study. Overall, these hamster-egg studies reported an average frequency of 8.2 aberrations per 100 sperm metaphases (814/9926). Under the assumption that the effective target region for the sperm ACM assay from 1q12 to 1pter is 4%–5% of the haploid genome (Mendelsohn et al. 1973), the extrapolation of the ACM data (125 abnormal/50,421 sperm) to the genome yields an estimate of 5.5%–6.5% sperm carrying structural aberrations (fig. 4).
Figure 4.
Genomewide comparison of the sperm ACM and hamster-egg cytogenetic methods. Bars A–E show the frequency of structural aberrations per 100 sperm metaphases evaluated in the following hamster-egg studies: A, Brandriff et al. (1988); B, Estop et al. (1995); C, Jenderny et al. (1992); D, Martin et al. (1987); E, Benet et al. (1992). The contribution made by three types of damage are shown, top to bottom: hatched, fragments of unknown origin (200/814 aberrations); unblackened, chromosome breaks (428/814 aberrations); and black, rearrangements (186/814 aberrations). The weighted mean (number of aberrations/total sperm ± SE) is also shown (sixth bar). Bars F (Van Hummelen et al. 1996) and G (Baumgartner et al. 1999) show the estimated frequencies of sperm carrying rearrangements in two previous studies that used a more limited FISH method. The ACM bar represents the overall frequency of sperm carrying structural abnormalities estimated from the data of the current study. The arrow reflects the estimate that 1q12 to 1pter represents 4%–5% of the haploid genome (see Discussion section). The portion contributed by partial chromosomal duplications and deletions is represented in black.
Comparisons by aberration type
Sperm aberrations detected by the hamster-egg method can be classified into three groups: rearrangements, chromosome breaks, and fragments. The frequency of sperm with rearrangements was ∼2% (186/9926) across hamster-egg studies. When the frequency of partial chromosomal duplications and deletions detected by the ACM assay was extrapolated across the haploid genome, it was not significantly different from the frequency of sperm with rearrangements observed by the hamster-egg method (fig. 4). This extrapolated frequency is also consistent with the earlier findings of Van Hummelen et al. (1996) and Baumgartner et al. (1999) from our lab using more limited sperm FISH analyses.
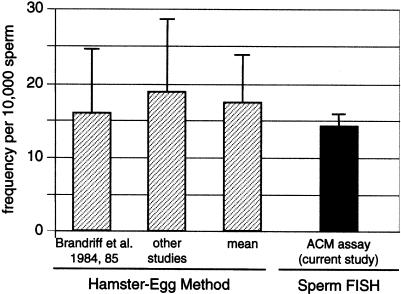
Sperm carrying the AC∼CM or A∼CM FISH phenotypes comprised 58% of all structural abnormalities detected by the ACM assay (table 2). These phenotypes could represent chromosomal breaks, translocations, inversions, or partial duplications involving the 1cen-1q12 region. In the hamster-egg system, chromosomal breaks were the only type of aberration affecting the 1cen-1q12 region and occurred with a frequency of 17.8 per 104 sperm metaphases (Brandriff et al. 1984, 1985; Martin et al. 1987; Estop et al. 1991; Jenderny et al. 1992). When compared against the ACM assay in figure 5, this frequency was not statistically different (P=.5) from the 14 breaks per 104 sperm (72/50,421) detected by FISH. This suggests that the ACM assay provides a valid measure of chromosomal breaks for the 1cen-1q12 region. Acentric fragments are an ambiguous category of damage observed using the hamster-egg method that cannot be resolved with confidence using the ACM assay (fig. 4).
Figure 5.
Comparison of the frequencies of sperm with breaks in 1cen-1q12, as determined by FISH and hamster-egg methods. The first bar represents the mean frequency (±SE) of sperm with breaks in 1cen-1q12 in a hamster-egg study (11 total donors, 2,468 sperm [Brandriff et al. 1984, 1985]) that included the same men evaluated in the current study. The second bar shows the average of three other labs: those of Martin et al. (1987) (30 donors, 1,582 sperm), Estop et al. (1991) (7 donors, 555 sperm), and Jenderny et al. (1992) (8 donors, 450 sperm). The mean represents the weighted average of all four hamster-egg studies.
