Abstract
The fibroblast growth factor–receptor 3 (FGFR3) Lys650 codon is located within a critical region of the tyrosine kinase–domain activation loop. Two missense mutations in this codon are known to result in strong constitutive activation of the FGFR3 tyrosine kinase and cause three different skeletal dysplasia syndromes—thanatophoric dysplasia type II (TD2) (A1948G [Lys650Glu]) and SADDAN (severe achondroplasia with developmental delay and acanthosis nigricans) syndrome and thanatophoric dysplasia type I (TD1) (both due to A1949T [Lys650Met]). Other mutations within the FGFR3 tyrosine kinase domain (e.g., C1620A or C1620G [both resulting in Asn540Lys]) are known to cause hypochondroplasia, a relatively common but milder skeletal dysplasia. In 90 individuals with suspected clinical diagnoses of hypochondroplasia who do not have Asn540Lys mutations, we screened for mutations, in FGFR3 exon 15, that would disrupt a unique BbsI restriction site that includes the Lys650 codon. We report here the discovery of three novel mutations (G1950T and G1950C [both resulting in Lys650Asn] and A1948C [Lys650Gln]) occurring in six individuals from five families. Several physical and radiological features of these individuals were significantly milder than those in individuals with the Asn540Lys mutations. The Lys650Asn/Gln mutations result in constitutive activation of the FGFR3 tyrosine kinase but to a lesser degree than that observed with the Lys540Glu and Lys650Met mutations. These results demonstrate that different amino acid substitutions at the FGFR3 Lys650 codon can result in several different skeletal dysplasia phenotypes.
Introduction
Hypochondroplasia (MIM 146000) is an autosomal dominant skeletal dysplasia that was probably first described by Ravenna (1913) but not generally recognized until much later, the term being coined by Maroteaux and Lamy (1960). Although many reports characterizing the phenotypic features have since appeared in the literature (Kozlowski 1965; Beals 1969; Walker et al. 1971; Hall and Spranger 1979; Wynne-Davies et al. 1981; Maroteaux and Falzon 1988), it is sometimes difficult to make a definitive diagnosis of hypochondroplasia solely on the basis of physical and radiological findings, especially in young children and milder cases. Hypochondroplasia and achondroplasia share many phenotypic features (e.g., apparent rhizomelia, limitation of elbow extension, tibial bowing, exaggerated lumbar lordosis, metaphyseal flaring, shortening of the pedicles, narrowing of the lumbar interpediculate distance, squared ilia, shortened femoral necks, brachydactyly, macrocephaly, and distinctive facial features). However, in hypochondroplasia these features tend to be milder, and some are often subtle or absent in any given individual. For these reasons, the diagnosis is frequently not made until later in childhood, when growth delay is first noted; and, consequently, reliable data about the incidence and prevalence of hypochondroplasia have not been compiled.
McKusick et al. (1973) first proposed that achondroplasia and hypochondroplasia are allelic disorders, on the basis of both the similarity of the clinical and radiological features and their analysis of a hypochondroplasia/achondroplasia compound heterozygote. This hypothesis was confirmed later, with the discovery of distinctive mutations, for each disorder, in the fibroblast growth factor–receptor 3 (FGFR3) gene located on human chromosome 4 (4p16.3). Two recurrent mutations in exon 10 (G1138A and G1138C [both resulting in Gly380Arg]) account for ⩾99% of all cases of achondroplasia (Shiang et al. 1994; Rousseau et al. 1994; Bellus et al. 1995a), and two recurrent mutations in exon 13 (C1620A and C1620G [both resulting in Asn540Lys]) account for ∼50%–70% of all cases of hypochondroplasia (Bellus et al. 1995b, 1996; Prinos et al. 1995; Prinster et al. 1998; Ramaswami et al. 1998; Grigelioniene et al. 2000). The etiology of the hypochondroplasia phenotype in most of the remaining individuals remains unknown. Genetic-locus heterogeneity has been established in a few multiplex families with exclusion of linkage to 4p16.3 (Stoilov et al. 1995; Rousseau et al. 1996; Grigelioniene et al. 2000). In addition, several other FGFR3 mutations have been reported recently in isolated families (Ile538Val [Grigelioniene et al. 1998], Asn540Thr [Deutz-Terlouw et al. 1998], Asn328Ile [Winterpacht et al. 2000], and Asn540Ser [Mortier et al. 2000]).
Given that there are now at least two different types of hypochondroplasia (those with known FGFR3 mutations and those without), attempts have been made to identify phenotypic differences between these two groups (Rousseau et al. 1996; Prinster et al. 1998; Ramaswami et al. 1998; Grigelioniene et al. 2000). These studies have demonstrated that patients with FGFR3 Asn540Lys mutations tend to be more disproportionate, have larger heads and shorter hands, and tend to exhibit more of the characteristic radiological findings than are seen in patients without the FGFR3 Asn540Lys mutation. We have been screening selected areas of the FGFR3 coding region for new point mutations in patients with suspected diagnoses of hypochondroplasia who do not have the FGFR3 Asn540Lys mutation. We report here the discovery of three novel FGFR3 mutations (G1950T and G1950C [both resulting in Lys650Asn] and A1948C [Lys650Gln]) occurring in six individuals from five families. A quantitative analysis of selected physical and radiological features of these individuals reveals that they have a milder phenotype than that seen in patients with the FGFR3 Asn540Lys mutation. We also demonstrate that the Lys650Asn/Gln mutations result in constitutive activation of the FGFR3 tyrosine kinase but to a lesser degree than is seen in TD2 (thanatophoric dysplasia type II) Lys650Glu mutations and in SADDAN (severe achondroplasia with developmental delay and acanthosis nigricans) syndrome/TD1 (thanatophoric dysplasia type I) Lys650Met mutations (Kitoh et al. 1998; Tavormina et al. 1999).
Patients and Methods
Recruitment of Patients
Patients were recruited by several of the authors and other collaborating physicians. All patients in whom a diagnosis of hypochondroplasia was considered possible by the referring physician were eligible to be included in the study. Blood samples and clinical data were obtained after patients or their parents gave informed consent. The protocol was approved by the institutional-review-board committees at the University of Colorado and the National Institutes of Health.
