Abstract
Pyridoxine-dependent epilepsy (PDE) is a rare autosomal recessive disorder characterized by generalized seizures in the first hours of life and responding only to pyridoxine hydrochloride. The pathogenesis of PDE is unknown, but an alteration in the binding of pyridoxal 5-phosphate to glutamic acid decarboxylase (GAD) has been postulated in patients with PDE. Results are reported for genetic linkage analyses in four families with consanguineous parents and in one family with nonconsanguineous parents. The GAD1 (2q31) and GAD2 genes (10p23) were tested and excluded. A genomewide search was subsequently performed, using microsatellite markers at an average distance of 10 cM, and the search revealed linkage of the disease-causing gene to markers on chromosome 5q31.2-q31.3 (maximum LOD score [Zmax] 8.43 at recombination fraction [θ] 0 and Zmax=7.58 at θ=0 at loci D5S2017 and D5S1972, respectively). A recombination event, between loci D5S638 and D5S463, in one family defined the distal boundary, and a second recombination event between loci D5S2011 and D5S2017 in another family defined the proximal boundary of the genetic interval encompassing the PDE gene (5.1 cM). Ongoing studies may lead to the identification of the disease-causing gene.
Pyridoxine-dependent epilepsy (PDE [MIM 226100]) is a rare autosomal recessive disorder characterized by a combination of various seizure types that usually occurs in the first hours of life and is unresponsive to standard anticonvulsants, responding only to immediate administration of pyridoxine hydrochloride (vitamin B6) (Hunt et al. 1954; Waldinger and Berg 1964; Nabbout et al. 1999). The dependence is permanent, and the interruption of daily pyridoxine supplementation leads to the recurrence of seizures. The pathogenesis of vitamin B6–dependent seizures is unknown, but some researchers have postulated that a reduced synthesis of γ-aminobutyric acid (GABA), resulting from diminished activity of the glutamic acid decarboxylase (GAD), could be responsible for the lowered seizure threshold in vitamin B6–dependent patients (Scriver and Whelan 1969; Yoshida et al. 1971). GAD requires pyridoxal 5-phosphate (PLP) as a coenzyme for activity, and an alteration in the binding of PLP to GAD has also been postulated in vitamin B6–dependent patients (Scriver and Whelan 1969; Scriver and Gibson 1995). Biochemical findings in affected individuals included (a) decreased levels of GABA in the brain and cerebrospinal fluid (CSF), (b) increased levels of glutamate in CSF and the cerebral cortex, and (c) decreased levels of PLP in the frontal cortex (Lott et al. 1978; Baumeister et al. 1994). Study of GAD activity in the kidney of an affected proband has revealed a complete deficiency of GABA synthesis, with a restoration to normal levels when the coenzyme is added (Yoshida et al. 1971).
In this article, we report the genetic linkage analysis of four families with consanguineous parents and of one family with nonconsanguineous parents. The clinical and electroencephalographic features of these families have been reported elsewhere (Nabbout et al. 1999). The diagnosis of PDE was based on the presence of generalized seizures that responded only to vitamin B6 administration in the first days of life. The families were of Turkish (family 1), French (family 2), Maurician (family 3), and Algerian ancestries (families 4 and 5).
DNA extraction and microsatellite analyses were performed as described elsewhere (Belin et al. 1998). The homozygosity-mapping strategy in consanguineous families was based on the assumption that affected individuals from the same kindred are homozygous by descent, for the disease-causing gene mutation. Microsatellite DNA markers from the entire genome were chosen from the Généthon map (Dib et al. 1996), and two-point linkage analyses using the MLINK option of the linkage package (Lathrop et al. 1985) were performed. We assumed the frequency of the disease allele to be .001 and set the penetrance at 100%, assuming an autosomal recessive mode of inheritance. We took into account the inbreeding loops, but allele frequencies were not available in the populations studied.
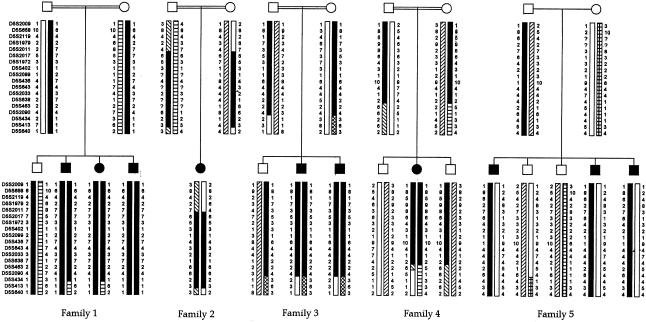
We first tested and excluded the GAD1 (2q31) and GAD2 (10p.23) genes, because these genes were regarded a priori as excellent candidates (data not shown). Then, we performed a genomewide search, with the use of microsatellite markers at an average distance of 10 cM, and found linkage of the disease-causing gene to markers on chromosome 5q31.2-q31.3 (maximum LOD score [Zmax] 8.43 at recombination fraction [θ] 0 and Zmax=7.58 at θ=0, for markers AFMb074xg1 and AFMa190bx1, respectively, at loci D5S2017 and D5S1972, respectively) (table 1). The probands were homozygotes at loci D5S2017, D5S1972, D5S402, D5S2099, D5S436, D5S643, D5S2033, and D5S638, in all consanguineous families. In family 5, affected individuals were genoidentical but were different from unaffected sibs. A recombination event between loci D5S2011 and D5S2017 in family 2 defined the proximal boundary of the genetic interval encompassing the disease-causing gene. In addition, a second recombination event, between D5S638 and D5S463, in family 4 defined the distal boundary of the genetic interval encompassing the PDE gene (5.1 cM) (fig. 1).
Table 1.