Etiology of Chromosomal Breaks in Sperm
Our study provides direct quantitative evidence that chromosomal breaks preexist in human sperm before fertilization. Prior hamster-egg studies could not pinpoint the origin of chromosome breaks, owing to an inherent limitation of that methodology. The strong agreement between the frequency of breaks within 1cen-1q12 measured by FISH and the hamster-egg method provides direct evidence that these breaks already existed within the fertilizing sperm, and that few if any were induced after fertilization. Furthermore, this interpretation suggests that there is no selection at fertilization against sperm carrying chromosome breaks, which contradicts findings using certain animal models (Sonta et al. 1991; Chayko and Martin-DeLeon 1992) but supports the findings of Van Hummelen et al. (1997) for a reciprocal translocation carrier, Honda et al. (2000) for a Robertsonian carrier, and Marchetti et al. (1999) in a study using mice.
There is other evidence that spermiogenesis (i.e., post-meiosis) is inherently susceptible to chromosomal damage. Spermatid development is accompanied by dramatic nuclear and chromatin changes. Nucleosome structures are converted into toroids, as histones are replaced by transition proteins and then finally protamines (Balhorn 1982; Brewer et al. 1999). Nuclear volume is markedly reduced coincidentally with greatly diminished DNA repair capabilities (Sega 1976; Working and Butterworth 1984; Joshi et al. 1990). There is compelling animal literature that male postmeiotic cells are extremely sensitive for the induction of chromosomal damage in the form of dominant lethality and heritable translocations in offspring of chemically treated male mice (Shelby et al. 1986, 1987; Hales and Robaire 1990; Russell et al. 1992; Adler et al. 1994; Generoso et al. 1995, 1996; Marchetti et al. 1997). Alkaline elution studies have also found DNA strand breaks in sperm (Singh et al. 1989). Consistent with these findings, our baseline frequencies of breaks in 1q12 in sperm appear to be higher than for lymphocytes: 8 breaks per 104 haploid lymphocyte equivalents (Rupa et al. 1997b) versus the 14 per 104 sperm observed in our study (P=.007).
Characterization of Break Sites within the 1q12 Region of Sperm
Nonrandom distributions of spontaneous and chemically induced chromosomal breakpoints in humans have been previously studied by G-banding (Morad et al. 1973; Funes-Cravioto et al. 1974; Reeves and Margoles 1974; Aula and von Koskull 1976; Kucerova and Polivkova 1976; Morad and El Zawahri 1977; Barrios et al. 1989; Warburton 1991; Estop et al. 1995). Similar breakpoint locations were found in normal and exposed individuals (Tucker et al. 1994) and breakpoints appeared to cluster within the heterochromatic and centromeric regions of certain chromosomes (Johnson et al. 1999). However, none of these studies utilized molecular cytogenetics to characterize the breakpoint locations. Using FISH, we observed a highly significant nonrandom distribution of breakpoints within the subcentromeric 1q12 region. About half of the breaks occurred near the alpha and classical satellite junction, and ∼30% were located in the middle of 1q12 (fig. 3). These sites correspond roughly to two radiation-induced hot spots observed in human lymphocytes by G-banding (Aymé et al. 1976; Barrios et al. 1989). Additional studies are warranted to investigate (a) the relationship between 1q12 break sites in sperm and chromosome-breakpoint locations in newborns, (b) donor variation in baselines and sensitivities to exposure, and (c) the generality of our findings to other men.
Etiology of Chromosomal Duplications and Deletions in Sperm
In this study, duplications to deletions of 1p and 1cen occurred in ∼1:1 ratios (table 1). This symmetry may be a product of meiotic segregation of reciprocal translocations involving 1p which would be expected to produce equal frequencies of 1p duplications and deletions (i.e., adjacent I segregation) and, more rarely, equal frequencies of sperm with duplications and deletions of 1cen (i.e., adjacent II segregation). Although pericentric inversions and unequal sister-chromatid exchanges involving chromosome 1 might produce symmetrical frequencies of duplications and deletions of 1p36.3, they are not predicted to produce duplications or deletions of the 1cen-1q12 region. Also, paracentric inversions would not be expected to yield the observed duplication and deletion phenotypes. Assuming all duplications and deletions measured by the sperm ACM assay are derived from reciprocal translocations that are randomly distributed along the genome, we calculate that as many as 5/1,000 sperm in healthy men might carry a balanced translocation.