Clinical Data
The referring physicians collected clinical and auxological data, and copies of radiographs from all patients with FGFR3 Lys650Asn/Gln mutations were analyzed by one of the authors (G.A.B.). Metacarpophalangeal profiles (MCPs) were generated on the basis of hand radiographs, according to the method of Poznanski (1984). Quantification of other radiological parameters (pelvic index, fibula:tibia length ratio, and L1:L4 interpediculate-distance ratio) was performed according to the method of Matsui et al. (1998). SDs (Z scores) for height and head circumference were calculated from standard growth charts. The two-tailed t-test (Glantz 1997) was used to compare data from patients with either Asn540Lys or Lys650Asn/Gln mutations.
Case Summaries
Family 1: patient 1 (G1950C [Lys650Asn])
This girl was the product of a normal pregnancy, labor, and delivery to average-stature parents (mother, 166 cm; father, 178 cm). Her growth was uneventful until age 3.5 years, when it slowed substantially and did not increase for 12 mo. She was evaluated at age 5 years, for short stature. Her height at age 5 years was 97.4 cm (<3d percentile for age 5 years/50th percentile for age 3.5 years), and her head circumference was >95th percentile. Her physical examination revealed mild frontal bossing, tibial bowing, and some limitation of elbow extension. A skeletal survey was obtained but was not considered to be diagnostic of any skeletal dysplasia. Results of thyroid studies were normal, but bone age showed discrepancy between hand bones (4.5 years) and wrist bones (2.5 years). Karyotype and levels of alkaline phosphatase, calcium, and phosphorus were normal. Cognitive, verbal, and social development were appropriate for age. The patient's growth velocity has decreased slightly with time, and at age 15 years 5 mo her height was 141.9 cm (<3d percentile for age 15 years 5 mo/50th percentile for age 10.5 years), and head circumference was 56 cm (∼95th percentile).
Family 2: patient 2 and patient 3 (G1950C [Lys650Asn])
The girl in family 2 (patient 2) was the product of a normal pregnancy. Growth was reported to be normal during the 1st year of life but then slowed by age 18 mo. She was evaluated for short stature at age 2 years, and no specific causes of growth delay were found. She has had no other significant medical problems, and her development has been completely appropriate. Growth velocity has remained relatively constant. At age 5 years, the patient's height was 96.2 cm (<3d percentile for age 5 years/50th percentile for age 3 years 6 mo), and her head circumference measured 50.5 cm (50th percentile). She has mild lumbar lordosis and normal alignment of her lower legs. Upper extremities show full range of motion, and the patient has normal-appearing hands and feet. The parents’ heights are relatively short (mother, 152 cm; father, 163 cm).
This girl's father (patient 3) has a history of spondylolisthesis and knee pain. His height is shorter than would be expected on the basis of his parents' height (mother, 157.5 cm; father, 183 cm), and his upper:lower segment ratio is 1.14 (>2 SD), indicating disproportionately short legs. His head circumference is large, measuring 60.8 cm (>98th percentile).
Family 3: patient 4 (G1950T [Lys650Asn])
This girl was born at 36 wk gestation, after a twin pregnancy complicated by late preeclampsia. Delivery was by cesarean section; birth weight was 2,894 g (75th percentile), and birth length was 48.3 cm (75th percentile). Acceleration of head-circumference growth was noted at age 3 wk. Linear growth was tracking between the 30th and 50th percentiles until age 1 year and then dropped to the 10th percentile. Head circumference, which had been at the 75th percentile, increased to >2 SD above the 95th percentile by age 15 mo. Developmental progress has been appropriate, although she has been noted to lag 1–2 mo behind her fraternal twin. Parental heights are low average (mother, 155 cm; father, 173 cm), and the patient's fraternal twin is at the 30th–35th percentiles for height and weight. Physical examination at age 15 mo was significant for a height of 73.5 cm (10th percentile) and occipitofrontal circumference (OFC) of 51.3 cm (>2 SD above 95th percentile). There was significant frontal bossing but no evidence of craniosynostosis. The patient had subtle epicanthal folds, a flattened nasal bridge, noticeable lumbar lordosis, subtle rhizomelia, and tibial bowing. Her hands were small, with significant shortening of the phalanges and a “trident” appearance. She had full range of motion at all joints and extra folds of skin on the thighs and upper arms.
Family 4: patient 5 (A1948C [Lys650Gln])
This boy was born vaginally at 35 wk gestation. Results of prenatal ultrasonography at 18 wk gestation showed larger than expected head size, and repeat ultrasounds at 26 and 31 wk gestation revealed shorter than expected extremities. DNA testing for achondroplasia was offered to the patient's parents but was declined. Birth weight, length, and head circumference were all between the 75th and 90th percentiles. At age 15 mo, the patient was evaluated for progressive macrocephaly and had a normal head CT. Hearing and vision are normal, but the patient is thought to have mild delays in motor and speech/language development. At age 18 mo he was referred for genetic evaluation. The parents' heights are average to tall (mother, 168 cm; father, 183 cm). At age 18 mo, the patient's height was 78.1 cm (10th percentile), and his OFC was 53 cm (>95th percentile for age 18 mo/50th percentile for age 10 years). He was noted to have a scaphocephalic head with a large open anterior fontanelle, frontal bossing, mildly depressed nasal bridge, mild shortening of upper and lower extremities with full range of motion, relatively short hands with bilateral single palmar crease, and mild hypotonia.
Family 5: patient 6 (G1950C [Lys650Asn])
This boy's birth weight and length were 4.029 kg and 58.4 cm, respectively. No problems were noted until age 9 mo, when tibial bowing was detected. The patient began walking at age 11 mo, and his tibial bowing increased in severity. Orthopedic and genetic evaluations were performed at age 2 years. Physical examination was notable for a height of 87.6 cm (25th–50th percentile) and head circumference of 52.5 cm (>95th percentile). The forehead was slightly prominent but did not have frontal bossing. Levels of calcium, phosphorus, parathyroid hormone, 1-25 vitamin D, and alkaline phosphatase were normal, and x-rays of the lower extremities were suggestive of achondroplasia/hypochondroplasia with widening of the proximal tibial and distal femoral metaphyses and narrow greater sciatic notches. The parent’s heights are tall to average (mother, 180.3 cm; father, 176.5 cm). Both parental head circumferences exceeded the mean by >+2 SD deviations.