Two-Point LOD Scores for Linkage of the Pyridoxine-Dependent Epilepsy Locus to Chromosome 5q31 Markers
|
LOD Score at θ = |
|||||||||
| Marker | Genetic Distancea (cM) | .00 | .05 | .10 | .15 | .20 | .30 | Zmax | θ |
| D5S658 | 2.7 | −99 | 5.14 | 4.80 | 4.22 | 3.55 | 2.13 | 5.14 | .05 |
| D5S2017 | 0 | 8.43 | 7.31 | 6.20 | 5.10 | 4.05 | 2.15 | 8.43 | .00 |
| D5S1972 | .2 | 7.58 | 6.57 | 5.58 | 4.60 | 3.66 | 1.97 | 7.58 | .00 |
| D5S402 | .8 | −99 | 2.36 | 2.06 | 1.67 | 1.28 | .61 | 2.36 | .05 |
| D5S436 | 1.9 | −99 | 5.02 | 4.48 | 3.82 | 3.12 | 1.79 | 5.02 | .05 |
From locus D5S2017 according to the Généthon map.
Figure 1.
Pedigrees and genotype data for markers spanning the PDE-locus interval. Markers are given in order, from the p telomere to the q telomere. The region of homozygosity is blackened.
The physical map of the 5q31.3-qter has been reported by Kostrzewa et al. (1998). More than 50 genes and >130 expressed sequence tags have been assigned to distal chromosome 5q. Among them, the GABA-A–receptor gene cluster and the glutamate receptor gene (GRIA1) could be regarded as candidate genes in epilepsy; however, they are reported to map to chromosomes 5q33 and 5q32, respectively—that is, outside the genetic interval defined by our study (GeneMap’99). At least three genes mapped to the critical region—namely, the fibroblast growth factor-1, glucocorticoid receptor, and KIAAO555 genes. Whether any of these genes is involved in PDE is under investigation.
In conclusion, we have excluded the primary involvement of GAD in the pathogenesis of PDE and have found linkage of the disease-causing gene to the 5q31 region, in the vicinity of a GABA-A–receptor gene cluster and of a glutamate-receptor gene, but both genes were excluded by our linkage data. We hope that ongoing studies will identify the disease-causing gene and help to explain the pathogenesis of pyridoxine dependence in PDE and, more generally, help to elucidate the pathogenesis of epileptic seizures in humans.
Electronic-Database Information
The accession number (OMIM) and URLs for data in this article are as follows:
- Online Mendelian Inheritance in Man, http://www.ncbi.nlm.nih.gov/Omim (for PDE [MIM 226100])
- GeneMap’99, http://www.ncbi.nlm.nih.gov/genemap99/
- Généthon, http://www.genethon.fr/genethon_en.html
References
- Baumeister FA, Wieland G, Shin YS, Egger J (1994) Glutamate in pyridoxine-dependent epilepsy: neurotoxic glutamate concentration in the cerebrospinal fluid and its normalization by pyridoxine. Pediatrics 94:318–321 [PubMed] [Google Scholar]
- Belin V, Cusin V, Girlich D, Toutain A, Moncla A, Vekemans M, Le Merrer M, Munnich A, Cormier-Daire V (1998) SHOX mutations in dyschondrosteosis (Leri-Weill syndrome). Nat Genet 19:67–69 [DOI] [PubMed] [Google Scholar]
- Dib C, Fauré S, Fizames C, Samson D, Drouot N, Vignal A, Millasseau P, Marc S, Hazan J, Seboun E, Lathrop M, Gyapay G, Morissette J, Weissenbach J (1996) A comprehensive genetic map on the human genome based on 5,264 microsatellites. Nature 380:152–154 [DOI] [PubMed] [Google Scholar]
- Hunt A, Stokes J, McCrory W, Stroud H (1954) Pyridoxine dependency: report of a case of intractable convulsions in an infant controlled by pyridoxine. Pediatrics 13:140–143 [PubMed] [Google Scholar]
- Kostrzewa M, Krings BW, Dixon MJ, Eppelt K, Köhler A, Grady DLN (1998) Integrated physical mapping and transcript map of 5q31.3-qter. Eur J Hum Genet 6:266–274 [DOI] [PubMed] [Google Scholar]
- Lathrop GM, Lalouel JM, Julier C, Ott J (1984) Strategies for multilinkage analysis in humans. Proc Natl Acad Sci USA 81:3433–3446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott IT, Coulombe T, Di Paolo RV, Richardson EP Jr, Levy HL (1978) Vitamin B6–dependent seizures: pathology and chemical findings in brain. Neurology 28:47–54 [DOI] [PubMed] [Google Scholar]
- Nabbout R, Soufflet C, Plouin P, Dulac O (1999) Pyridoxine dependent-epilepsy: a suggestive electroclinical pattern. Arch Dis Child Fetal Neonatal Ed 81:F125–F129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scriver CR, Gibson KM (1995) Disorders of β- and γ-amino acids in free and peptide-linked forms. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited disease. Vol 1, 7th ed. McGraw-Hill, New York, pp 1349–1368 [Google Scholar]
- Scriver CR, Whelan DT (1969) Glutamic acid decarboxylase (GAD) in mammalian tissue outside the central nervous system and its possible relevance to hereditary vitamin B6 dependency with seizures. Ann NY Acad Sci 166:83–96 [DOI] [PubMed] [Google Scholar]
- Waldinger C, Berg RB (1963) Signs of pyridoxine dependency manifest at birth in siblings. Pediatrics 32:161–168 [PubMed] [Google Scholar]
- Yoshida T, Tada K, Arakawa TS (1971) Vitamin B6 dependency of glutamic acid decarboxylase in the kidney from a patient with vitamin B-6 dependent convulsion. Tohoku J Exp Med 104:195–198 [DOI] [PubMed] [Google Scholar]