Relative Frequencies and Donor Variation for Chromosomal Abnormalities in Sperm
The results of the ACM FISH assay indicate that structural chromosomal defects are more common than numerical abnormalities in sperm of healthy men. Adjusting for the ∼8-fold difference in relative genome content, the frequency of breaks within the 1q12 region was much higher than duplications and deletions (P<.05). The aggregate frequency of chromosomal breaks, duplications, and deletions detected for chromosome 1 was 24.8 per 104 sperm (125/50,421). This was significantly higher (P<2×10-8) than the 10.1/104 sperm (51/50,421) carrying numerical abnormalities of chromosome 1 (disomy, nullisomy, and diploidy). The frequency of sperm with an extra copy of chromosome 1 (8.9±0.7 per 104 sperm) corresponds well to the combined frequency of disomy 1 (1.7/104) plus diploidy (6.6/104) detected, in an earlier study, among ∼120,000 sperm from these same men (Van Hummelen et al. 1996).
There was no significant variation among donors in the frequencies of sperm with two copies of chromosome 1 (range 7.9–10.6 per 104 sperm), nullisomy 1 (0–3.0 per 104 sperm), or chromosomal breakage within the 1cen-1q12 region (12–17.2 per 104 sperm). Furthermore, there was no significant variation in the frequencies of sperm with terminal or centromeric duplications or deletions among men. The breakpoint distributions among the four donors in our study (fig. 3B) were more variable compared to average (fig. 3A) than predicted by Poisson statistics, but the effect is small (P=.049) and a larger study is warranted to investigate variation of breakpoint distributions among men.
Clinical Relevance
By use of prior FISH methodologies for sperm aneuploidy, several paternal risk factors have been identified for sperm disomies, such as paternal age (Griffin et al. 1995; Martin et al. 1995; Robbins et al. 1995; Rousseaux et al. 1998), chemotherapy (Monteil et al. 1997; Robbins et al. 1997a; Martin et al. 1999), and cigarette smoking (Robbins et al. 1997b; Rubes et al. 1998). Our method for detecting chromosomal breaks and rearrangements in sperm provides an important new approach for measuring exposure to chromosome-breaking agents and assessing genetic predisposition to such damage. Pairing our ACM assay with multicolor FISH assays for sperm aneuploidy (e.g., the X-Y-21-18 assay [Van Hummelen et al. 1997] and the X-Y-21 assay [Baumgartner et al. 1999]) promises to provide a robust approach for detecting paternally transmissible chromosomal damage of both the numerical and the structural type.
Acknowledgments
The authors wish to thank Drs. Francesco Marchetti, Paul Van Hummelen, Brenda Eskenazi, and Kirby Johnson for their helpful comments. We would also like to thank Drs. Dan Pinkel and Rick Segrave at the University of California, San Francisco, for generously providing the pUC1.77 plasmid clone. This work was performed under the auspices of the U.S. Department of Energy by LLNL, contract W-7405-ENG-48, with funding support from NIEHS Superfund P4ZES04705 and tuition and travel support for E.S. from West Virginia University. This paper is published with the approval of the director of the West Virginia Agriculture, Forestry, and Consumer Sciences Experiment Station, as Scientific Paper 2770.