Mutation Analysis
Genomic DNA was isolated from blood samples by established methods (Bellus et al. 1995a). PCR amplification of FGFR3 exons 10, 13, and 15 were performed as described elsewhere (Bellus et al. 1995a, 1995b; Tavormina et al. 1995). Screening for Gly380Arg mutations in exon 10 was performed by digestion with restriction enzymes SfcI and MspI. Screening for exon 13 Asn540Lys mutations was performed by digestion with restriction enzymes AluI and BspMI (Bellus et al. 1996; Prinster et al. 1998), and screening for mutations in exon 15 codons 649–651 was performed by digestion with restriction enzyme BbsI (Tavormina et al. 1995). Products of restriction-enzyme digestion were analyzed by agarose gel electrophoresis and were visualized by staining with ethidium bromide.
PCR products for sequencing of the Lys650Asn mutations were generated by use of nested primers. The forward nested primers were labeled with fluorescein Cy-5, and the reverse nested primers were labeled with Cy-5.5. Sequencing was performed by the two-dye PCR Sequencing Reaction protocol provided by Visible Genetics. The sequence of the PCR products was determined by the Long Read Tower System from Visible Genetics. Sequencing of the Lys650Gln mutations was performed by use of the gel-purified exon 15 PCR product, with a nested sequencing primer, [32P]-dCTP, and a Thermo Sequenase Cycle Sequencing kit (USB). Reaction products were separated on a standard polyacrylamide sequencing gel and were visualized by autoradiography.
FGFR3 Transfection and Autophosphorylation Studies
Point mutations in an FGFR3 cDNA construct were created according to methods described elsewhere (Webster et al. 1996) and were confirmed by sequencing. Transfection, immunoprecipitation, and autophosphorylation studies were performed as described elsewhere (Webster et al. 1996; Tavormina et al. 1999).
Results
Loss of the FGFR3 Exon 15 BbsI Site
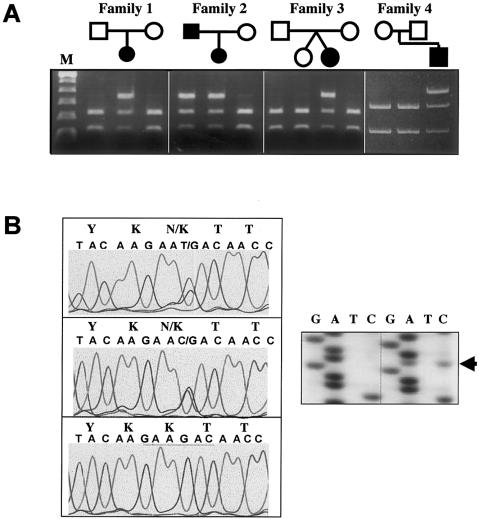
In an ongoing study, we have screened for FGFR3 mutations in 211 individuals with suspected diagnoses of hypochondroplasia and have found that 110 (52.1%) have Asn540Lys mutations (C1620A or C1620G). Of the 101 individuals without Asn540Lys mutations, 11 (10.9%) were shown to have the common FGFR3 achondroplasia mutation (G1138A [Gly380Arg]). By digestion with BbsI, we screened the remaining 90 individuals for alterations in FGFR3 exon 15, and we identified 6 individuals from five families who lost the BbsI site on one allele. Loss of a unique BbsI restriction site in exon 15 has been used to screen for FGFR3 A1948G (Lys650Glu) and A1949T (Lys650Met) mutations found in TD2 (Tavormina et al. 1995) and SADDAN syndrome (Tavormina et al. 1999), respectively. The 6-bp recognition sequence of BbsI spans codons 649–651, and loss of this site will detect any missense mutation in codon 650, as well as any changes in both the last position of codon 649 and the first two positions of codon 651. Polymorphisms do not commonly occur within this sequence, since none were found in screening >100 normal chromosomes (Tavormina et al. 1995). In these six individuals, we determined that the loss of the BbsI site occurred sporadically in three cases and cosegregated with disproportionate and short stature in another family (fig. 1A). Sequencing of the PCR products revealed the presence of three novel mutations that result in Lys650Asn substitutions (produced by both G1950C and G1950T) and in Lys650Gln (A1948C) substitutions (fig. 1B).
Figure 1.
BbsI digestion and DNA sequencing of FGFR3 exon 15 PCR products. A, BbsI digestion of exon 15 PCR products for four families. The mutant allele is not digested in affected individuals. Lane M, 100-bp DNA-ladder standard. B, Automated DNA sequencing, which reveals the presence of a heterozygous G1950C/K650N mutation in patient 1 (top) and a heterozygous G1950T/K650N in patient 4 (middle). Patients 2, 3, and 6 were also found to have G1950C/K650N mutations (data not shown). Control sequence (bottom) is depicted with theBbsI restriction site underlined. Sequencing autoradiography of patient 5 (left gel) shows the presence of a heterozygous A1948C/K650Q mutation (arrow) not seen in the control (right gel).
Quantitative Analysis of Auxological and Radiological Data
On review of photographs (fig. 2) and medical records, it was our general impression that these patients tended to have a milder hypochondroplasia phenotype than we have seen in patients with Asn540Lys mutations. To confirm this impression, a quantitative analysis of auxological and radiological parameters was performed. The auxological parameters were compared with data that we have obtained from 38 individuals with established FGFR3 Asn540Lys mutations. Quantification of radiological data from our patients with FGFR3 Lys650Asn/Gln mutations was performed according to the method of Matsui et al. (1998), and comparison was made with their data set from eight patients with established Asn540Lys mutations. In addition, MCPs were determined for all individuals with Lys650Asn/Gln mutations and were compared both with Prinster et al.'s (1998) data from patients with Asn540Lys mutations and with Hall and Spranger's (1979) data from patients with radiologically confirmed diagnoses of hypochondroplasia.