References
- Adler ID, Reitmeir P, Schmoller R, Schriever-Schwemmer G (1994) Dose response for heritable translocations induced by acrylamide in spermatids of mice. Mutat Res 309:285–291 [DOI] [PubMed] [Google Scholar]
- Aula P, von Koskull H (1976) Distribution of spontaneous chromosome breaks in human chromosomes. Hum Genet 32:143–148 [DOI] [PubMed] [Google Scholar]
- Aymé S, Mattei J, Mattei M, Aurran Y, Giraud F (1976) Nonrandom distribution of chromosome breaks in cultured lymphocytes of normal subjects. Hum Genet 31:161–175 [DOI] [PubMed] [Google Scholar]
- Balhorn R (1982) A model for the structure of chromatin in mammalian sperm. J Cell Biol 93:298–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrios L, Miro R, Caballin M, Fuster C, Guedea F, Subias A, Egozcue J (1989) Cytogenetic effects of radiotherapy: breakpoint distribution in induced chromosome aberrations. Cancer Genet Cytogenet 41:61–70 [DOI] [PubMed] [Google Scholar]
- Baumgartner A, Van Hummelen P, Lowe XR, Adler ID, Wyrobek AJ (1999) Numerical and structural chromosomal abnormalities detected in human sperm with a combination of multicolor FISH assays. Environ Mol Mutagen 33:49–58 [DOI] [PubMed] [Google Scholar]
- Benet J, Genesca A, Navarro J, Egozcue J, Templado C (1992) Cytogenetic studies in motile sperm from normal men. Hum Genet 89:176–180 [DOI] [PubMed] [Google Scholar]
- Brandriff B, Gordon L, Ashworth L, Watchmaker G, Carrano A, Wyrobek A (1984) Chromosomal abnormalities in human sperm: comparisons among four healthy men. Hum Genet 66:193–201 [DOI] [PubMed] [Google Scholar]
- Brandriff B, Gordon L, Ashworth L, Watchmaker G, Moore D, Wyrobek AJ, Carrano AV (1985) Chromosomes of human sperm: variability among normal individuals. Hum Genet 70:18–24 [DOI] [PubMed] [Google Scholar]
- Brandriff B, Gordon LA, Moore DH, Carrano AV (1988) An analysis of structural aberrations in human sperm chromosomes. Cytogenet Cell Genet 47:29–36 [DOI] [PubMed] [Google Scholar]
- Brandriff B, Gordon LA (1990) Human sperm cytogenetics and the one-cell zygote. In: Allen J, Bridges B, Lyon M, Moses M, Russell L (eds) Banbury report 34: Biology of mammalian germ cell mutagenesis. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, pp 183–194 [Google Scholar]
- Brandriff B, Meistrich M, Gordon L, Carrano A, Liang J (1994) Chromosomal damage in sperm of patients surviving Hodgkin's disease following MOPP (nitrogen mustard, vincristine, procarbazine, and prednisone) therapy with and without radiotherapy. Hum Genet 93:295–299 [DOI] [PubMed] [Google Scholar]
- Brewer L, Corzett M, Balhorn R (1999) Protamine-induced condensation and decondensation of the same DNA molecule. Science 286:120–123 [DOI] [PubMed] [Google Scholar]
- Chandley AC (1991) On the paternal origin of de novo mutation in man. J Med Genet 28:217–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chayko C, Martin-DeLeon P (1992) The murine Rb(6.16) translocation: alterations in the proportion of alternate sperm segregants effecting fertilization in vitro and in vivo. Hum Genet 90:79–85 [DOI] [PubMed] [Google Scholar]
- Cooke HJ, Hindley J (1979) Cloning of human satellite III DNA: different components are on different chromosomes. Nucleic Acids Res 6:3177–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopp E, Papp T, Schiffmann D (1997a) Detection of hyperdiploidy and chromosome breakage affecting the 1 (1cen-q12) region in lentigo malignant melanoma (LMM), superficial spreading melanoma (SSM) and congenital nevus (CN) cells in vitro by the multicolor FISH technique. Cancer Lett 120:157–163 [DOI] [PubMed] [Google Scholar]
- Dopp E, Schuler M, Schiffmann D, Eastmond DA (1997b) Induction of micronuclei, hyperdiploidy, and chromosomal breakage affecting the centric/pericentric regions of chromosomes 1 and 9 in human amniotic fluid cells after treatment with asbestos and ceramic fibers. Mutat Res 377:77–87 [DOI] [PubMed] [Google Scholar]
- Eastmond DA, Rupa DS, Hasegawa L (1994) Detection of hyperdiploidy and chromosome breakage in interphase human lymphocytes following exposure to the benzene metabolite hydroquinone using multicolor fluorescence in situ hybridization with DNA probes. Mutat Res 322:9–20 [DOI] [PubMed] [Google Scholar]
- Estop AM, Cieply K, Vankirk V, Munn S, Carver K (1991) Cytogenetic studies in human sperm. Hum Genet 87:447–451 [DOI] [PubMed] [Google Scholar]
- Estop AM, Marquez C, Munne S, Navarro J, Cieply K, Van Kirk V, Martorell MR, Benet J, Templado C (1995) An analysis of human sperm chromosome breakpoints. Am J Hum Genet 56:452–460 [PMC free article] [PubMed] [Google Scholar]
- Funes-Cravioto F, Yakovienko K, Kuleshov N, Zhurkov V (1974) Localization of chemically induced breaks in chromosomes of human leucocytes. Mutat Res 23:87–105 [DOI] [PubMed] [Google Scholar]
- Generoso WM, Witt KL, Cain KT, Hughes L, Cacheiro NL, Lockhart AM, Shelby MD (1995) Dominant lethal and heritable translocation tests with chlorambucil and melphalan in male mice. Mutat Res 345:167–180 [DOI] [PubMed] [Google Scholar]
- Generoso WM, Sega GA, Lockhart AM, Hughes LA, Cain KT, Cacheiro NL, Shelby MD (1996) Dominant lethal mutations, heritable translocations, and unscheduled DNA synthesis induced in male mouse germ cells by glycidamide, a metabolite of acrylamide. Mutat Res 371:175–183 [DOI] [PubMed] [Google Scholar]
- Genesca A, Miro R, Caballin MR, Benet J, Germa JR, Egozcue J (1990) Sperm chromosome studies in individuals treated for testicular cancer. Hum Reprod 5:286–290 [DOI] [PubMed] [Google Scholar]
- Griffin DK, Abruzzo MA, Millie EA, Sheean LA, Feingold E, Sherman SL, Hassold TJ (1995) Non-disjunction in human sperm: evidence for an effect of increasing paternal age. Hum Mol Genet 4:2227–2232 [DOI] [PubMed] [Google Scholar]
- Hales BF, Robaire B (1990) Reversibility of the effects of chronic paternal exposure to cyclophosphamide on pregnancy outcome in rats. Mutat Res 229:129–134 [DOI] [PubMed] [Google Scholar]
- Hassold TJ (1998) Nondisjunction in the human male. Curr Top Dev Biol 37:383–406 [DOI] [PubMed] [Google Scholar]
- Hecht F, Hecht B (1984) Fragile sites and chromosome breakpoints in constitutional rearrangements II. Spontaneous abortions, stillbirths and newborns. Clin Genet 26:174–177 [DOI] [PubMed] [Google Scholar]
- Honda H, Miharu N, Samura O, He H, Ohama K (2000) Meiotic segregation analysis of a 14;21 Robertsonian translocation carrier by fluorescence in situ hybridization. Hum Genet 106:188–193 [DOI] [PubMed] [Google Scholar]
- Howard-Peebles P (1999) Fragile X: from cytogenetics to molecular genetics. In: Gersen S, Keagle M (eds) The principles of clinical cytogenetics. Humana Press Inc., Totowa, New Jersey, pp 425–442 [Google Scholar]
- Jenderny J, Jacobi M, Ruger A, Rohrborn G (1992) Chromosome aberrations in 450 sperm complements from eight controls and lack of increase after chemotherapy in two patients. Hum Genet 90:151–154 [DOI] [PubMed] [Google Scholar]
- Johnson K, Brenner D, Nath J, Tucker J, Geard C (1999) Radiation-induced breakpoint misrejoining in human chromosomes: random or non-random? Int J Radiat Biol 75:131–141 [DOI] [PubMed] [Google Scholar]