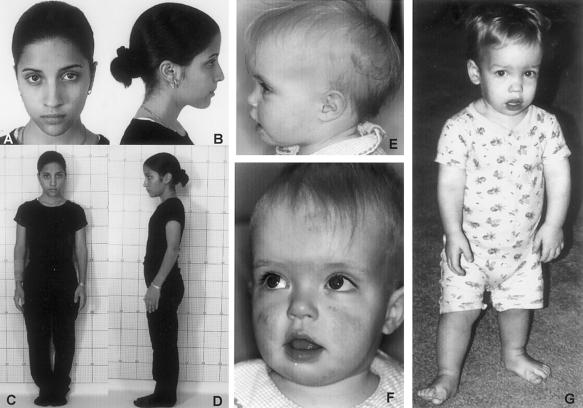
Figure 2.
Photographs of patients. Note the mild shortening of the arms and normal facial appearance in patient 1, who has G1950C (Lys650Asn) and is shown at age 15 years (A–D), whereas patient 4, who has G1950T (Lys650Asn) and is shown at age 15 mo (E and F), and patient 5, who has A1948C (Lys650Gln) and is shown at age 20 mo (G), have more-prominent dysmorphic facial features.
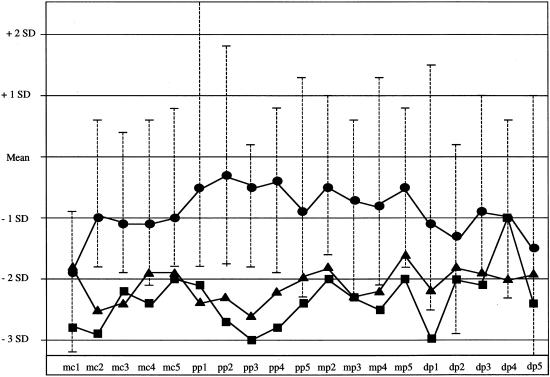
As shown in table 1, patients with Lys650Asn/Gln mutations have a statistically significant smaller height deficit than is seen in patients with Asn540Lys mutations (Z=-1.87 [SD=0.99] vs. Z=-3.25 [SD = 1.09]; P<.02). Disproportion of the upper extremities was similar in both groups, as indicated by the arm span:height ratio. The degree of macrocephaly appeared to be greater in the patients with Lys650Asn/Gln, but it did not quite reach statistical significance. Narrowing of the lumbar interpediculate distances was significantly milder in patients with Lys650Asn/Gln mutations than in patients with Asn540Lys mutations (fig. 3). Both groups showed the typical exaggerated metaphyseal flaring of the proximal tibiae, but disproportionate elongation of the fibulae was significantly less in the patients with Lys650Asn/Gln mutations (fig. 4). The pelvic index was essentially normal in patients with Lys650Asn/Gln and in patients with Asn540Lys mutations. Finally, the MCP of patients with Lys650Asn/Gln mutations was substantially different from that observed in patients with Asn540Lys mutations and in patients with radiologically confirmed hypochondroplasia (figs. 4 and 5). Although there is nearly equivalent shortening of the distal phalanges in all three groups, the patients with Lys650Asn/Gln exhibit generally less disproportion in the metacarpals, proximal phalanges, and middle phalanges.
Table 1.
Phenotypic Data for Patients with either Asn540Lys Mutations or Lys650Asn/Gln Mutations
|
Mean ± SD [No. of Patients] |
|||
| Asn540Lysa | Lys650Asn/Gln | Pb | |
| Auxological data: | |||
| Height (Z score) | −3.25 ± 1.09 [36] | −1.87 ± .99 [6] | <.02 |
| Arm span:height | .995 ± .17 [28] | .982 ± .04 [5] | NS |
| OFC (Z score) | +1.72 ± 1.11 [37] | +2.48 ± 1.65 [6] | <.2 |
| Radiological data: | |||
| L1:L4 interpediculate-distance ratio | 1.02 ± .09 [8] | .91 ± .07 [6] | <.05 |
| Fibula:tibia length ratio | 1.07 ± .03 [8] | 0.91 ± .07 [6] | <.005 |
| Pelvic index | .37 ± .04 [8] | .40 ± .02 [6] | NS |
Data are from Matsui et al. (1998).
NS = not significant.
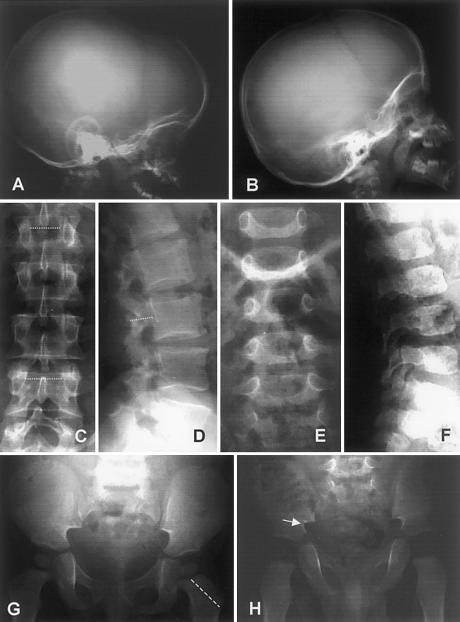
Figure 3.
Radiographs of patients. The skull radiograph of patient 4 at age 15 mo (A) exhibits macrocephaly and frontal bossing, whereas that of patient 6 at age 2 years (B) is less disproportionate. AP spines of patient 3 at age 39 years (C) and of patient 5 at age 18 mo (E) show some widening of the interpediculate distances (i.e., width of the spinal canal), proceeding from L1 to L4, although this is less than normal. Shortening of the lumbar pedicles as seen in the lateral spine views (i.e., depth of spinal canal) is observed in patient 3 at age 39 years (D) but is less severe in patient 5 at age 18 mo (F). The pelvis of patient 4 at age 15 mo (G) is relatively normal, except for shortened femoral necks, whereas patient 5 at age 18 mo (H) shows shortening of the femoral necks and narrowing of the greater sciatic notch.