- Joshi D, Yick J, Murray D, Meistrich M (1990) Stage-dependent variation in the radiosensitivity of DNA in developing male germ cells. Radiat Res 121:274–281 [PubMed] [Google Scholar]
- Kamiguchi Y, Mikamo K (1986) An improved, efficient method for analyzing human sperm chromosomes using zona-free hamster ova. Am J Hum Genet 38:724–740 [PMC free article] [PubMed] [Google Scholar]
- Kucerova M, Polivkova Z (1976) Banding technique used for the detection of chromosomal aberrations induced by radiation and alkylating agents TEPA and epichlorohydrin. Mutat Res 34:279–290 [DOI] [PubMed] [Google Scholar]
- Marchetti F, Lowe X, Bishop J, Wyrobek AJ (1997) Induction of chromosomal aberrations in mouse zygotes by acrylamide treatment of male germ cells and their correlation with dominant lethality and heritable translocations. Environ Mol Mutagen 30:410–417 [DOI] [PubMed] [Google Scholar]
- Marchetti F, Lowe X, Bishop J, Wyrobek J (1999) Absence of selection against aneuploid mouse sperm at fertilization. Biol Reprod 61:948–954 [DOI] [PubMed] [Google Scholar]
- Martin R, Hildebrand K, Yamamoto J, Rademaker A, Barnes M, Douglas G, Arthur K, Ringrose T, Brown I (1986) An increased frequency of human sperm chromosomal abnormalities after radiotherapy. Mutat Res 174:219–225 [DOI] [PubMed] [Google Scholar]
- Martin R, Rademaker A, Hildebrand K, Long-Simpson L, Peterson D, Yamamoto J (1987) Variation in the frequency and type of sperm chromosomal abnormalities among normal men. Hum Genet 77:108–114 [DOI] [PubMed] [Google Scholar]
- Martin R, Rademaker A, Hildebrand K, Barnes M, Arthur K, Ringrose T, Brown I, Douglas G (1989) A comparison of chromosomal aberrations induced by in vivo radiotherapy in human sperm and lymphocytes. Mutat Res 226:21–30 [DOI] [PubMed] [Google Scholar]
- Martin R, Ko E, Rademaker A (1991) Distribution of aneuploidy in human gametes: comparison between human sperm and oocytes. Am J Med Genet 39:321–331 [DOI] [PubMed] [Google Scholar]
- Martin R, Spriggs E, Ko E, Rademaker A (1995) The relationship between paternal age, sex ratios, and aneuploidy frequencies in human sperm, as assessed by multicolor FISH. Am J Hum Genet 57:1395–1399 [PMC free article] [PubMed] [Google Scholar]
- Martin R (1998) Human sperm chromosome complements in chemotherapy patients and infertile men. Chromosoma 107:523–527 [DOI] [PubMed] [Google Scholar]
- Martin R, Ernst S, Rademaker A, Barclay L, Ko E, Summers N (1999) Analysis of sperm chromosome complements before, during, and after chemotherapy. Cancer Genet Cytogenet 108:133–136 [DOI] [PubMed] [Google Scholar]
- McFadden D, Friedman J (1997) Chromosome abnormalities in human beings. Mutat Res 396:129–140 [DOI] [PubMed] [Google Scholar]
- Mendelsohn M, Mayall L, Bogart B, Moore D, Perry B (1973) DNA content and DNA-based centromeric index of the 24 human chromosomes. Science 179:1126–1129 [DOI] [PubMed] [Google Scholar]
- Monteil M, Rousseaux S, Chevret E, Pelletier R, Cozzi J, Sele B (1997) Increased aneuploid frequency in spermatozoa from a Hodgkin's disease patient after chemotherapy and radiotherapy. Cytogenet Cell Genet 76:134–138 [DOI] [PubMed] [Google Scholar]
- Morad M, Jonasson J, Lindsten J (1973) Distribution of mitomycin C induced breaks on human chromosomes. Hereditas 74:273–281 [DOI] [PubMed] [Google Scholar]
- Morad M, El Zawahri M (1977) Non-random distribution of cyclophosphamide-induced chromosome breaks. Mutat Res 42:125–130 [DOI] [PubMed] [Google Scholar]
- Olson SB, Magenis RE (1988) Preferential paternal origin of de novo structural chromosome rearrangements. In: Daniel A (ed) The cytogenetics of mammalian autosomal rearrangements. Liss, New York, pp 583–599 [Google Scholar]
- Parada LA, Limon J, Iliszko M, Czauderna P, Gisselsson D, Hoglund M, Kullendorff CM, Wiebe T, Mertens F, Johansson B (2000) Cytogenetics of hepatoblastoma: further characterization of 1q rearrangements by fluorescence in situ hybridization: an international collaborative study. Med Pediatr Oncol 34:165–170 [DOI] [PubMed] [Google Scholar]
- Pellestor F, Sele B, Jalbert H (1987) Chromosome analysis of spermatozoa from a male heterozygous for a 13;14 Robertsonian translocation. Hum Genet 76:116–120 [DOI] [PubMed] [Google Scholar]
- Reeves B, Margoles C (1974) Preferential location of chlorambucil-induced breakage in the chromosomes of normal human lymphocytes. Mutat Res 26:205–208 [DOI] [PubMed] [Google Scholar]
- Robbins WA, Segraves R, Pinkel D, Wyrobek AJ (1993) Detection of aneuploid human sperm by fluorescence in situ hybridization: evidence for a donor difference in frequency of sperm disomic for chromosomes 1 and Y. Am J Hum Genet 52:799–807 [PMC free article] [PubMed] [Google Scholar]
- Robbins WA, Baulch JE, Moore DH, Weier HU, Blakey D, Wyrobek AJ (1995) Three probe fluorescence in situ hybridization to asses chromosome X, Y and 8 aneuploidy in sperm of 14 men from two healthy groups: evidence for a paternal age effect on sperm aneuploidy. Reprod Fertil Dev 7:1–11 [DOI] [PubMed] [Google Scholar]
- Robbins WA (1996) Cytogenetic damage measured in human sperm following cancer chemotherapy. Mutat Res 355:235–252 [DOI] [PubMed] [Google Scholar]
- Robbins WA, Meistrich ML, Moore D, Hagemeister FB, Weier HU, Cassel MJ, Wilson G, Eskenazi B, Wyrobek AJ (1997a) Chemotherapy induces transient sex chromosomal and autosomal aneuploidy in human sperm. Nat Genet 16:74–78 [DOI] [PubMed] [Google Scholar]
- Robbins WA, Vine MF, Truong KY, Everson RB (1997b) Use of fluorescence in situ hybridization (FISH) to assess effects of smoking, caffeine, and alcohol on aneuploidy load in sperm of healthy men. Environ Mol Mutagen 30:175–183 [DOI] [PubMed] [Google Scholar]
- Rousseaux S, Hazzouri M, Pelletier R, Monteil M, Usson Y, Sele B (1998) Disomy rates for chromosomes 14 and 21 studied by fluorescent in-situ hybridization in spermatozoa from three men over 60 years of age. Mol Hum Reprod 4:695–699 [DOI] [PubMed] [Google Scholar]
- Rubes J, Lowe X, Moore DH, Perreault S, Slott V, Everson D, Selevan S, Wyrobek AJ (1998) Smoking cigarettes is associated with increased sperm disomy in teenage men. Fertil Steril 70:715–723 [DOI] [PubMed] [Google Scholar]
- Rudak E, Jacobs PA, Yanagimachi R (1978) Direct analysis of the chromosome constitution of human spermatozoa. Nature 274:911–913 [DOI] [PubMed] [Google Scholar]
- Rupa DS, Hasegawa L, Eastomand DA (1995) Detection of chromosomal breakage in the 1 cen-1q12 region of interphase human lymphocytes using multicolor fluorescence in situ hybridization with tandem DNA probes. Cancer Res 55:640–645 [PubMed] [Google Scholar]
- Rupa DS, Eastmond DA (1997) Chromosomal alterations affecting the 1cen-1q12 region in buccal mucosal cells of betel quid chewers detected using multicolor fluorescence in situ hybridization. Carcinogenesis 18:2347–2351 [DOI] [PubMed] [Google Scholar]
- Rupa DS, Hasegawa LS, Eastmond DA (1997a) Detection of chromosomal alterations affecting the 1cen-1q12 region in irradiated granulocytes and lymphocytes by multicolour FISH with tandem DNA probes. Mutagenesis 12:195–200 [DOI] [PubMed] [Google Scholar]