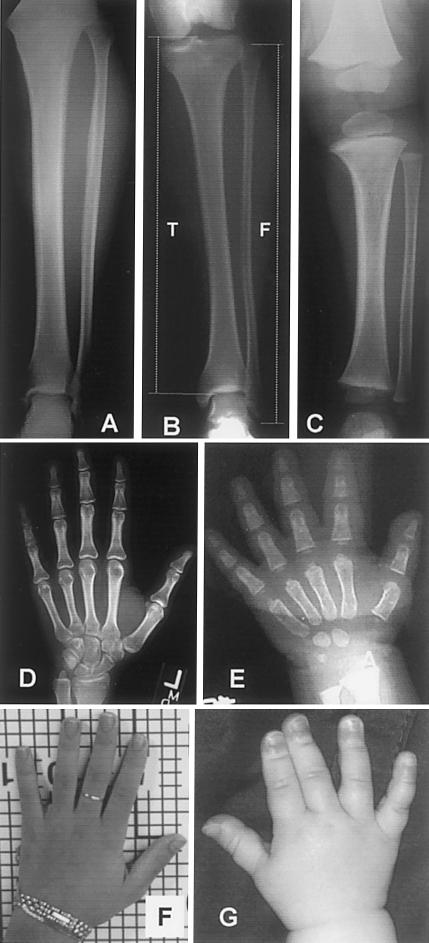
Figure 4.
Radiographs and photographs of extremities. In patient 3 at age 39 years (A), patient 1 at age 13 years (B), and patient 4 at age 15 mo (C), the fibula:tibia length ratio is close to normal. There is moderately exaggerated flaring of the distal femoral and proximal tibial metaphyses in the younger patient (C). There is mild to moderate shortening of the metacarpals and phalanges in patient 1 at age 15 years (D and F), which is more pronounced in patient 4 at age 15 mo E and G).
Figure 5.
MCP. The lengths of the metacarpals and phalanges were measured on the basis of radiographs of the hands of each patient, and Z scores were determined according to the method of Poznanski (1984); the average Z score for each metacarpal (mc1–mc5) and phalanx (pp1–pp5, mp2–mp5, and dp1–dp5) is shown.• = FGFR3 Lys650Asn/Gln (n=6); ▪ = FGFR3 Asn540Lys (n=9) (Prinster et al. 1998); ▴ = radiologically confirmed hypochondroplasia (n=21) (Hall and Spranger 1979). The range in Z scores for each bone is shown for the patients with Lys650Asn/Gln mutations.
Lys650Asn/Gln: Constitutive Activation of FGFR3 Tyrosine Kinase
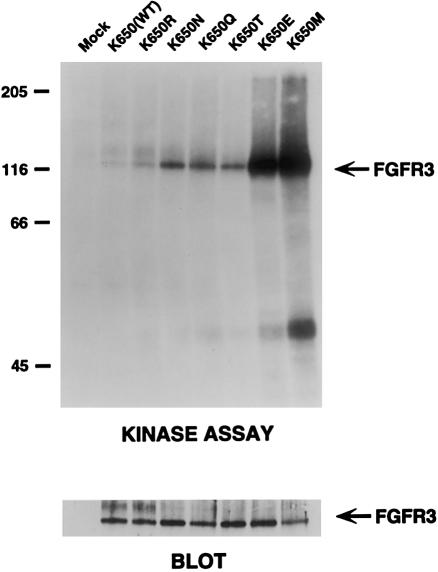
To compare the effect of the FGFR3 Lys650Asn mutations (which were discovered prior to the Lys650Gln mutations) with that of other FGFR3 mutations at codon 650, a series of FGFR3 cDNA constructs representing all of the possible amino acid substitutions resulting from one-nucleotide substitutions in codon 650 were made. These mutant constructs were transiently expressed in NIH 3T3 cells, and cell extracts were immunoprecipitated with an FGFR3 antibody. A [32P]-ATP autophosphorylation assay was used to test the for constitutive kinase activity in the immunoprecipitates, and they were evaluated by gel electrophoresis. As shown in figure 6, the Lys650Asn/Gln and Lys650Thr mutations result in similar degrees of constitutive FGFR3 tyrosine kinase activation (approximately three- to fivefold greater than that in the wild type). The activities of these three mutations are considerably less than those of the Lys650Glu and Lys650Met mutations that are associated with TD2 and SADDAN syndrome, and the latter two mutations are, respectively, ∼10 and ∼18 times greater than that of the wild type.
Figure 6.
Autophosphorylation of FGFR3 cDNA mutant constructs. FGFR3 cDNA constructs comprising all of the possible amino acid substitutions resulting from single nucleotide changes of the Lys650 codon were synthesized and transfected into NIH 3T3 cells. FGFR3 was immunoprecipitated from cell lysates and incubated with [32P]-ATP in an autophosphorylation assay. Equal amounts of immunoprecipitate (lower panel) were then separated by gel electrophoresis and were exposed to x-ray film. The locations of molecular-weight markers (in kD) are shown on the left. The relative activity of the mutations, compared with that in the wild type, was determined by densitometry and is as follows: K650R, 1.5×; K650N, 3.7×; K650Q, 4.9×; K650T, 3.1×; K650E, 9.6×; and K650M, 18.1×.
Discussion
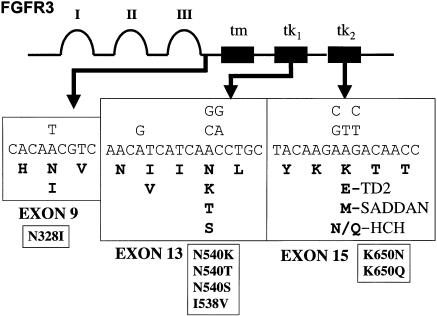
We have discovered within the FGFR3 Lys650 codon three new mutations that result in a mild hypochondroplasia phenotype. We now know of nine missense mutations, which result in seven different amino acid substitutions within four FGFR3 codons that cause hypochondroplasia (fig. 7). The two mutations that result in Asn540Lys substitutions remain, by far, the most common (52% in this study). However, the Lys650Asn/Gln mutations that we have reported here are reasonably common, being detected in 2.8% (6 of 211) of the total number of patients screened and in 6.7% (6 of 90) of patients without other known FGFR3 mutations. These mutations may account for an even higher proportion of true cases of hypochondroplasia, since the inclusion criteria for patients in this study were relatively relaxed and other causes of short stature had not yet been completely eliminated in many of the patients in whom we did not detect FGFR3 mutations.
Figure 7.
FGFR3 hypochondroplasia mutations. A schematic of the FGFR3 protein is shown, and the areas within the three exons where hypochondroplasia missense mutations occur are shown in greater detail. The nucleotide substitutions are shown above, and the amino acid substitutions are shown below; for comparison, the mutations causing TD2 and SADDAN syndrome are also shown.