- Rupa DS, Schuler M, Eastmond DA (1997b) Detection of hyperdiploidy and breakage affecting the 1cen-1q12 region of cultured interphase human lymphocytes treated with various genotoxic agents. Environ Mol Mutagen 29:161–167 [PubMed] [Google Scholar]
- Russell LB, Hunsicker PR, Shelby MD (1992) Melphalan, a second chemical for which specific-locus mutation induction in the mouse is maximum in early spermatids. Mutat Res 282:151–158 [DOI] [PubMed] [Google Scholar]
- Sega GA (1976) Molecular dosimetry of chemical mutagens. Measurement of molecular dose and DNA repair in mammalian germ cells. Mutat Res 38:317–326 [DOI] [PubMed] [Google Scholar]
- Shelby MD, Cain KT, Hughes LA, Braden PW, Generoso WM (1986) Dominant lethal effects of acrylamide in male mice. Mutat Res 173:35–40 [DOI] [PubMed] [Google Scholar]
- Shelby MD, Cain KT, Cornett CV, Generoso WM (1987) Acrylamide: induction of heritable translocations in male mice. Environ Mutagen 9:363–368 [DOI] [PubMed] [Google Scholar]
- Singh NP, Danner DB, Tice RR, McCoy MT, Collins GD, Schneider EL (1989) Abundant alkali-sensitive sites in DNA of human and mouse sperm. Exp Cell Res 184:461–470 [DOI] [PubMed] [Google Scholar]
- Sonta S, Yamada M, Tsukasaki M (1991) Failure of chromosomally abnormal sperm to participate in fertilization in the Chinese hamster. Cytogenet Cell Genet 57:200–203 [DOI] [PubMed] [Google Scholar]
- Sutherland G (1991) Chromosomal fragile sites. Genet Anal Tech Appl 8:161–166 [DOI] [PubMed] [Google Scholar]
- Tucker J, Senft J (1994) Analysis of naturally occurring and radiation-induced breakpoint locations in human chromosomes 1, 2 and 4. Radiat Res 140:31–36 [PubMed] [Google Scholar]
- Tusell L, Alvarez R, Caballin MR, Genesca A, Miro R, Ribas M, Egozcue J (1995) Induction of micronuclei in human sperm-hamster egg hybrids at the two-cell stage after in vitro gamma-irradiation of human spermatozoa. Environ Mol Mutagen 26:315–323 [DOI] [PubMed] [Google Scholar]
- Van Hummelen P, Lowe XR, Wyrobek AJ (1996) Simultaneous detection of structural and numerical chromosome abnormalities in sperm of healthy men by multicolor fluorescence in situ hybridization. Hum Genet 98:608–615 [DOI] [PubMed] [Google Scholar]
- Van Hummelen P, Manchester D, Lowe X, Wyrobek AJ (1997) Meiotic segregation, recombination, and gamete aneuploidy assessed in a t(1;10)(p22.1;q22.3) reciprocal translocation carrier by three- and four-probe multicolor FISH in sperm. Am J Hum Genet 61:651–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton D (1991) De novo balanced chromosome rearrangements and extra marker chromosomes identified at prenatal diagnosis: clinical significance and distribution of breakpoints. Am J Hum Genet 49:995–1013 [PMC free article] [PubMed] [Google Scholar]
- Working PK, Butterworth BE (1984) An assay to detect chemically induced DNA repair in rat spermatocytes. Environ Mutagen 6:273–286 [DOI] [PubMed] [Google Scholar]
- Wyrobek A, Alhborn T, Balhorn R, Stanker L, Pinkel D (1990) Fluorescence in situ hybridization to Y chromosomes in decondensed human sperm nuclei. Mol Reprod Dev 27:200–208 [DOI] [PubMed] [Google Scholar]
- Wyrobek AJ (1993) Methods and concepts in detecting abnormal reproductive outcomes of paternal origin. Reprod Toxicol 7:3–16 [DOI] [PubMed] [Google Scholar]
- Wyrobek AJ, Marchetti F, Sloter E, Bishop J (2000) Chromosomally defective sperm and their developmental consequences. In: Anderson D (ed) Human monitoring after environmental and occupational exposure to chemical and physical agents. NATO ASI Series (in press) [Google Scholar]