The facts that these new FGFR3 mutations cause different amino acid substitutions within the same codon that is affected in both TD2 and SADDAN syndrome and result in lower levels of ligand-independent FGFR3 kinase activation further highlights the importance of the Lys650 residue in the biological function of FGFR3. Lys650 is located within the activation loop of the FGFR3 tyrosine kinase domain (Mohammadi et al. 1996), and amino acid substitutions at this position have been shown to have a dramatic effect on the constitutive activation of FGFR3-mediated receptor autophosphorylation (Webster et al. 1996; Tavormina et al. 1999). The activation loop of FGFRs is thought to block substrate binding until FGF binding and receptor dimerization alter its conformation, permitting both autophosphorylation of the receptor and phosphorylation of intermediate signaling molecules (Mohammadi et al. 1996). Webster et al. (1996) have shown that the Lys650 codon plays a critical role in stabilizing the FGFR3 activation loop in an inactive conformation, since different mutations of this residue constitutively activate the tyrosine kinase to varying degrees whereas mutations of adjacent codons have little effect. In addition, mutations of this residue may preclude the need for receptor dimerization at the cell membrane to activate the tyrosine kinase. A similar construct of Xenopus FGFR2 (Lys652Glu) is not inhibited by dominant-negative FGFR constructs that competitively inhibit receptor dimerization (Neilson and Freisel 1996). Raffioni et al. (1998) have shown that chimeric PDGFR-FGFR3 Lys650Glu and Lys650Met constructs expressed in PC12 cells produce only small amounts of mature-size (i.e., glycosylated) receptor protein but exhibit strong ligand-independent autophosphorylation of a smaller, presumably intracellular, form of the receptor.
Our findings establish a clear correlation, in human skeletal dysplasias, between the level of constitutive FGFR3 tyrosine kinase activation due to different amino acid substitutions at the Lys650 codon and the severity of the disturbance in endochondrial ossification. Although the overall TD2 phenotype is more severe than that of SADDAN, and although, in our assay, the Lys650Met mutations were more active than the Lys650Glu mutations, dysplasia of the long bones is generally more severe in SADDAN syndrome than in TD2. These results add further support to the theory that FGFR3 normally functions as a negative regulator of bone growth and that FGFR3 mutations causing skeletal dysplasias result in inappropriate activation of FGFR3 signaling (Colvin et al. 1996; Deng et al. 1996; Naski et al. 1996; Webster et al. 1996).
Aside from the skeletal findings, it is interesting that the patients with Lys650Asn/Glu mutations do not exhibit significant developmental delay or cognitive impairments (although mild delays were suspected in patients 4 and 5), acanthosis nigricans, or craniosynostosis. Abnormal brain development is a feature of TD1, TD2 (Ho et al. 1984), and SADDAN syndrome (Bellus et al. 1999). Postnatal onset of acanthosis nigricans occurs in SADDAN syndrome, in Crouzonodermatoskeletal syndrome (Ala391Glu mutations) (Meyers et al. 1995), and in rare cases of TD1 (Arg248Cys mutations) with prolonged survival (Baker et al. 1997). In both TD2 and Crouzonodermatoskeletal syndrome, craniosynostosis is frequently observed with acanthosis nigricans. The reason why only some FGFR3 mutations are strongly correlated with these phenotypic features is not currently understood. It is likely that some amino acid substitutions do more than modulate ligand-independent activation of the FGFR3 intracellular kinase domain. Raffioni et al. (1998) have demonstrated that PDGFR-FGFR3 chimeras with either Lys650Glu or Lys650Met mutations vary considerably in their ability to activate downstream signaling pathways and to induce neurite outgrowth in PC12 cells, even though both mutations result in comparable constitutive activation of FGFR3 tyrosine kinase.
It is notable that we have found several phenotypic differences between patients with FGFR3 Asn540Lys mutations and those with FGFR3 Lys650Asn/Gln mutations. The difference, in head circumference, between individuals with Lys650Asn/Gln mutations and those with Asn540Lys mutations was not statistically significant in this study, but it is interesting that two of our patients came to medical attention primarily because of progressive macrocephaly with head circumferences that were significantly above the 95th percentile. Given the severity of the craniofacial findings in both TD2 and SADDAN syndrome, it seems possible that mutations at this position may have a greater effect on craniofacial growth than is seen for other FGFR3 mutations associated with hypochondroplasia.
The observation that individuals with FGFR3 Lys650Asn/Gln mutations have milder radiological features than are seen in individuals with Asn540Lys mutations is also interesting. It is notable that, even though complete skeletal surveys were obtained on all six patients with Lys650Asn/Gln mutations, a radiological diagnosis of hypochondroplasia versus achondroplasia was made in only one patient (patient 6), whose height was between the 25th and 50th percentiles. All the other patients showed some features consistent with hypochondroplasia, but these features were sufficiently mild or few in number that this diagnosis could not be made with confidence. Limb disproportion is a common finding among all types of hypochondroplasia. It is interesting that, even though two patients with Lys650Asn mutations were noted to have tibial bowing, the fibula:tibia length ratios of the patients with Lys650Asn/Gln mutations are significantly less disproportionate than those in patients with the Asn540Lys mutations.
It is perhaps most surprising that patients with Lys650Asn/Gln mutations were significantly taller than patients with the Asn540Lys mutations. Even though our one adult patient (patient 3) had significant skeletal disproportion, his height (163 cm) was sufficient to prevent him from being perceived by his family or acquaintances as having dwarfism. Parental heights can influence the height of patients with skeletal dysplasias, but abnormally great parental height does not account for the decreased height deficit observed in our patients. In our patients with Lys650Asn/Gln mutations, the average height of unaffected mothers is 163.1 cm (range 152–180 cm), and the average height of unaffected fathers is 178.7 cm (range 173–183 cm), both of which are near the 50th percentile. In the largest clinical series on hypochondroplasia published to date, Maroteaux and Falzon (1988) reported the average height of adult men to be 146 cm (±4.98 cm), with their tallest patient being 153 cm. Molecular diagnosis was not available at that time, but it is safe to assume that their patients represented a mixture of individuals with and without the FGFR3 Asn540Lys mutation. Our six adult patients with Asn540Lys mutations ranged in height from 128 cm to 142.2 cm. It is notable that some adult patients with either FGFR3 Ile538Val mutations (Grigelioniene et al. 1998) or FGFR3 Asn540Ser mutations (Winterpacht et al. 2000) were also relatively tall, with maximum heights of ∼157 cm in women and 167.9 cm in men, which are, respectively, −1 SD and −1.3 SD from the mean.
Together, these results support the prediction by Rimoin (1975) that individuals with mild forms of hypochondroplasia would blend in with individuals on the shorter end of the normal height spectrum and would not necessarily come to medical attention. Given both the relatively high rate of missense mutations in the FGFR3 gene (Bellus et al. 1995a) and the fact that, in terms of free energy, there is probably a small difference between the active and inactive states of FGFR tyrosine kinase domains (Mohammadi et al. 1996), we suspect that other FGFR3 missense mutations (e.g., Lys650Thr) will be found to cause mild forms of disproportionate and short stature. On the basis of our findings, we believe that FGFR3-mutation analysis is an appropriate study for all patients with short stature, disproportionate limbs, and any radiological changes consistent with hypochondroplasia. Furthermore, it seems likely that some FGFR3 polymorphisms may also play a role in the multifactorial genetic determination of adult height.
Acknowledgments
We thank all the families who participated in this study. We would also like to acknowledge Ginny Szabo, Geralyn Swindle, and Desiree Kelly for excellent technical assistance and Iain McIntosh and Arthur Aylsworth for help with preparation of the manuscript. This study was partially funded by National Institute of Arthritis and Musculoskeletal and Skin Diseases grant 1K08AR002075 (to G.A.B.), by the Genentech Foundation for Growth and Development, and by National Institute of Dental and Craniofacial Research grant R01-DE12581 (to D.J.D.).
Electronic-Database Information
The accession number and URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for hypochondroplasia [MIM 146000])
References
- Baker KM, Olson DS, Harding CO, Pauli RM (1997) Long-term survival in typical type 1 thanatophoric dysplasia. Am J Med Genet 70:427–436 [PubMed] [Google Scholar]
- Beals RK (1969) Hypochondroplasia: a report of five kindreds. J Bone Joint Surg Am 51-A:728–736 [PubMed] [Google Scholar]
- Bellus GA, Bamshad MJ, Przylepa K, Dorst J, Lee RR, Hurko O, Jabs EW, Curry CJ, Wilcox WR, Lachman RS, Rimoin DL, Francomano CA (1999) Severe achondroplasia with developmental delay and acanthosis nigricans (SADDAN): phenotypic analysis of a new skeletal dysplasia caused by the fibroblast growth factor receptor 3 Lys650Met mutation. Am J Med Genet 85:53–65 [PubMed] [Google Scholar]
- Bellus GA, Hefferon TW, Ortiz de Luna RI, Hecht JT, Horton WA, Machado M, Kaitila, McIntosh I, Francomano CA (1995a) Achondroplasia is defined by recurrent G380R mutations of FGFR3. Am J Hum Genet 56:368–373 [PMC free article] [PubMed] [Google Scholar]
- Bellus GA, McIntosh I, Smith EA, Ayslworth AS, Kaitila I, Horton WA, Greenhaw, FA, Hecht JT, Francomano CA (1995b) A recurrent mutation in the tyrosine kinase domain of fibroblast growth factor receptor 3 causes hypochondroplasia. Nat Genet 10:357–359 [DOI] [PubMed] [Google Scholar]
- Bellus GA, McIntosh I, Szabo J, Aylsworth A, Kaitila I, Francomano CA (1996) Hypochondroplasia: molecular analysis of the fibroblast growth factor receptor 3 gene. Ann N Y Acad Sci 785:182–187 [DOI] [PubMed] [Google Scholar]
- Colvin JS, Bohne BA, Harding GW, McEwen DG, Ornitz DM (1996) Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet 12:390–397 [DOI] [PubMed] [Google Scholar]
- Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P (1996) Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 84:911–921 [DOI] [PubMed] [Google Scholar]
- Deutz-Terlouw PP, Losekoot M, Aalfs CM, Hennekam RC, Bakker E (1998) Asn540Thr substitution in the fibroblast growth factor receptor 3 tyrosine kinase domain causing hypochondroplasia. Hum Mutat Suppl 1:S62–S65 [DOI] [PubMed] [Google Scholar]
- Glantz SA (1997) Primer of biostatistics, 4th ed. McGraw Hill, New York, pp 65–107 [Google Scholar]
- Grigelioniene G, Eklof O, Aurencikas E, Ollars B, Hertel NT, Dumanski JP, Hagenas L (2000) The Asn540Lys mutation in FGFR3 and phenotype in hypochondroplasia. Acta Paediatr 89:1072–1076 [DOI] [PubMed] [Google Scholar]
- Grigelioniene G, Hagenas L, Eklof O, Neumeyer L, Haereid PE, Anvret M (1998) A novel missense mutation Ile538Val in the fibroblast growth factor receptor 3 in hypochondroplasia. Hum Mutat 11:333 [DOI] [PubMed] [Google Scholar]
- Ho KL, Chang CH, Yang SS, Chason JL (1984) Neuropathologic findings in thanatophoric dysplasia. Arch Neuropathol (Berl) 63:218–228 [DOI] [PubMed] [Google Scholar]
- Hall BD, Spranger J (1979) Hypochondroplasia: clinical and radiological aspects in 39 cases. Radiology 133:95–100 [DOI] [PubMed] [Google Scholar]
- Kitoh H, Brodie SG, Kupke KG, Lachman RS, Wilcox WR (1998) Lys650Met substitution in the tyrosine kinase domain of the fibroblast growth factor receptor gene causes thanatophoric dysplasia type I. Hum Mutat 12:362–363 [PubMed] [Google Scholar]
- Kozlowski K (1965) Hypochondroplasia. Pol Rev Radiol Nucl Med 29:450–459 [Google Scholar]
- Maroteaux P, Falzon P (1988) Hypochondroplasia: review of 80 cases. Arch Fr Pediatr 45:105–109 [PubMed] [Google Scholar]
- Maroteaux P, Lamy M (1960) Les chondropdystrophies genotypiques. L’Expansion Scientifique Francaise, Paris, pp 26–27 [Google Scholar]
- Matsui Y, Yasui N, Kimura T, Tsumaki N, Kawabata H, Ochi T (1998) Genotype phenotype correlation in achondroplasia and hypochondroplasia. J Bone Joint Surg Br 80:1052–1056 [DOI] [PubMed] [Google Scholar]
- McKusick VA, Kelly TE, Dorst JP (1973) Observations suggesting allelism of the achondroplasia and hypochondroplasia genes. J Med Genet 10:11-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers GA, Orlow SJ, Munro IR, Pryzlepa KA, Jabs EW (1995) Fibroblast growth factor receptor 3 (FGFR3) transmembrane mutation in Crouzon syndrome with acanthosis nigricans. Nat Genet 11:462–464 [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Schlessinger J, Hubbard SR (1996) Structure of the FGF receptor tyrosine kinase domain reveals a novel autoinhibitory mechanism. Cell 86:577–587 [DOI] [PubMed] [Google Scholar]
- Mortier G, Nuytinck L, Craen M, Renard JP, Leroy JG, DePaepe A (2000) Clinical and radiological features of a family with hypochondroplasia owing to a novel Asn540Ser mutation in the fibroblast growth factor receptor 3 gene. J Med Genet 37:220–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naski, MC, Wang Q, Xu J, Ornitz DM (1996) Graded activation of fibroblast growth factor receptor 3 by mutations causing achondroplasia and thanatophoric dysplasia. Nat Genet 13:233–237 [DOI] [PubMed] [Google Scholar]
- Neilson KM, Freisel R (1996) Ligand-independent activation of fibroblast growth factor receptors by point mutation in the extracellular, transmembrane and kinase domains. J Biol Chem 271:25049–25057 [DOI] [PubMed] [Google Scholar]
- Poznanski AK (1984) The hand in radiologic diagnosis, 2d ed. Vol 1. WB Saunders, Philadelphia, pp 31–66 [Google Scholar]
- Prinos P, Costa T, Sommer A, Kilpatrick MW, Tsipouras P (1995) A common FGFR3 mutation in hypochondroplasia. Hum Mol Genet 4:2097–2101 [DOI] [PubMed] [Google Scholar]
- Prinster C, Carrera P, Delmaschio M, Weber G, Maghnie M, Vigone MC, Mora S, Tonini G, Rigon F, Beluffi G, Severi F, Chiumello G, Ferrari M (1998) Comparsion of clinical-radiological and molecular findings in hypochondroplasia. Am J Med Genet 75:109–112 [DOI] [PubMed] [Google Scholar]
- Raffioni S, Zhu YZ, Bradshaw RA, Thompson LM (1998) Effect of transmembrane and kinase domain mutations on fibroblast growth factor receptor 3 chimera signaling in PC12 cells. J Biol Chem 273:35250–35259 [DOI] [PubMed] [Google Scholar]
- Ramaswami U, Rumsby G, Hindmarsh PC, Brook CG (1998) Genotype and phenotype in hypochondroplasia. J Pediatr 133:99–102 [DOI] [PubMed] [Google Scholar]
- Ravenna F (1913) Achondroplasie et chondrohypoplasie: contribution clinique. N Iconogr Salpetriere 26:157–184 [Google Scholar]
- Rimoin DL (1975) The chondrodystrophies. Adv Hum Genet 5:1–118 [DOI] [PubMed] [Google Scholar]
- Rousseau F, Bonaventure J, Legeai-Mallet L, Pelet A, Rozet JM, Maroteaux P, Le Merrer F, Munnich A (1994) Mutations in the gene encoding fibroblast growth factor receptor 3 in achondroplasia. Nature 371:252–254 [DOI] [PubMed] [Google Scholar]
- Rousseau F, Bonaventure J, Legeai-Mallet L, Schmidt H, Weissenbach J, Maroteaux P, Munnich A, Le Merrer F (1996) Clinical and genetic heterogeneity of hypochondroplasia. J Med Genet 33:749–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiang R, Thompson LM, Zhu YZ, Church DM, Fielder TJ, Bocian M, Winokur ST, Wasmuth JJ (1994) Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell 78:335–342 [DOI] [PubMed] [Google Scholar]
- Stoilov I, Kilpatrick MW, Tsipouras P, Costa T (1995) Possible genetic heterogeneity in hypochondroplasia. J Med Genet 32:492–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavormina PL, Bellus GA, Webster MK, Bamshad MJ, Fraley AE, McIntosh I, Szabo J, Jiang W, Jabs EW, Wilcox WR, Wasmuth JJ, Donoghue DJ, Thompson LM, Francomano CA (1999) A novel skeletal dysplasia with developmental delay and acanthosis nigricans is caused by a Lys650Met mutation in the fibroblast growth factor receptor 3 (FGFR3) gene. Am J Hum Genet 64:722–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavormina PL, Shiang R, Thompson LM, Zhu YZ, Wilkin DJ, Lachman RS, Wilcox WR, Rimoin DL, Cohn DH, Wasmuth JJ (1995) Thanatophoric dysplasia (types I and II) caused by distinct mutations in fibroblast growth factor receptor 3. Nat Genet 9:321–328 [DOI] [PubMed] [Google Scholar]
- Walker BA, Murdoch JL, McKusick VA, Langer LO, Beals RK (1971) Hypochondroplasia. Am J Dis Child 122:95–104 [DOI] [PubMed] [Google Scholar]
- Webster MK, d'Avis PY, Robertson SC, Donoghue DJ (1996) Profound ligand-independent kinase activation of fibroblast growth factor receptor 3 by the activation loop mutation responsible for a lethal skeletal dysplasia, thanatophoric dysplasia type II. Mol Cell Biol 16:4081–4087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterpacht A, Hilbert K, Stelzer C, Schweikardt T, Decker H, Segerer, Spranger J, Zabel B (2000) A novel mutation in FGFR-3 disrupts a putative N-glycosylation site and results in hypochondroplasia. Physiol Genomics 2:9–12 [DOI] [PubMed] [Google Scholar]
- Wynne-Davies, R, Walsh WK, Gormley J (1981) Achondroplasia and hypochondroplasia: clinical variation and spinal stenosis. J Bone Joint Surg Br 63:508–515 [DOI] [PubMed] [Google Scholar]